This study presents the results of experiments conducted on the granulation of copper sulfide (Cu2S) melt. The granulometric characteristics of the samples are calculated as a function of the melt temperature. An increase in the melt temperature to 1,350°C promotes the formation of particles with a particle size of –10.0 +5.0 mm, and a decrease in the melt temperature to 1,250°C promotes the formation of particles with smaller size grades (i.e., –1.6 +1.0, –1.0 +0.63, and –0.63 +0.063 mm). With an increase in temperature, the mean squared deviation of particle size from the average value and the degree of polydispersity decreases to some extent. This study also estimates the influence of the melt temperature on the shape of the granules. The cooling and spheroidization times of particles during granulation at the melt temperatures of 1,250°C, 1,300°C, and 1,350°C were calculated. In the temperature range under consideration for all of the investigated size grades of particles, the cooling time exceeded the spheroidization time, which contributed to the formation of spherical particles. The melt temperature did not significantly affect the chemical composition of Cu2S granules. The main phase component of the granules was chalcosine (Cu2S) with a monoclinic lattice of more than 80%. Chalcosine (Cu2S) with a hexagonal lattice of 8% to 9% and digenite (Cu1.78S) with 4% to 12% were also identified. Dispersed inclusions of metallic copper were also detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The bulk of copper (Cu) is recovered from sulfide concentrates and intermediates [1,2,3]. Their processing technologies are based on pyrometallurgical processes associated with the formation of sulfur dioxide, part of which enters the atmosphere [4]. The development of autogenous processes in Cu metallurgy increased the Cu content in matte to 55%; therefore, further hydrometallurgical processing of Cu became reasonable. Recently, along with the pyrometallurgical methods, hydrometallurgical processing techniques, such as lixiviating in solutions of various solvents, pressure leaching, and bioleaching [5,6,7], have become more widespread. In particular, Chizhikov et al. [8] proposed the electrochemical dissolution of sulfide anodes in sulfuric acid solution to obtain a cathode metal. Electrolysis was implemented at various enterprises [9, 10] but did not become widely utilized in the industry because of the difficulties associated with the low mechanical strength and uneven dissolution of massive sulfide anodes.
Selivanov et al. [11] proposed an alternative method for the electrochemical processing of granular sulfide alloys in the form of a bulk anode. Its novelty consists in a special method for melt preparation, i.e., granulation [12, 13], which is characterized by a high crystallization rate. Cooling of a sulfide melt by granulation leads to the formation of an ultradispersed structure, the stabilization of high-temperature nonstoichiometric phases, a decrease in the proportion of the metal component up to its complete elimination, and a significant increase in the reaction surface [14].
The proposed electrolysis method with a bulk anode can be adapted for the processing of copper sulfide (Cu2S) materials to obtain elemental metallic Cu and sulfur (S). This study presents the results of experiments conducted on the granulation of pure Cu2S as the predominant phase of industrial Cu-containing intermediates.
This Study Aims to investigate the influence of the granulation modes of Cu2S melt on various characteristics of the particles obtained.
The Scientific Novelty of this study consists in the assessment of the effect of the cooling rate of Cu2S particles during granulation on their granulometric characteristics, such as shape, size, material homogeneity, and degree of polydispersity, as well as on the structure and phase composition depending on the size of the granules.
Research Methodology
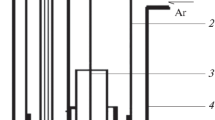
Sulfide melt was granulated by the pool method on a laboratory unit (Fig. 1).
Cu2S of extra pure grade, containing 80% Cu and 20% S, was used as a starting material for the preparation of the granules. According to the Cu2S phase diagram, the melting point of a compound with this composition is 1,130 °C. The temperatures for granulation were selected experimentally. Cu2S weighing 200 g was heated in an electric resistance furnace to temperatures of 1,250 °C, 1,300 °C, or 1,350 °C. The melt was kept at a given temperature for 10 min, poured into a container with water, and stirred. The resulting granules were dried. The granule size grades were determined by the sieve analysis method using standard sets of sieves. The granulometric characteristics of the samples were calculated according to the known equations [15]. The proportion (Ni) of the granulated product in each fraction of the sample was calculated using the following equation:
where mi is the mass of the i th fraction.
The average size (di) of the material particles is determined using the following equations:
where dav is the geometric mean diameter of the particles (mm) and dmax and dmin are the maximum and minimum diameters of the i th fraction granule (mm), respectively.
To assess the homogeneity of the bulk material, the σ value was used, which characterized the mean squared deviation of particle size from the average value:
The degree of polydispersity, which is the coefficient of variation, was calculated as the ratio of the mathematical variance to the average linear size:
The final shape of the granule particles depends on whether the drop from which this particle was formed has time to spheroidize (τsph) and solidify (τcool), or there will be no conditions for this [13]. If the cooling time exceeds the spheroidization time (i.e., τcool > τsph), then the particles are spherical. By contrast, if τcool < τsph, then the particles are nonspherical. Under the condition that τcool < τsph, the shape of the particles is splintery and branched. To estimate the cooling time of the Cu2S melt and the shape of the granules, calculations were performed using the following equation:
where dd is the drop size, mm, ρc is the density of the Cu2S melt (i.e., 5,700, 5,500, and 5,400 kg/m3 at the melt temperatures of 1,250 °C, 1,300 °C, and 1,350 °C, respectively) [16], Cc is the heat capacity of the Cu2S melt at the melt temperatures of 1,250 °C to 1,350 °C (it is assumed to be 0.62 kJ/kg·°C), tm, tc, tg are the temperatures of the melt, onset of crystallization (tc = 1,130 °C), and gas (tg = 100 °C), respectively, and α is the coefficient of melt–gas heat transfer (103 W/m2 ·°C) [15].
On the basis of the equality of the volumes of the drop and the cylinder (strand) transformed into a drop, the spheroidization time was estimated using the following equation:
where η is the coefficient of dynamic viscosity (i.e., 4.3·10–3 at 1,200 °C and 3.7·10–3 N s/m2 at 1,300 °C [17]) and δ is the coefficient of surface tension (i.e., 380·10–3, 390·10–3, and 395·10–3 N/m at the melt temperatures of 1,250 °C, 1,300 °C, and 1,350 °C, respectively [16]).
Analytical devices, namely, infrared absorption analyzer (CS-230), optical atomic emission spectrometer with inductively coupled plasma (Spectroflame MODULA S), and atomic absorption spectrometer (Solar M6), were used to verify the composition of the products. X-ray phase analysis was performed on an XRD-7000 Maxima diffractometer (Shimadzu) in Cu-Kα radiation in the scattering angle (2θ) range of 10° to 70°. The microstructure of Cu granules was examined using an Olympus GX51 optical microscope.
Research Results
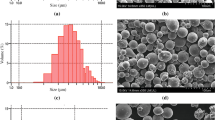
In the course of the experiments, the granules obtained were quenched at different temperatures. The resulting granules have a gray/blue color and a near-spherical shape. However, splintery particles are also observed. The results of sieving by size grades are presented in Table 1 and Fig. 2.
The intensity of pouring the melt into water (or the jet diameter) affects the granulation performance. Table 2 and Fig. 2 show that the maximum amount of the fraction with the size grade –5.0 +2.5 mm was obtained.
An increase in the melt temperature from 1,250 °C to 1,300 °C and 1,350 °C leads to an increase in the amount of the fraction with the size grade –10.0 +5.0 mm. By contrast, at 1,250 °C, the yield of smaller particles, i.e., the size grades –1.6 +1.0, –1.0 +0.63, and –0.63 +0.063 mm, increases.
Thus, an increase in the overheating temperature promotes the formation of larger granules, whereas a decrease in the melt temperature to 1,250 °C promotes the formation of smaller granules. The calculated values of the average diameter of the material (Table 2) also confirm this conclusion. With an increase in temperature, the mean squared deviation of particle size from the average value and the degree of polydispersity decrease to some extent.
According to the calculations of the cooling and spheroidization times (Table 3) at the crystallization temperature of 1,130 °C, in the investigated temperature range for particles of all size grades, the condition τcool > τsph is met, which contributes to the formation of spherical particles.
The metallographic data presented in Fig. 3 reveal that the granules are formed by the sulfide phase and have micropores and microcracks in their volume. In some granules, regardless of the size grade, metallic Cu inclusions are detected. Crystallization of the metal phase is caused by nonequilibrium cooling (quenching) of the high-temperature state. With an increase in the cooling rate typical for particles of smaller fractions, the formation of more metallic Cu inclusions can be expected.
The chemical analysis results of the fractions with the size grades –5.0 +2.5, –2.5 +1.6, and –1.6 +1.0 mm presented in Table 4 indicate that the temperature of the melt and the particle size grade have no effect on the chemical composition of the granules. An insignificant decrease in the Cu content with a decrease in the granule size indicates local crystallization of metallic Cu and a decrease in its amount in the sulfide phase.
According to the X-ray phase analysis, in accordance with the ICDD database, PDF4 (Card No. 96-900-8288), the main phase component (Table 5) of all of the investigated samples is the Cu2S phase, which has a monoclinic lattice. The Cu2S phase with a hexagonal lattice (Card No. 96-231-0610) was detected in a smaller amount. In some samples, the phase of nonstoichiometric Cu2S of the digenite modification (Cu1.78S) was also detected. The structure of digenite is characterized by a deficit of Cu in the crystal lattice relative to the stoichiometric composition.
The presence of digenite in the samples also revealed that part of Cu is crystallized in the form of a metallic phase. Its content was not calculated by the X-ray phase analysis because of its insignificant amount, uneven distribution, and overlapping peaks. The absence of the Cu1.78S phase in the samples quenched at a temperature of 1,300 °C is rather random. However, with an increase in the overheating temperature and a decrease in the cooling rate of particles (valid for particles with a large diameter), the fraction of the stoichiometric compound Cu2S is assumed to increase. By contrast, with a decrease in the overheating temperature and particle size, the formation of metallic Cu inclusions and nonstoichiometric Cu2S of the digenite modification (Cu1.78S) can be expected.
The results obtained can be used to elaborate and optimize the granulation mode of alloys based on Cu2S in subsequent electrochemical processing.
Conclusions
-
1.
Granulation of molten Cu2S at temperatures of 1,250 °C, 1,300 °C, and 1,350 °C was performed in a laboratory unit. An increase in the melt temperature to 1,350 °C promotes the formation of larger granules (i.e., size grade –10.0 +5.0 mm), and a decrease in the melt temperature to 1,250 °C promotes the formation of smaller granules (i.e., size grades –1.6 +1.0, –1.0 +0.63, and –0.63 +0.063 mm). With the increase in temperature, the mean squared deviation of particle size from the average value and the degree of polydispersity decrease to some extent.
-
2.
The cooling and spheroidization times at the melt temperatures of 1,250 °C, 1,300 °C, and 1,350 °C were calculated. In the entire temperature range under consideration, the cooling time of the particles significantly exceeds the spheroidization time, which contributes to the formation of spherical particles.
-
3.
The main phase component of the granules is chalcosine (Cu2S) with a monoclinic lattice of more than 80%. Chalcosine (Cu2S) with a hexagonal lattice of 8% to 9% and digenite (Cu1.78S) with 4% to 12% were also identified. As the melt temperature and particle size decrease, the formation of digenite and metallic Cu inclusions can be predicted.
This study was supported by the Russian Foundation for Basic Research and the Sverdlovsk Region within the Scientific Project No. 20-48-660020.
References
R. B. Gordon, “Production residues in copper technological cycles,” Resources, Conservation and Recycling, 36, 2, 87–106 (2002).
S. S. Naboychenko, N. G. Ageev, A. P. Doroshkevich, et al., Processes and Devices of Non-Ferrous Metallurgy: Textbook for Universities [in Russian], GOU VPO UGTU–UPI, Yekaterinburg (2005).
M. Wang and W. Li X. Chen, “Substance flow analysis of copper in production stage in the U.S. from 1974 to 2012,” Resources, Conservation and Recycling, 105, 36–48 (2015).
R. Moskalyk and A. Alfantazi, “Review of copper pyrometallurgical practice: today and tomorrow,” Minerals Engineering, 16(10), 893–919 (2003).
D. Dreisinger, “Copper leaching from primary sulfides: Options for biological and chemical extraction of copper,” Hydrometallurgy, 83, 10–20 (2006).
F. Letowski, B. Kolodziej, M. Czernecki, et al., “A new hydrometallurgical method for the processing of copper concentrates using ferric sulphate,” Hydrometallurgy, 4(2), 169–184 (1979).
B. Xu, H. Zhong, and T. Jiang, “Recovery of valuable metals from Gacun complex copper concentrate by two-stage countercurrent oxygen pressure acid leaching process,” Miner. Eng., 24(10), 1082–1083 (2011).
D. M. Chizhikov, L. V. Pliginskaya, Z. F. Gulyanitskaya, et al., Patent USSR No. 280858, Electrochemical Method for Processing Copper-Nickel Matte, submitted 05/08/1968, published 09/03/1970, Bul. No. 28.
D. M. Chizhikov, Z. F. Gulyanitskaya, N. A. Gurovich, and I. N. Kitler, Hydrometallurgy of Sulfide Alloys and Mattes [in Russian], Izdatelstvo AN SSSR, Moscow (1962).
V. V. Mikhailov, V. P. Schastlivy, and G. R. Pevzner, “Aspects of the use of electrolysis of nickel sulfide anodes at foreign plants,” Bul. CIIN. Tsvetn. Metallurg., No. 20, p. 33 (1971).
E. N. Selivanov, O. V. Nechvoglod, L. Yu. Udoeva, et al., Patent RF No. 2434065, Method of Processing Sulfide Copper-Nickel Alloys, submitted 08/18/2010; published. 11/20/2011, Bul. No. 32.
R. Maharjan and S. H. Jeong, “High shear seeded granulation: Its preparation mechanism, formulation, process, evaluation, and mathematical simulation,” Powder Technol., 366, 667–688 (2020).
S. S. Naboichenko, Non-Ferrous Metal Powders [in Russian], Metallurgiya, Moscow (1997).
E. N. Selivanov, O. V. Nechvoglod, and V. G. Lobanov, “The effect of the nickel sulphide alloys structure on their electrochemical oxidation parameters,” IFAC Proceedings Volumes, 46(16), 259–262 (2013).
L. Yu. Udoeva, E. N. Selivanov, S. E. Klein, and N. I. Selmenskikh, “Granulation of copper-nickel matte: dispersion, microstructure, and reactivity,” Tsvetn. Metall., No. 12, 20–35 (2013).
S. E. Vaysburd, Physicochemical Properties and Structural Aspects of Sulfide Melts [in Russian], Metallurgiya, Moscow (1996).
A. V. Vanyukov and V. Ya. Zaitsev, The Theory of Metallurgical Processes [in Russian], Metallurgiya, Moscow (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 1, pp. 71–75, January, 2021.
Rights and permissions
About this article
Cite this article
Nechvoglod, O.V., Sergeeva, S.V. & Agafonov, S.N. Study of the Influence of the Granulation Modes of Cu2S Melt on the Granulometric and Structural Characteristics of Particles. Metallurgist 65, 82–89 (2021). https://doi.org/10.1007/s11015-021-01135-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01135-8