Investigations of the process of liquid-phase sintering of powder compositions of the Cu–Al system were carried out. As a result, the dependence of their final porosity indicators on the corresponding initial factors, comprising sintering temperatures and the number of constituent components, was revealed. Based on the obtained experimental data, the dependences of the microstructure of Cu–Al system compositions having a 25% aluminum content on sintering temperature and aluminum concentration are presented. In the course of the research, the dependence of the aluminum solid phase distribution in the Cu–Al system on the sintering temperature was studied at various concentrations. Experimental data on determining the porosity of the Cu–Al system compacts, the amount of the solid phase component in the liquid, as well as the amount of aluminum in the solid phase after sintering, depending on its initial concentration, are presented. The sintering of Cu–Al system compacts is shown to proceed with the participation of the liquid phase. In this case, changes in the dimensions of the sintered briquettes, on which the stability of the resulting powder blanks depends, turns out to be important. The technique and results of dilatometric studies of changes in compact dimensions depending on the sintering temperature and the initial porosity of the workpieces are presented. Additionally, the dependence of the relative change in the sample length and the medium temperature on the aluminum composition concentration was investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the creation of metal powder compositions for various technical purposes, an important role is played by the choice of makeup and ratios of components. Currently, among the many powder materials produced in various countries, copper-based compositions occupy a special place. The main reason for their demand consists in their good pressing processability characteristics. In addition, blanks obtained from these materials have low porosity and the most durable frame following sintering, thus explaining their use in various fields of industry. However, due to the high cost of copper and its inclusion in the list of strategic elements, the problem of minimizing its percentage in compositions with the condition of preserving the most important valuable properties of manufactured products is becoming increasingly important. From this point of view, the creation of new powder compositions of the Cu–Al system having the above-mentioned properties can be considered a very urgent scientific and technical problem [1,2,3].
The large difference between the melting temperatures of Cu (1089°C) and Al (659°C) accounts for the relevance of studying their interaction during sintering. The highly plastic properties of copper and aluminum ensure the process of pressing a mass comprised of metal powders to be carried out without high energy costs, in particular, high pressing pressure. Thus, obtaining finely porous compacts of a given composition presents no technological difficulties that also affect technical and economic efficiency, i.e., involving low material and labor costs, constituting the main object of this study.
Sintering in the Cu–Al system occurs with the participation of the aluminum liquid phase. The features of the liquid-phase sintering of this system, including in combustion mode, were investigated in [4,5,6,7,8,9]. At temperatures exceeding the eutectic (548°C), one of the reasons for the increase in the linear dimensions of compacts is hypothesized to consist in an increase in their porosity caused by the diffusion of aluminum atoms from the liquid to the solid phase. Earlier [10], a similar explanation was given for the observed increase in the size of Fe–Cu system compacts during their sintering in the temperature ranges exceeding the melting point of copper. Presumably, this can be explained in terms of the diffusion of copper atoms from the melt into iron particles.
According to the generally accepted point of view, the authors of [4,5,6,7,8,9,10] also assumed the liquid-to-solid diffusion of atoms to begin after saturation of the melt with the dissolving component of the solid phase.
In the considered systems under certain sintering conditions, along with the volumetric increase of compacts, in some cases their shrinkage is observed. In [4,5,6,7,8], this was explained by the rearrangement of solidphase particles in liquid and solid-phase sintering, while, in [8], the process of dissolution-precipitation was proposed as the main reason.
Based on the fact that the destruction of the solid-phase frame by a liquid alloy should cause a change in the shape of the compact, the author of [10] excludes the possibility of a shrinkage mechanism by regrouping particles, since no such destruction is observed.
Thus, the conclusion can be drawn about the mechanism of volumetric changes in compacts under liquid-phase sintering, in particular, their relationship and sequence, having yet to be investigated in full. For example, in the above scientific papers, the issue about the consistency of particle rearrangement and increase in the volume of compacts is not considered.
In [11], a model of the volumetric change of compacts in the liquid-phase combining the components is proposed. This model was developed on the basis of the idea that the dissolution of the solid phase components in the liquid melt is first preceded and then accompanied by the diffusion of atoms in the opposite direction. In addition, this model is based on a thermodynamic evaluation of the driving forces of such processes as the formation of alloys and shrinkage of compacts of interacting systems.
According to this model, the sign of the change in the volume of individual particles of the solid phase during its interaction with the liquid phase is determined by the predominant direction of the diffusion flux of atoms at the interface. The transition of the solid phase atoms into the melt is accompanied by a decrease in the particle size [12], while the diffusion of atoms from the liquid phase into the solid leads to an increase in their volume [13].
When both flux directions exist, the sign and magnitude of the volumetric change in particles are determined by the difference in diffusion fluxes [14]. The granules constituting the solid phase, being in mechanical contact with each other, form a relatively rigid compact frame. Therefore, a change in the volume of the solid phase particles making up the frame of the compact in one direction or another affects the volume as a whole. Such a model provides for an accurate calculation of the final porosity M (%) of a compact sintered in the presence of a liquid phase, if the initial porosity M0, atomic concentration of the additive K (at. %), its quantity following sintering, as well as the Ksol amount of solid phase component in Kliq liquid, are known:
where R is a coefficient (0 < R <1) taking into account the possible rearrangement and difference in the conditions of solubility of solid phase particles in the melt, the positions of liquid and solid phase formation, as well as in free zones.
The volumetric changes in Cu–Al system compacts in a liquid-phase system were investigated on the basis of the theoretical premises presented above.
The present study is aimed at investigating the formation processes for the linear dimensions and final porosity of compacts from a Cu–Al composition during their liquid-phase sintering, in particular, the essence of the influence of the initial parameters (initial porosity and medium temperature) on the course of this process.
The scientific novelty of the study involves the established features of volumetric changes and some regularities of exothermic sintering of powder compositions consisting of dispersed particles of the Cu–Al system. Criteria of plasticity and the packing nature of the Cu–Al system porous compositions with a specific concentration of constituent components are determined.
The practical significance of the study lies in the creation of new composite materials with improved mechanical and technological properties, as well as increased economic performance. A method for the exothermic sintering of aluminum alloys having a high copper content has been investigated with a view to reducing energy consumption during production and shortening the process duration. The materials produced by this method can be used in such industrial areas as the production of aircraft, electrical equipment, and shipbuilding, where, along with low specific gravity and production costs, high resistance to mechanical wear, dynamic loads, and corrosion is required.
Initial Materials and Research Methods
Based on the theoretical premises presented above, the volumetric changes in Cu–Al system compacts in a liquid-phase system were investigated. For this, a powder mixture was prepared, and a vacuum dilatometer was constructed.
PMS-2 electrolytic copper powder and ACD-2 aluminum powder were used to prepare a powder charge. The charge mixtures consisted of 15, 25, 35, and 45% aluminum powder. The rest part of the mixture included copper powder. The initial porosity of raw compacts with a diameter of 12 mm and a height of 14–18 mm, constructed for the study of changes in linear dimensions during sintering, varied in the range from 10 to 40%.
The sintering process was carried out for 1 h at temperatures of 550, 680, 880, and 980°C in a vacuum of 0.1 Pa. Taking into account the good shape preservation of the samples after sintering, their volume was calculated by measuring the linear dimensions when determining porosity.
In order to measure the linear dimensions of the Cu–Al system compacts in the process of liquid-phase sintering, a specially designed vacuum dilatometer with H117 light-beam oscillograph was used for measuring the length of the sintered compact and reference sample, as well as determining the temperature both of the compact itself and its medium. A thermocouple probe made of a thin chromel-alumel alloy wire was used as a temperature sensor, one of the connections being placed in the channel of the pierced compact.
Along with positive features, the sintering of powder compositions with the participation of a liquid phase also has negative aspects. So, for example, if the presence of a liquid phase strengthens the powder porous frame, then the same phase provides no stable dimensions of the briquette to be obtained after sintering. Therefore, dilatometric studies of the linear dimensions of the Cu–Al system compacts during liquid-phase sintering represent a very important aspect of this research.
The design of the above dilatometer promotes for determining the change in the linear dimensions of the compact with an accuracy of 10−5 cm (± 2 mm).
Results of Experimental Studies
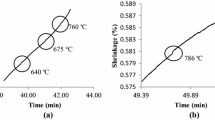
Figure 1a shows a dilatogram obtained as a result of studying the sintering process of a briquette pressed from copper powder with up to 25 at.% of grade ASD-1 aluminum powder at a temperature of 500°C.
Dependence of the dimensions (1–5) and the temperature (1′–5′) of Cu–25Al (a) and Cu–45Al (b) system compacts on their M0 initial porosity during sintering: (a) 1, 1′ – 15%; 2, 2′ – 25%; 3, 3′ – 35%; 4, 4′ – 45%; 5, 5′ – 55%; (b) 1, 1′ – 18%; 2, 2′ – 26%; 3, 3′ – 36%; 4, 4′ – 46%; 5, 5′ – 56%; the relative change in the length of the reference sample (6) and the temperature of the medium (7).
We note that the relative density of the briquette was 67%. At the moment of the liquid phase formation (melting of the eutectic), which occurred at the 30th minute of the sintering process, the sample temperature significantly exceeded the furnace temperature. In such cases, at a temperature of 500°C, the size of the compact increases abruptly within a 10-fold advance of the coefficient of thermal expansion.
In the course of the conducted studies, an increase in the compact dimensions unassociated with thermal expansion was revealed to coincide in time with the formation of a thermal effect. This phenomenon is due to the diffusion of aluminum atoms in copper as the liquid alloy flows over the surface of copper particles during the formation of intermetallic compounds. In this case, the greater the porosity of the raw compacts, the lower their size increase, which is in good agreement with theoretical predictions. In reality, based on formula (1), the change in the porosity of the compact as a result of its sintering can be represented in the form of two terms [2,3,4].
The temporal coincidence of a sharp increase in the temperature of the compact with an increase in its dimensions leads to the idea that the final volumetric changes of the Cu–Al system during sintering are associated with the formation of intermetallic compounds, since it is their formation that is accompanied by the release of heat. With a low content of aluminum additive (K = 0.25) across the entire investigated range of porosity values, the first term is greater in absolute value than the second; this corresponds to an increase in the size of compacts (ΔM > 0). With an increase in porosity, the self-decrease was experimentally established to occur in this parameter.
A somewhat different pattern is observed with an increase in the amount of aluminum to 45% in the mixture (see Fig. 1b). With an increase in the porosity of the compacts, their volumetric increase gradually decreases, giving way to shrinkage \( \left(\overline{\varDelta M}<0\right). \)
In this case, in formula (2), the first term is less than the second at some values of M0 due to a decrease in the Ksol∙(1 – K) – Kliq∙K difference, as well as the R coefficient as K increases (Table 1).
In the case when the porosity of the “raw” compact reaches 43–46%, its increase, ahead of shrinkage, practically ceases (see Fig. 1b; curves 4, 5). However, this does not indicate an increase in the amount of copper particles. The appearance of the thermal effect indicates the formation of intermetallic compounds on their surface.
The shrinkage of compacts without preliminary increase, first of all, indicates the destruction of the rigid frame at a given amount of solid phase. It can be easily shown that the volume of the solid phase in the compact is equal to the following value:
Using formula (3) it is possible to estimate the value n , at which the shrinkage of the compact occurs without a preliminary increase. Substituting the K = 0.45 and M0 = 55% values into formula (3), n = 0.30 is obtained for curve 5. For curve 4, M0 = 45% and n = 0.35. The destruction of the rigid frame can be assumed to possibly correspond to the existing concepts of particle rearrangement (provided that this does not occur for any initial porosity and amount of liquid alloy). Formally, the concept of rearrangement can include the approximation of the particle centers caused by their dissolution in a liquid alloy.
Figure 2 demonstrates the effect of the aluminum concentration on the nature of the volumetric increase in compacts at M0 = 0.3 constant initial porosity.
Changes in the dimensions (1–5) and temperature (1′–5′) of the Cu-Al system compacts with M0 = 28% porosity during sintering depending on the KAl concentration of aluminum: 1, 1′ – 15%; 2, 2′ – 25%; 3, 3′ – 35%; 4, 4′ – 45%; 5, 5′ – 55%; the relative change in the length of the reference sample (6) and the temperature of the medium (7).
At an aluminum content of 55%, shrinkage occurs without a significant preliminary increase in the volume of compacts. This indicates the destruction of the compact frame at the time of aluminum melting. According to calculations and formula (3), in this case, n = 0.36, similar to the above example. In the range of aluminum concentration from 15 to 45%, a sharp increase is observed in the compact dimensions followed by a gradual decrease.
At the initial moment, a noticeable increase in Δl/l is possibly due to the melting and dissolution of intermetallic compounds formed on the surface of copper particles during a volumetric increase in compacts. In this case, it is difficult to make any assumption about the at least partial destruction of the frame and subsequent rearrangement of solid phase particles. Since, with further exposure, a certain drop in Δl/l with a decreasing rate is associated with the cooling of the samples after the exothermic effect, it depends less on the initial porosity and the amount of aluminum in them. The absence of a volumetric increase in compacts with a sufficiently small amount of a solid phase in them appears to be a direct indicator of the rearrangement of solid phase particles in the presence of a liquid phase.
Let us note some features of the liquid-phase sintering of the Cu–Al system associated with this process. First, contrary to the data presented in the existing literature, the rearrangement is not the first in a series of sequential sintering stages [3, 4]. Rather, it is surrounded by the diffusion flow of atoms from the liquid to the solid phase, causing an increase in the rigid frame, if any. In this case, the direction of atom flow towards the solid phase is not completely opposite to the flow direction of atoms saturating the liquid alloy. However, at the moment of creating a contact between the solid and liquid phases, the diffusion flow into the solid phase noticeably prevails over the reverse process. Therefore, the transition of the solid phase into the liquid phase can be attributed to the second stage of sintering. Second, despite the fact that the rearrangement of particles represents a special mechanism for changing compacts, it cannot be attributed to a separate case – for example, to the third stage of sintering – since it coincides in time of appearance with the first and second stages. The transition of atoms from the liquid to the solid phase, occurring simultaneously with diffusion and causing a rearrangement of particles, is accompanied by an increase in volume due to the Kirkendall effect.
An important point to note is that, despite its distortion, the shape of the compact is not completely lost when the rigid frame is destroyed. However, a further decrease in the amount of the second phase, especially due to an increase in the concentration of the low-melting component, leads to a complete loss of the compact shape.
Conclusions
-
1.
Volumetric changes in Cu–Al compacts during liquid-phase sintering conform to equation (1). According to this, the dissolution of the solid phase components in the liquid melt can be argued to be preceded and then accompanied by the oppositely directed diffusion of atoms.
-
2.
The volumetric enlargements of compacts revealed as a result of dilatometric studies that occur during sintering with the participation of the liquid phase are due to the diffusion of atoms from the liquid to the solid phase. Such increases in dimensions outpace the shrinkage in the liquid phase associated with the dissolution of the particles of one of its components.
-
3.
When the amount of the solid phase comprises the order of 1/3 of the total volume of the compact, its shrinkage occurs without preliminary increase upon appearance of the liquid phase. This indicates the possibility of fracture of the rigid frame and shrinkage under these conditions due to a rearrangement of particles without significant changes in the shape of the compact.
-
4.
Contrary to the data presented in the existing literature, the rearrangement is not the first in a series of sequential sintering stages [3, 4]. Rather, it is surrounded by the diffusion flow of atoms from the liquid to the solid phase, causing, if anything, an increase in the rigid frame.
-
5.
At the initial stage of the rigid frame destruction, despite its distortion, the shape of the compact is preserved to a certain extent. However, over time, an increase in the concentration of a low-melting component in the system leads to a complete loss of the compact shape.
References
S. M. Gorbatyuk, A. N. Pashkov, A. Yu. Zarapin, and A. D. Bardovskii, “Development of technology for the production of metalmatrix composite materials based on aluminum by hot pressing,” Metallurg, No. 12, 54–58 (2018).
E. A. Chernyshov, E. A. Romanov, and E. A. Romanova, “Obtaining a highly reinforced dispersion-hardened composite material based on aluminum by the method of internal oxidation,” Metallurg, No. 8, 70–81 (2018).
A. T. Mamedov, Structural and Anti-Friction Powder Materials [in Russian], Elm, Baku (2006).
G. Petzow and W. A. Kaysser, “Influence of sintering and thermomechanical treatment on microstructure and properties of W–Ni–Fe alloys,” in: G.S. Upadhyaya (editor), Sintered Metal-Ceramic Composites, Elsevier Science Publ., Amsterdam (1984).
K. V. Savitskii, et al., “Influence of dispersion of aluminum powder on sintering of Cu-Al alloy in the presence of a liquid phase,” Poroshkovaya Metallurgiya, No. 11, 20–25 (1985).
K. V. Savitskii, et al., “Investigation of the mechanism of sintering of metal-ceramic alloys of copper and aluminum in the presence of a liquid phase,” Poroshkovaya Metallurgiy, No. 1, 5–11 (1986).
F. F. Nia and S. L. Davies, “Production of Al-Cu-Si alloys by PM methods,” Powder Metallurgy, 25, No. 4. 209–215 (1982).
J. Puckert, W.A. Kaysser, and G. Petzow, Int. J. Powder Metal, and Powder Techn., 20, 301 (1984).
W. Kehl and H. F. Fischmeister, “Observation on dimensional changes during sintering of Al-Cu compacts,” Sintering Theory and Practice. Material Science Monograph, 14, 269–274 (1981).
G. Bocrstigel, Erscheinungsbild und Ursachen von Volumenanderungen beim Sintern von Preblingen aus Eisen-Kupfer und Eisen-Kupfer-Graphit-Pulvermischungen-Stahl und Eisen, 79, 1187–1201 (2009).
A. P. Savitskii, “Features of the sintering process of binary systems,” Poroshkovaya Metallurgiya, No. 7-8, 62–69 (2000).
A. P. Savitskii, E. S. Kim, and L. S. Martsunova, “Shrinkage of briquettes during liquid-phase sintering,” Poroshkovaya Metallurgiya, No. 9/10, 9–13 (2000).
A. P. Savitskii and N. N. Burtsev, “Growth of briquettes during liquid-phase sintering,” Poroshkovaya Metallurgiya, No. 11/12, 31–38 (2009).
M. Khansen and K. Anderko, Structures of Double Alloy [In Russian], Vol. 1, Metallurgizdat, Moscow (1962).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 64, No. 12, pp. 65–70, December, 2020.
Rights and permissions
About this article
Cite this article
Yagubov, E.K. Features of Structural Formation in a Cu–Al Powder System. Metallurgist 64, 1307–1314 (2021). https://doi.org/10.1007/s11015-021-01120-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01120-1