This study describes the conversion processes of charge materials and the chemical processes in the charge and hearth zones of furnaces that smelt industrial silicon and high-silicon ferroalloys. The primary factor that determines the efficiency of the charge mode and the technological process is the coefficient of excess carbon in the furnace bath. The fluctuations in the effective capacity of the furnace; its distribution over the zones of the arc, charge, and melt; and the active resistances of these zones are caused by the regular deviations of this coefficient from unity. Such deviations are induced by uncontrolled changes in the humidity of the reducing agents.
Carbon deficiency and excess modes affect the state of charge and melt zones and the distribution of electric energy on the arc discharge power. Transient processes that occur when the composition of the charge on the dosing unit is changed, as well as the direct supply of the reducing agent to the furnace mouth and reflecting changes in the charge zone conductivity, are studied. Results reveal that transient processes are accompanied by high material and energy losses and their duration, depending on the volume of the bath and the effective capacity of the furnace, can exceed 4 h.
On the basis of the analysis of the changes in charge conductivity and arc power, the negative consequences of the layer-by-layer charge loading method adopted for smelting industrial silicon are presented; these consequences are based on the alternation of charge supply, with an excess and shortage of the reducing agent. In the absence of reliable methods for the continuous monitoring of the moisture content of reducing agents in the charge stream, the coefficient of carbon excess must be evaluated by changing the resistance of the melt zone, which reflects changes in the size of the carbide layer that is formed due to the imbalance of silicon carbide and silicon monoxide. Such evaluation can determine the degree of imbalance of the reducing agent in the mixture and apply the currently required control effect on the composition of the charging material in a timely manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In any electrothermal technology of carbothermal metal reduction, the properties of charge materials, particularly the electrical conductivity of carbonaceous reducing agents (such as coke, semicoke, charcoal, and mineral coal) are emphasized. An important factor is ash content. The requirements for this parameter are much stricter in the production of industrial silicon compared with that in the production of ferroalloys. The stability of the properties of the charge materials is crucial to a successful manufacturing process and high efficiency.
However, the ash and volatile substance contents of reducing agents are relatively stable, but their moisture content depends on many related circumstances, including season, weather, and other climatic conditions.
Therefore, a huge challenge in controlling the process of smelting industrial silicon and high-silicon ferroalloys is the regulation of the dosage mode of the charge. The complexity of the silicon reduction process is caused not only by its high temperature and several simultaneous intermediate and related reactions but also by the lack of operational information on the melting process. The lack of reliable data on the ratio of silica to solid carbon content in the reducing agents fed to the furnace mouth does not provide the main function of the charge mode, i.e., stabilization at a given level of the carbon excess coefficient. Therefore, the completeness of the silicon reduction reaction. This phenomenon is caused by the uncontrolled fluctuations in the moisture and ash content of reducing agents [1, 2]. One method of controlling the moisture content of carbonaceous reducing agents in the charge stream is the use of neutron sensors [3]; although this method has often been discussed and occasionally tested in industrial conditions for more than three decades, it is not constantly applied in practice.
Majority of all chemical reactions in the working volume of the furnace occurs in the charge zone, and the main silicon reduction reaction caused by the interaction of its carbide and monoxide is implemented in a narrow active zone, which is represented by the walls of the near-electrode crucibles. This reaction represents the limiting stage of the process and requires a high temperature (more than 1800°C), which must be provided by electric arcs. The intensity of silicon reduction and furnace productivity depends on their power.
Carbon-reducing agents conduct electric current and thus determine the electrical conductivity of the entire charge zone. The higher the content of the reducing agent and the higher the conductivity of the charge, the greater the current branching along the charge and the less power released in the arc. Therefore, violations of the dosing mode of the charge affect the characteristics of the electrode and electric modes and the general course of the process. As a result of the changes in the moisture content of the reducing agents and in the charge mode parameters due to the high inertia of the furnace, long transient processes accompanied by considerable material and energy losses occur. Under these conditions, the reduction of these unjustified costs is possible only through the operational monitoring of the state of the charge and hearth zones and the timely application of control actions on the parameters of the main production modes. To assess the measure and time of these effects, experimental studies on the processes in the transformation zones of charge materials are required.
Theoretical Treatment
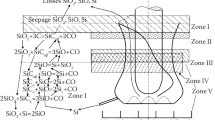
The specific electrical resistivity of a sufficiently small amount of the charge varies along the height of the bath due to the chemical transformation of the charge materials and changes in temperature. In accordance with the process conditions and the composition of the condensed phases, the charge zone of the furnace bath can be divided into three zones: low, medium, and high temperature [4]. In the low-temperature zone, the main reaction is silica gasification:
Another process that is possible in this zone is the reaction of silicon monoxide with carbon, both formed in the low-temperature zone and brought from the medium- and high-temperature zones of the furnace bath:
In the medium-temperature zone, the condensed phases are represented only by silica and silicon carbide. The only process in this zone is the gasification of silica:
In the high-temperature zone exceeding 1800°С, the concentration of SiO, which is also formed through Eq. (3), exceeds that in equilibrium with silicon. This enables to spend part of SiO in this zone on the destruction of silicon carbide by
In addition, in the high-temperature zone, SiO can also be formed by
Thus, in the upper horizons of the charge zone, silica, reducing agents, and silicon carbide are found in the upward flow of SiO; only carbide is found in the high-temperature lower zone, and there is silica in the case of a lack of carbon in the charge.
Depending on the production process, liquid silicon, solid silicon carbide (with an excess of carbon in the charge), and magma-like silica (in the case of a considerable carbon deficiency) may be found in the hearth (melt zone). Under certain conditions, the coexistence of all three products is possible. The most common Eq. (5) in this zone is the interaction of molten quartzite and liquid silicon with the formation of SiO. If carbide accretion occurs in the melt zone, then the formed SiO is used to destruct carbide via Eq. (4). Such ideas are consistent with the results of excavations of furnaces after their shutdown [5] and the laboratory studies on the silicon reduction process [6, 7].
Methodology and Results of an Industrial Experiment
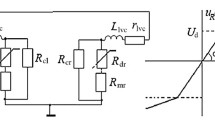
According to the electrical equivalent circuit of the working space of furnaces smelting silicon and highsilicon ferroalloys [8], the payload resistance of the furnace comprises series-connected resistances of the hearth (melt) and the arc, shunted by the resistance of the charge. From the point of view of ensuring the maximum of the arc component of the power, the melt resistance Rm should be minimal because it limits the arc current and the voltage drop across the arc. By contrast, the charge resistance Rc should be increased to limit the current flowing through the charge zone and increase the share of the arc power in the total power of the furnace. Two major factors determine the charge resistance: (1) the fractional composition of the ore and carbon (reduction) parts of the charge and (2) the solid carbon content in the charge zone of the furnace. The first parameter is selected as a result of many years of practice and does not undergo any remarkable fluctuations during the longterm operation of the furnaces; by contrast, the second parameter depends largely on the type of reducing agent and its properties and mainly on moisture content.
Thus, moisture content fluctuations cause disturbances in the charge mode and affect the parameters of the electric mode due to the change in the charge resistance Rc and the payload resistance. To correct the charge mode, two control actions are used: (1) changing the weighed amount of reducing agents on the dosing unit and (2) adding the carbon or ore component of the charge directly to the furnace mouth.
These effects cause transient processes, whose duration depends on the size of the bath and the bulk density of the electric power supplied to the furnace. As the charge with the new composition moves to the hightemperature zone, Rc increases with a decrease in the weighed amount of the reducing agent or decreases with an increase in the weighed amount. Rc has an inverse dependence on the content of the reducing agent; thus, using the parameter inverse to resistance (i.e., the charge conductivity σc) is convenient to analyze the transient processes.
We control these parameters using an automated system that is based on the analysis of the dynamic current–voltage characteristic of the circuit, that is, the interdependence of the instantaneous values of the voltage and current of the electrode. The system determines the parameters of the equivalent circuit of the working space and the resistance of the charge and the melt [9]. In contrast to studies [10,11,12], which only determined the distribution of currents and powers along the branches of the arc and charge, our method can additionally determine voltage drops, which are connected in series on the arc and the melt resistance; consequently, the power becomes noticeable in separate areas of the working space of the furnace.
Figure 1 presents the characteristics of the charge conductivity σc and its rate of change σ′c = dσc/dt for two consecutive (with an interval of 2 h) increases in the weighed amount of carbon in the burden by 5 kg. Dots represent measurement data, and lines represent their smoothed values. Data were obtained by analyzing the state of the 48 MVA ore-reducing furnace, which smelted FSX-48 grade ferrochrome silicon. The conductivity from one steady-state value transfers gradually to higher levels. Moreover, its derivative reaches a maximum twice during the interval under consideration, and its values close to zero at certain points indicate that the transient processes caused by the change in the weighed amount are almost complete.
The maximum rate can be explained by the fact that at these times, the entire low-temperature zone where the carbide and solid carbon of the reducing agent coexist are already filled with a new composition charge. The subsequent slowdown in the change of the conductivity of the charge is associated with the lower specific conductivity of silicon carbide compared with that of the reducing agent and the remarkably small sizes of the medium and high-temperature zones. The rates of escape and conversion of the charge materials depend on the power and size of the furnace bath. For this unit, the duration of the complete renewal of the charge was approximately 4 h, and the duration of filling the low-temperature zone with a new charge was 2.5 h.
The response to the corrective addition to the mouth of any component of the charge considerably differs from that to the change in composition. In this case, the charge was not renewed, and only a narrow local zone with a predominant content of carbon or ore material was formed. Charge conductivity increases temporarily (depending on the type of additive) or decreases; at the end of the transition process, it returns to its previous level. However, the use of such additives is not always reasonable. This relates to a greater extent to additives of reducing agents because an excess of carbon causes an excessive deposition of silicon carbide on the hearth, which is expressed as an increase in the melt resistance and limitation of the current and arc power.
The characteristics of the charge conductivity and its rate of change caused by the supply of 200 kg of coke to the mouth during the transition process are presented in Fig. 2. The additive starts to affect the conductivity of the charge 30 min after it is fed to the mouth. The increase in conductivity is associated with the increase in the temperature of the local zone. After approximately 1 h, the rate of change in conductivity reaches its maximum value. Moreover, the rate starts to decrease gradually because silicon carbide is produced intensely at this time. After 2.5 h since the application of the effect, the charge conductivity reaches a maximum value, i.e., the local addition of a reducing agent reaches the boundary of the zones of low and medium temperatures. The further decrease in charge conductivity is due to the minimal effect of excess carbide in the SiC–SiO2 mixture located in the medium-temperature zone.
Any transient process in smelting of silicon or ferroalloys is always associated with either the loss of power and productivity of the furnace or the loss of silicon in the form of SiO. Energy losses are first associated with the loss of the arc load and accordingly with a decrease in the intensity of silicon reduction in the modes of the excess reducing agent. In addition, energy and productivity losses are inherent in modes with a developed arc discharge, wherein the losses of SiO with exhaust gases increase remarkably due to the lack of a reducing agent. Therefore, the charge mode control should be prioritized over other technological modes.
The approach to controlling the charge mode during smelting of ferroalloys differs from the approach adopted for the smelting of industrial silicon. In the production of high-silicon ferroalloys, the imbalance of the reducing agent is assessed visually using the secondary characteristics of the furnace operation. Naturally, the operator notices the changes that have occurred in the operation of the furnace later and thus does not have enough time to perform the necessary control action. Nevertheless, he corrects the process course after a rather long period.
Another approach to control has been adopted in the production of industrial silicon. Having agreed that directly controlling the carbon imbalance in the furnace is impossible, production engineers have adopted a technological scheme; according to this scheme, the process is performed with the accumulation of carbide and its subsequent destruction under a considerable deficiency of reducing agent. The so-called “light” and “heavy” downloads are used for this scheme when the ratio of quartzite and reducing agent weights is ± 10% different from the stoichiometric composition.
In the practice of silicon smelting, the rule of the electrode burst during the transmission of a work shift has been adopted as an implicit requirement; that is, by the end of the shift, the furnace operator should bring the electrodes to the level of their upper stroke limiter by feeding “light” loads. In this manner, the operator who replaces him can start work by loading “heavy” batches of the charge and receive the finished product in intensive mode. However, to switch to a mode of high recovery intensity, several irrational modes with their inherent energy and material losses must be implemented.
Figure 3 presents the characteristics of the change in the conductivity of the charge zone and the share of power released in the arcs. The process is extremely unstable; thus, with a charge average conductivity per day of 0.144 mOhm−1, the standard deviation is 0.048 mOhm−1, which is 33.6%. In this case, the fluctuation range is 0.317 mOhm−1, which exceeds the average value more than twice. The instability of the charge conductivity causes instability of the power arcs. The average value and standard deviation of the power released in the arcs are equal to 1.86 ± 0.48 MW, with a range of oscillation of 2.86 MW. The increase in the charge conductivity caused by its supply with an excess of carbon corresponds to a decrease in the arc power. In this case, the reaction of the SiC production develops predominantly. Settling on the hearth, excess carbide forms a deposit and increases Rm. Consequently, the rate of recovery process and the productivity of the furnace decrease, and the specific energy consumption (SEC) increases.
A different scenario is observed during the transition to loading a charge with a lack of carbon. From the excessive production of silicon dioxide and monoxide in the melt zone, the reactions of gasification SiO2 + Si = 2SiO and the chemical destruction of the already existing carbide deposit SiC + SiO = 2Si + CO occur. The increase in Rc and decrease in Rm occurring in this transition process cause an increase in the arc power and intensity of silicon reduction. Silicon accumulated in carbide during the period of excess carbon mode also contributes to an increase in its degree of extraction into the alloy and furnace productivity. In this mode, SEC is reduced. However, neither high productivity nor favorable electric mode can compensate for the energy losses of the previous transition of the furnace from the mode of carbon deficiency to the mode of its excess. Moreover, the excessive and intense production of SiO leads to its increased removal from the working space of the furnace, i.e., to losses not only of the material but also of the energy spent on the partial restoration of silicon.
The practical methods of regulating the charge mode under the condition of limited information on the content of solid carbon in the charge are interesting. In the practice of manufacturing high-silicon ferroalloys, the Stakhanov Ferroalloy Plant phenomenon is the most interesting among known techniques. The storage conditions of the reducing agents at this enterprise contribute to the maximum moisture content; therefore, practically no errors are found in determining the composition of the charge corresponding to the stoichiometry of the target reaction. Given that the energy consumption for the evaporation of additional moisture is negligible in comparison with the energy losses due to the imbalance of carbon, the technical and economic indicators of the process are much higher than the average indices of furnaces of other plants.
The Kuznetsk ferroalloy plant also has positive experiences in carbon deficiency modes. This technique can almost avoid the formation of a carbide hearth and power losses. Moreover, the increased loss of SiO is partially compensated by the capture and sale of finely divided silica. Technical and economic indicators are also considerably higher than the industry average.
The method of replacing part of the charge with silicon carbide is now increasingly used as one of the measures to reduce power fluctuations and increase production efficiency. The larger the amount of carbide additive placed into the reaction zone, the more its electrical resistance increases and stabilizes. Despite the indisputable advantages of this approach, it should only be regarded as a half measure because it does not solve the main task of process control, which ensures the completeness of the target reduction reaction due to the correct dosage of charge materials; moreover, it does not exclude the method of layer-by-layer charge loading.
Conclusion
The accuracy of the regulation of the mode of charge weighing and dosing has a decisive influence on the course of technological processes for the smelting of industrial silicon and high-silicon ferroalloys. Uncontrolled fluctuations in the solid carbon content of the charge lead to an unstable characteristic of melting and the appearance of intermittent transient processes from one low-efficient state to another (even more inefficient). The greater the carbon imbalance, the greater the material and energy losses during such oscillatory processes. The negative effect is remarkably increased because of the charge dosing method adopted in the silicon production, with alternating loads with a significant predominance of the ore or carbon component.
In the absence of methods for the continuous monitoring of the moisture content of reducing agents and the quick identification of the coefficient of excess carbon in the charge flow, the authors propose to evaluate it by changing the resistance of the melt zone. The melt zone reflects changes in the carbide layer size, which result from the imbalance of the products of the limiting stage of the reduction reaction (i.e., silicon carbide and silicon monoxide). This assessment can determine the degree of imbalance of the reducing agent in the charge and apply the currently required control effect on the composition of the charging material in a timely manner.
References
V.P. Vorobyov, Electrothermics of Recovery Processes [in Russian], Ural Branch of the Russian Academy of Sciences, Yekaterinburg (2009).
V.P. Vorobyov and A.V. Ignatiev, “Calculation of the basic electrical and geometric parameters of ore-thermal electric furnaces (OTEF) in the production of silicon alloys,” Elektrometallurgiya, No. 6, 31–35 (2012).
G.S. Vozhenikov and Yu.V. Belyshev, Radiometry and Nuclear Geophysics [in Russian], Publishing House of the Ural State Mining University, Yekaterinburg (2011).
K.S. Yolkin, Production of Metallic Silicon in Russia – State and Prospects: Proceedings of the VI International Congress “Non- Ferrous Metals and Minerals” (Krasnoyarsk, September 15–18, 2014): Light Metals LLC (2014).
M. Ksiazek, M. Tangstad, and E. Ringdalen, The Rapid Si-Furnace Excavation – Unique Chance to Investigate the Interior of the Furnace, INFACON-XV; SINTEF, Trondheim, Norway, 24-27.02.2018.
M. Ksiazek, S. Gradahl, E. A. Rotevant, and B. Wittgens, “Capturing and condensation of SiO gas from industrial Si furnace,” in: Advances in Molten Slags, Fluxes, and Salts: Proc. of the 10th Int. Conf. on Molten Slags, pp. 1153–1160.
J. Vangskasen and M. Tangstad, “Condensate in the metallurgical silicon process reaction mechanism,” in: The Thirteenth Int. Ferroalloys Cong.: Efficient Technologies in Ferroalloy Industry, Almaty, Kazakhstan (2013), pp. 283–289.
V.P. Vorobyov and A.V. Sivtsov, “Working zones of ferroalloy furnaces and equivalent electric payload equivalent circuits,” Elektrometallurgiya, No. 6, 12–14 (2001).
V.P. Vorobev and A.V. Sivtsov, Patent No. 2268556 RF, H IPC 05 B 7/148, The method of controlling the technology of electric arc reduction smelting; applied 04/01/2004; published 01/20/2006, Bull. No. 2.
G.A. Saevarsdottir, M.Th. Jonsson, and J.A. Bakken, “ArcElectrode interactions in silicon and ferrosilicon furnaces,” in: Proc. INFACON X, Cape Town, South Africa, 593-604 (Feb. 2004).
Y.A. Tesfahunegn, T. Magnusson, M. Tangstad, and G. Savarsdottir, Effect of Electric Tip Position on the Current Distribution in Submerged Arc Furnaces for Silicon Production: 10th Int. Symp., ISICA 2018, Jiujiang, China, Revised Selected Papers (October 13-14, 2018).
Y. A. Tesfahunegn, T. Magnusson, M. Tangstad, and G. Savarsdottir, “Dynamic current and power distributions in a submerged arc furnace,” Mater. Proc. Fundamentals, 3–14 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 64, No. 5, pp. 21–27, May, 2020.
Rights and permissions
About this article
Cite this article
Sivtsov, A.V., Yolkin, K.S., Kashlev, I.M. et al. Processes in the Charge and Hearth Zones of Furnace Working Spaces and Problems in Controlling the Batch Dosing Mode during the Smelting of Industrial Silicon and High-Silicon Ferroalloys. Metallurgist 64, 396–403 (2020). https://doi.org/10.1007/s11015-020-01008-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-020-01008-6