The subject of study was the Safyanovskaya Med company’s acidic underspoil water containing 0.17 g/liter of copper and 1.8 g/liter of zinc (pH 2.8–2.9). The goal was to study and develop a technology of cleaning water of impurities (copper, zinc, aluminum, iron, manganese), accompanied by extraction of copper and zinc in the form of commercial products. Copper extraction by cementation with metallic iron was studied. It was shown that this method could allow extracting 94–95% copper into a concentrate. The role of resolution processes of precipitated copper in cementation was established. To reduce the consumption of iron and to improve the quality of precipitated copper, it is proposed to carry out cementation in a washbox. The copper content in the concentrate was 27–28%, while the specific consumption of iron was 7.0–7.5 kg/kg of copper. During the studies of material composition of copper precipitate, the formation of cuprospinel, goethite, and bassanite phases and the precipitation of basic aluminum sulfates were detected. The extraction of zinc from the solution after copper removal was studied by precipitation with sodium sulfide. As a result, a concentrate was obtained with a zinc content of 49.6–50.9% with extraction of 99%. The process regularities for zinc concentrate dehydration were studied. By optimizing the crystallization conditions of the precipitate, a minimum final cake moisture content of 40–42% was reached. A process flow diagram for acidic underspoil water, including copper cementation and the precipitation of zinc sulfide, was developed. According to this diagram, the mother solution from zinc precipitation should be treated with lime until the pH reaches 10–10.5, followed by slurry settling. As a result, clean water of the following composition is obtained, mg/liter: 0.05 zinc, 0.01 copper, 0.02 aluminum, < 0.02 iron, 0.05 manganese. These values correspond to the quality of drinking water according to GOST R 51232-98.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Water used by mining companies is contaminated with ions of nonferrous metals; therefore, significant amounts of valuable components (mainly copper and zinc) are lost. Of interest are mine, quarry, and underspoil water of mining facilities that process copper-zinc pyrite ores [1, 2]. Rock weathering is accompanied by the formation of sulfatic solutions characterized by a complex salt composition and high concentrations of copper and zinc, which can be considered as commercial. The purification of such similar solutions is an important task.
Our goal here is to study and develop a technology of cleaning underspoil water of impurities (copper, zinc, aluminum, iron, manganese) and extracting copper and zinc in the form of commercial products.
The processing of solid zinc products with Zn ≥ 15–20% is of industrial interest [3, 4]. Such products can be Waelz processed [3,4,5]. Copper products can be processed if their Cu content is no less than 15% (as per GOST R 52998-2008). This suggests that it is reasonable to extract copper and zinc concentrates from underspoil water by the most simple methods: precipitation of almost insoluble compounds.
Here we discuss the results of purification of a solution by these methods for the Safyanovskaya Med company’s underspoil water as an example. Water samples of the following composition were tested, g/liter: 0.17 Cu 1.8 Zn, 1.65 K, 0.05 Na, 4.20 Mg, 0.84 Al, 0.23 Fe (III), 0.21 Mn, 0.60 Ca. Their pH = 2.8–2.9. This solution is typical for underspoil, mine, and quarry waters. It is obvious that the main impurities are copper, zinc, aluminum, iron, and manganese. Traditionally, copper is extracted from water by cementation with metallic iron [6,7,8,9,10]:
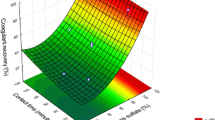
Two cementation methods were proposed. One method is cementation in an agitated reactor fed with a great amount of PZhR 200 iron powder (200 μm particle size) by atomizing under pressure. The laboratory reactor was of 2 liter capacity and had an impeller agitator providing a Reynolds number of 20,000. The duration of the process was 0.5 h, as recommended in [6]. The temperature was 20–22°C. Upon completion of the test, the solution was settled with a 0.1% solution of М338 Magnafloc flocculant (8.10 mliter/liter) for 1 h. After that, the solution was decanted and the precipitate was filtered out, washed out, dried, and weighed. The copper balance was drawn up after analysis of the precipitate and filtrate. Figure 1 shows how the amount of iron powder used affects the extraction of copper and the quality of the product.
The dependence of cementation indicators on the duration of the process was studied as well (Fig. 2). Simultaneously, it was analyzed whether it is possible to decrease the amount of precipitating agent by increasing the contact time. The optimal consumption of iron powder is 1.5 g/liter because it ensures the maximum extraction and quality of precipitate at the minimum specific consumption of iron (corresponding to 9.4 kg/kg of copper). Decreasing the consumption of iron powder to 0.70–1.20 g/liter leads to an abrupt decrease in extraction (Fig. 1). The contact time should not be increased to longer than 0.5 h to avoid the intensive resolution of copper precipitate (Fig. 2). For example, it is completely dissolved in 4 h. The resolution of copper is not accompanied by an increase in the concentration of iron (III) in the solution; therefore, the process can be explained by the following reaction with atmospheric oxygen [6]:
It should also be noted that an increase in the duration of cementation degrades the quality of the precipitate, which can be attributed to the precipitation of compounds of metallic impurities.
Cementation is characterized by high consumption of iron: no less than 7.0–7.5 kg/kg of copper according to experimental data. This consumption is due to side processes in full compliance with the stoichiometry of the reactions
This level of iron consumption makes the cementation process with powder unprofitable. Profitability can be achieved with cheap iron waste (scrap, crop, etc.).
The specific consumption of iron is reduced in the second method: cementation in a washbox with iron scrap loaded on sieves. It was assumed that supplying acidic water to vibrating sieves with scrap would promote copper cementation followed by removal of the copper rim from the scrap surface. This is how the intradiffusion resistance to the cementation process would be eliminated [7,8,9,10], and copper would partially accumulate in the concentrate tank and be carried away as slime by decopperized water.
Cementation was carried out in a laboratory washbox with two MOD-0.02 SKL movable sieves (manufactured by the Zavod Trud company, Novosibirsk) with a surface area of 0.01 m2 each. The precipitating agent was scrap (crop) iron with a specific surface area of 0.55 m2 /kg, which was determined by low-temperature adsorption of nitrogen. Each sieve was loaded with 300 g of scrap (which is the sieve capacity). For cementation, the solution was allowed to pass through two sieves consequently, the machine operating. The solution was supplied through a feeding nozzle to each sieve sequentially. No additional water was supplied. When cementing in a washbox, the goal was to find operating conditions that would ensure stable minimum concentration of copper in the mother solution for a long time.
The influence of the feed rate of the solution and the frequency of sieve vibration on the cementation indicators was studied as well. To this end, 25 liter of solution were fed at different frequencies of sieve vibration and a water supply of 25 liter/h. Before each test, the copper rim was removed from the scrap surface in an ultrasonic bath. The frequency dependence of the copper concentration in the mother solution is extremal: the optimal frequency falls within the range 100–200 min–1 (Table 1). Further increase in the frequency leads to an increase in the copper concentration in the mother solution, which can be attributed to the slip of more solution through the channels in the vibrating layer of the precipitating agent (channel effect).
The optimal specific capacity was determined as follows. The solution was supplied in volumes of ≈ 10 liter at a frequency of 100–200 min–1 and capacity gradually reduced from 25 liter/h. The copper concentration in the mother solution was determined. A stable residual copper concentration of ≈ 10 mg/liter was achieved by gradually decreasing the feed rate of the solution to 15 liter/h.
Next, the operation of the machine was tested for a long time at the optimal feed rate of the solution (0.7–0.8 m3 /m2 corresponding to a 15 liter/h flow) onto the sieves. In the process, 20 to 40 liter fractions of mother solution were obtained, and about 350 liter of solution was processed. As the scrap level on the sieve decreased, a new material was loaded to the sieve level. The copper concentration in the mother solution was no lower than 9–10 mg/liter (≈ 94–95% extraction) even after decrease in the specific feed rate of the solution to the sieves to 0.05 m3 /(m2∙ h), which can be explained by the resolution of copper, according to reaction (2).
The precipitates obtained in different ways were subject to X-ray diffraction (XRD) analysis in a Shimadzu XRD-7000 diffractometer. The diffraction patterns were interpreted using an IPS-6 program. The XRD analysis failed to detect the basic aluminum sulfates because of their X-ray amorphous properties. Based on the XRD and chemical data, the phase compositions of copper precipitates were determined (Table 2, Fig. 3). The content of metallic iron was confirmed by measurements with a Satmagan 135 magnetic analyzer. The precipitates obtained in the optimal conditions were subjected to chemical and XRD analyses.
The low copper concentration in the products is due to not only the necessity of maintaining an excess of iron, but also the presence of great amounts of basic aluminum sulfates in the precipitate, which was confirmed by the chemical analysis. The basic aluminum sulfates form because during cementation at an excess amount of iron, the pH of the medium increases from 2.8–2.9 to 4.0–4.5 because of reaction (3). At pH = 4.0–4.5, the basic aluminum sulfates are actively precipitated. This, in turn, degrades the quality of the precipitate.
As follows from the data obtained, the precipitate is strongly contaminated by not only basic aluminum sulfates, but also by iron oxides and calcium sulfate. Iron oxides were detected in phases similar in structure to cuprospinel and goethite, while calcium sulfate was found in the form of a compound similar in structure to bassanite (CaSO4∙ 0.5H2O). The presence of iron oxides is attributed to the precipitation of iron hydrates (III) whose structure becomes similar to the crystalline structure of goethite after drying. The cuprospinel phase can be identified as dried iron hydrates in which the iron is isomorphically replaced by partially precipitated (at pH = 4.0–4.5) copper hydrate (II) or (I) or by a mechanical mixture of copper and iron hydroxides.
To improve the quality of the precipitate produced using an iron powder, it was proposed to subject it to dry or wet magnetic separation.
This process allows not only enriching the precipitate with copper, but also separating the unreacted metallic iron (which can be returned into the process) into the magnetic fraction. A 25-T wet magnetic analyzer was used for magnetic separation. The magnetic-field strength was maintained at a level of 1500 Oe. Originally, the nonmagnetic fraction was not separated because all of the precipitate was magnetic. This suggests that the cementation is accompanied by processes similar to galvanic precipitation of copper on iron. Recording pinhole X-ray diffraction patterns with the EDX spectrometer of a EVO MA 15 microscope made it possible to reveal that the iron particles are coated by a ≈ 1 μm film of copper precipitate. Hence, the cementation process was complicated by the intradiffusion of the solution through the copper film. Therefore, for the purposes of commercial- scale cementation, it is reasonable to use a washbox to avoid the intradiffusion resistance of the thin copper film. As a result, the quality of the precipitate was improved, the specific consumption of iron decreased from 9.4 to 7.0–7.5 kg/kg of copper, and the content of residual iron in the precipitate decreased as well.
To extract zinc sulfide, commercial NaHS with a concentration of 33% was used. This reagent is cheap and readily available. The process occurs according to the following reaction [11]:
After cementation, the solution had the following composition, g/liter: 0.01 Cu, 0.05 Fe(III), 1.30 Fe(II), 1.8 Zn, 1.5 K, 0.05 Na, 3.98 Mg, 0.60 Al, 0.18 Mn, 0.64 Ca.
The results of extraction of zinc concentrate are presented in Fig. 4. The reagent was supplied and the slurry was agitated for no longer than 15 min to avoid the resolution of zinc [12]. The temperature was 20–22°C. During precipitation, pH reduces from 4.0–4.5 to 2.5–2.7, according to (5). This range of pH is favorable for the extraction of zinc sulfide [11,12,13,14,15,16]. The NaHS consumption ≈ 1.60 g/liter (0.89 kg/kg of zinc) corresponding to 100% stoichiometric amount according to (5) is optimal. This value is explained by the fact that the sulfide ion is not used for the side reduction of iron (III) in the solution. The content of zinc in the concentrate reaches 50.9–49.6%, which meets the requirements of GOST R 54922-2012 to the quality of zinc concentrate. The concentration of zinc in the mother solution decreased to 0.2 mg/liter. Lower concentrations in the mother solution were failed to be achieved because of the partial resolution of the precipitate in the presence of atmospheric oxygen [12].
The zinc-sulfide slurry had extremely poor filterability. The filtration of zinc-sulfide slurries was studied in a vacuum filter and in a Labox 25 filter press (25 cm2 filtration area). Various fabric filters were tested. The slurry was preliminarily thickened to a solid content of ≈ 2% (maximum densification of precipitate). In the press filter, the pressure was increased to 1.6 MPa. None of the tested fabrics (even with a gas permeability lower than 1 m3 /(m2∙min) at a pressure of 200 kPa, according to GOST 12088-77) filtered out the solid phase. This is because of the ultrafine zinc sulfide, which is confirmed by the data obtained with a Helos N2791 laser particle-size analyzer.
The filtration problem was resolved by introducing ZnS from the previous stage of precipitation into the reaction system. It was shown that a substantial improvement of the filtrational properties is achieved at a 1 to 5% consumption of cycling zinc concentrate. This is due to the formation of larger precipitate particles in such conditions. The size of particles was 8 μm (90%) in the absence of seed (ZnS) and 39 and 44 μm (90%) in the presence of 1 and 5% ZnS, respectively. As a result, filtration started at a pressure of 1.6 MPa on fabrics with a gas permeability of 1 m3 /(m2∙min) at 200 kPa. In these conditions, the moister content of the cake ≈40–42% at a filtration rate of 0.03 t/(m2∙ h) of the solid phase. Further increase in the amount of ZnS did not lead to an increase in the filtration rate.
After precipitation of zinc, the mother solution should be treated with lime suspension. It was shown that after neutralization of the solution to pH = 9.5–10.5 and settling, the solution is finely cleaned of heavy nonferrous and light metals. The purified water had the following composition, mg/liter: 0.05 Zn, 0.01 Cu, 0.02 Al, < 0.02 Fe, 0.05 Mn. During settling, pH increased to 8.0–8.5 due to hydrolysis processes.
The studies resulted in a flow diagram of rational processing of underspoil water from nonferrous metal mining facilities (Fig. 5). Water cleaned of nonferrous metals is used for industrial purposes, primarily, for preparation of a mixture for filling the excavated space.
The diagram includes cementation of copper and thickening, filtration, and drying of the copper concentrate. The moisture content of slurries was experimentally determined using data from [17]. The copper precipitate slurry is clarified at an average linear rate of 2 m/h, achieving a ≈ 70% solid content in the thickened product. The vacuum filtration of copper precipitate slurry produces a cake of 11–12% moisture content at high filtration efficiency (0.5–0.6 t/(m2∙ h)).
Upon thickening and filtration of the precipitate, the loss of copper because of dissolution does not increase, despite the dependence of the content and extraction of copper on the consumption of iron powder (Fig. 2). This is because without intensive agitation the dissolution of copper abruptly slows down due to intradiffusion.
Zinc concentrate is extracted from the mother solution in the presence of sodium hydrosulphide. The product is thickened to a solid content of 1.8–2.0%, dehydrated to a moisture content of 40–42%, and, after drying, delivered to the consumer (zinc production plants). The linear rate of clarification was 0.5 m/h. The filtration rate under a pressure of 1.6 MPa was 0.03 t/(m2∙ h). The zinc content in the zinc concentrate was 49–51%, and the copper content in the copper concentrate was 27–28%. The extraction of copper and zinc from the solution was no less than 94 and 99%, respectively.
Dezinced water was treated with lime until pH = 9.5–10.5 and supplied to ponds for cleaning from fine suspensions. The purified water is used for industrial purposes (for preparation of a mixture for filling mines).
Conclusions
The concentration of copper and zinc from the Safyanovskaya Med company’s underspoil water has been studied. It has been shown how the precipitation conditions and the composition of water affect the composition of the products. To minimize the consumption of iron and to reduce the cost of the process, it has been proposed to carry out cementation in washboxes with iron waste. In these conditions, the intradiffusion resistance to the reaction is eliminated, which results in a concentrate richer than in the case of cementation with iron powder. An integrated flow diagram of water processing to extract copper (27–28% Cu) and zinc (49–51% Zn) concentrates has been developed. The extraction of copper and zinc was 94–95% and 99%, respectively. The quality of copper and zinc concentrates meets the requirements of GOST R 52998-2008 and GOST R 54922-2012, respectively. The operating conditions ensuring fine cleaning of the solution from nonferrous metals and obtaining commercial products have been established.
References
M. G. Viduetskii, I. F. Garifulin, V. A. Mal’tsev, and A. P. Purgin, “A technology of cleaning mine, underspoil, reused, and sewage water of mining and smelting facilities,” Tsvetn. Metally, No. 8, 9–14 (2017).
A. M. Pan’shin, M. G. Viduetskii, A. P. Purgin, et al., “Developing a technology of cleaning mine water use a KFM-series pneumatic flotation cell,” Tsvetn. Metally, No. 10, 93–97 (2014).
V. A. Chanturiya and A. P. Kozlov, “Innovative processes of advanced and comprehensive processing of man-made mineral raw materials,” in: Proc. Conf. “Tekhnogen-2012” on Fundamentals of Technologies of Processing and Recycling Man-Made Waste [in Russian], Ekaterinburg (2012), pp. 20–23.
S. A. Yakornov, A. M. Pan’shin, P. A. Kozlov, and D. A. Ivakin, “Current state of the art in technologies of leaching dusts of the iron and steel industry and the products of their pyrometallurgical processing (acid, ammonium, and alkaline technologies),” Tsvetn. Metally, No. 5, 37–43 (2017).
S. E. Klyain, P. A. Kozlov, and S. S. Naboichenko, Extraction of Zinc from Ore Raw Materials [in Russian], Izd. UGTU-UPI, Ekaterinburg (2009).
M. I. Alkatsev, Cementation Processes in Nonferrous Metallurgy [in Russian], Metallurgiya, Moscow (1981).
E-S. Z. El-Ashtoukhy and M. H. Abdel-Azi, “Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens,” Int. J. Mineral Process.,121, 65–69 (2013).
V. Cala-Rivero, J. C. Arranz-Gonzalez, V. Rodriguez-Gomez, et al., “A preliminary study of the formation of efflorescent sulfate salts in abandoned mining areas with a view to their harvesting and subsequent recovery of copper,” Minerals Eng.,129, 37–40 (2018).
Sajeda A. Al-Saydeh, Muftah H. El-Naas, and Syed J. Zaidi, “Copper removal from industrial wastewater: A comprehensive review,” J. Industr. Eng. Chemistry,56, 35–44 (2017).
M. Karavasteva, “Kinetics and deposit morphology of copper cementation onto zinc, iron and aluminium,” Hydrometallurgy,76, No. 1–2, 149–152 (2005).
G. M. Vol’dman and A. N. Zelikman, Theory of Hydrometallurgical Processes [in Russian], Moscow (2003).
B. D. Khalezov, Studies and Development of the Technology of Heap Leaching of Copper and Copper-Zinc Ores [in Russian], PhD Thesis, 05.16.02, Ekaterinburg (2008).
A. E. Lewis, “Review of metal sulphide precipitation,” Hydrometallurgy, No. 2, 222–234 (2010).
B. D. Khalezov, N. A. Vatolin, L. A. Ovchinnikova, and G. A. Pavlichenko, “Studying the extraction of copper and zinc sulfides from copper–zinc sulfate solutions,” GIAB, No. 1, 261–265 (2005).
L. P. Wang and Y. J. Chen, “Sequential precipitation of iron, copper and zinc from wastewater for metal recovery,” J. Envir. Eng., No. 1, 1–11 (2019).
L. P. Wang, J. Ponou, S. Matsuo, et al., “Selective precipitation of copper and zinc over iron from acid mine drainage by neutralization and sulfidization for recovery,” Soc. Materials Eng. for Resources for Japan, No. 2, 136–140 (2014).
G. G. Chuyanov, Auxiliary Beneficiation Processes. Dehydration and Dust Collection [in Russian], Izd. UGGU, Ekaterinburg (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 63, No. 11, pp. 8–14, November, 2019.
Rights and permissions
About this article
Cite this article
Klyushnikov, A.M. Study of Copper and Zinc Extraction from Underspoil Water. Metallurgist 63, 1135–1143 (2020). https://doi.org/10.1007/s11015-020-00933-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-020-00933-w