Exhaustion of rich or readily enriched precious metal ores leads to involvement in operation of stubborn ores from which effective extraction of precious metals by traditional methods is impossible. Within stubborn ores, a considerable part of gold is finely associated with sulphide minerals, generally with arsenopyrite and pyrite, and remains inaccessible for leaching even with fi ne comminution. Presence of an active carbonaceous agent occluding cyanide complexes of gold from solutions is another reason for stubbornness of gold-bearing ores. The joint negative effect of both factors on enrichment gives rise to doubly stubborn ores. A reduction in gold extraction indicators during hydrometallurgical processing for ores and concentrates with sorption and active organic carbon is overcome in most cases by using ion-exchange resins and activated carbon, whereas recovery of gold bonded in sulfides requires breakdown of the mineral crystal lattice. For this purpose, oxidizing roasting, and autoclave or bacterial oxidation of sulfides in a pulp are usually applied. The last two methods are now the most widespread. At the same time, roasting gold ores and concentrates is applied at a number of the overseas enterprises. This shows that hightemperature processes remain the main methods for preparing gold-bearing ores and concentrates for leaching. On the basis of results of calculations, research, and design and engineering development, and industrial tests the authors conclude that it is possible to increase extraction of gold and silver in products from the Mai and Badran fields after pyrolysis in a rotary drum furnace. Compared with oxidizing roasting environmental protection issues are resolved. Procedures and production equipment schemes are developed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The main reason preventing the spread of high-temperature processes in the practice of processing stubborn gold ores is failure to resolve ecological problems connected with discharge of oxide compounds of arsenic and sulfur. In addition, during roasting there is possible encapsulation of gold particles both due to melting of ore particles and also formation of oxide films at a gold surface. In this case, cinder should be reground and (or) subjected to alkali treatment in order to provide access of cyanide to a gold surface.
More promising for treatment of gold-arsenic and gold-antimony concentrates are pyrolysis and pyrohydrolysis with limited access of air or water vapor, which provides of a porous (loose) cinder structure and also removal of arsenic and sulfur in the form of solid products [1, 2] (Fig. 1).
In order to obtain good cyanide products, it is necessary to observe rigidly the optimum roasting temperature regime.
This task is complicated in view of the occurrence during roasting of chemical reactions whose thermal effects should be taken into account when calculating the thermal balance and roasting furnace construction [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16].
All processes are based on endothermic reactions, that are the main problem in industrial application of oxidation roasting (pyrolysis). In addition, carbon present in materials at 400–1000°C reacts with heated water vapor also by endothermic reactions [6]. The reactions are entirely reversible. Their occurrence according to the Le Chatelier rule facilitates a reduction in pressure (rarefaction).
It has been established [17, 18] that if the main carriers (gold collectors) are pyrite, arsenopyrite, chalcopyrite, their total decomposition is completed at 700–750°C. In this case, the gas phase carries volatile gaseous products: CO, CO2, As, and S. If the carriers are antimonite, tetrahedrite, and impoverished ore, their total decomposition occurs at 500–700°C.
The overall endothermic effect during pyrolysis without access of air or in an inert atmosphere (nitrogen, argon) may reach 120–150 kJ/mole or more.
During research and in production, there is a main task: to reduce the overall endothermic effect of pyrolysis to a minimum by changing conditions for its implementation.
The aim of the work is evaluation of the thermodynamic possibility of extracting precious metals from lean ores or dumps of mining and enrichment production, and calculation and construction of effective industrial units for increasing the degree of precious metal extraction. For this, the following tasks were resolved:
-
1)
evaluation of thermodynamic potentials of possible chemical processes for precious metal extraction;
-
2)
experimental determination of conditions for the occurrence of chemical processes with the aim of increasing the degree of gold extraction from concentrates and tailings; and
-
3)
construction of semi-industrial and industrial units for extracting precious metals from concentrates and wastes of the mining and metallurgical complex.
Research procedure. In order to conduct research, a pilot plant was used for roasting (pyrolysis) of concentrates. The unit is a rotary drum electric furnace MOTrVrP-225 (construction and manufacture of Factory Company of Metals, ZKM) that is fitted with a reaction tube of chamotte with lamellar graphite. The outer tube diameter is 255 mm, tube length 2300 mm; the furnace has three zones. The nominal electric power for the electric furnace is 60–63 kW. The maximum tube surface working temperature is 1320 ± 10°C.
Oxidizing roasting was carried out in two stages. The first stage at 400–450°C, and the second at 725–790°C. Distillation roasting (pyrolysis) was performed at different temperatures: 700 ± 100°С, 725 ± 10°С, 750 ± 10°С, and 800 ± 10°С. The dwell time for a specimen in the unit 10–30 min.
The starting gold sulfide concentrates were subjected to chemical, phase, and mineral analysis.
Similar studies have been conducted with cinders of oxidation and distillation roasting, and also pyrolysis sublimates after which cyaniding of concentrates and cinders was accomplished by a standard procedure.
The results obtained served as a basis for developing a technology for controlling pyrolysis and development of an equipment and production scheme.
Research results. Intensification of pyrolysis is achieved by introducing an oxidizing agent into the reaction zone: heated water vapor with a temperature above 400°C and air at a temperature above 400°C [17,18,19]. Thermodynamic calculations confirm the occurrence during pyrolysis and pyrohydrolysis (pyrolysis with addition of water vapor) of the following reactions:
Without oxygen:
With participation of water vapor and oxygen:
All these reactions, apart from reaction (1), are exothermic. It is well known [3, 6] that during pyrohydrolysis (in an atmosphere with water vapor) sublimation of sulfur commences from 150°C. Up to 450°C water vapor consumption has little effect on the degree of transformation, but at above 650°C there is an increase in water vapor diffusion rates, which facilitates a rapid increase in the degree of conversion. Heat treatment provides preparation of cinder with a low sulfur volume content (<1.2%) that is difficult to achieve during roasting by traditional methods.
A study of hydrolysis and pyrohydrolysis of stubborn sulfides concentrates [17,18,19] has shown that use of water vapor and air oxygen is only effective in the case of their limited application in volumes of 5–20%. In this case, a change is noted in pyrolysis heat engineering, the endothermic effect of the process approaches zero, and processes (3), (4), and (5) themselves with participation of water vapor and oxygen become exothermic.
The decomposition temperature for complex oxides is well known [6, 16]: for pyrite and arsenopyrite it is 300–700°C, and for chalcopyrite it is 500–700°C. During heat treatment of concentrates, the mineral decomposition temperature is reduced by 70–100°C compared with heat treatment in an air atmosphere. It has also been established that during heat treatment sulfur is released in an elementary state, arsenic in the form of trisulfide, and organic substances without breakdown. According to values of isobaric-isothermal potentials [13,14,15,16,17], reactions (6) and (7) do not proceed since the isobaric-isothermal potential ΔG is positive (at 700°C):
At 700°C, the value of the isobaric-isothermal potentials confirms the possibility of occurrence of the following reactions:
It has been established that sulfide minerals present in concentrates such as PbS, ZnS, Ag2S, Sb2S3, and others, react with water vapor similar to FeS2.
Under heat treatment conditions for sulfide concentrates, there is a complex process. The equation for reaction of arsenopyrite (1 mole) with pyrite (1.5 mole) and water (1 mole) at 700°C takes the form
Theoretically, the required consumption of sulfidizer is 1.5–2.0 mole. An increase in the amount of water more than 1 mole leads to oxidation of the arsenic sulfide formed, which is undesirable [3, 18].
During heat treatment, the following reaction is thermodynamically probable:
However, arsine (AsH3) breaks down at 230°C at atmospheric pressure.
Various researchers have established [6,7,8,9,10,11,12,13,14] that oxidation of sulfide materials at up to 600 ± 50°C in the presence of water vapor proceeds more slowly than in the presence of air. Starting from 600°C, the reaction rate increases and the process ceases in 25–70 min. It has also been established by experience that arsenic sulfide (As2S3) is a water vapor atmosphere does not undergo changes, it volatilizes and is carried by the flow into a cooler-condenser.
An increase in heat treatment temperature during pyrolysis and pyrohydrolysis above 750°C is undesirable [12,13,14,15, 18,19,20,21] for production and economic considerations, since heat treatment products lose their magnetic properties, sintering and compaction commences, which is unacceptable for cyaniding. Due to the endothermic nature of reactions, during performance of the process in industrial equipment (drum furnace) there is an increase in heat carrier consumption.
The best results for extraction of gold into cyanide solutions up to 98.7% are obtained with pyrolysis of sulfide gold-containing concentrates in a pilot plant furnace (retort) with a treatment time of 25 min and temperature of 750 ± 50°C [17]. After 10–15 min of charging material into an isothermal zone of the retort, the weight of solid residue stabilized. A dwell time for material in the reaction zone of more than 25–30 min led to an undesirable process, diffusion coarsening of sulfide grains followed by deterioration of cyaniding indices.
Pilot plant tests of pyrolysis in a Yakut concentrator were performed in a rotary tube furnace under industrial conditions of indirect heating in an argon atmosphere at 400–800°C. Roasting in two stages showed identical results with pyrolysis for an identical dwell time in the isothermal furnace zone (τ = 12 min). An increase in both roasting and pyrolysis temperatures above 750°C led to sintering and cinder melting, and an increase in their stubbornness [11,12,13,14,15,16,17,18,19].
According to phase analysis data with pyrolysis of cinder performed at the optimum temperature for 12 min, the content of cyanided gold increased as a result of obtaining porous loose cinder and an increase in the proportion of gold with an open surface. The maximum gold extraction was 92.3% with its content in absorption cyaniding tailings of 4.1%. Similar extraction indices (98.8%) have been obtained with cyaniding of two-stage oxidation roasting cinder.
Pyrolysis cinder consists of pyrite and arsenopyrite decomposition products, pyrrhotite and iron oxides. In the case of oxidation roasting, almost all the iron is converted into oxide form. In this case, sulfides lose 50–100% of sulfur and more than 20% of volume with a corresponding increase in porosity, which provides access of cyanide solution to the surface of finely disseminated gold in sulfide, and correspondingly an increase in extraction index.
The main sublimation components during pyrolysis are three phases, i.e., arsenic sulfide, arsenic oxide, and elementary sulfur. The composition of sublimates (in weight fractions of elements) corresponds to the formula As50S40O10.
Pyrolysis of gold sulfide concentrate with dispensed additives of water vapor and ammonium chloride has significant promise for application: for preparation (breaking down gold sulfide concentrates) with additions of 2–3%, and during roasting before melting with additions of 20–25%.
Results of these studies make it possible to conclude that there is a practical possibility of increasing the extraction indices for gold from raw material from enrichment plants by 18–30% after pyrolysis in a tubular rotary furnace with application of desublimaters (crystallizers, coolers) for arsenic sulfide and crystalline sulfur.
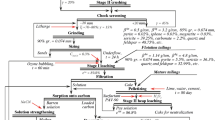
On the basis of pilot plant tests [20, 21], structures and a multipurpose rotary furnace have been designed and developed for indirect heating with burner units based on diesel fuel or fuel oil. Equipment and a production scheme have been developed for sublimation roasting (pyrolysis) of up to 500–550 kg/h of gold-containing concentrate containing gold at 40–60g/ton (Fig. 2).
The chain of equipment proposed may provide breakdown, preparation of raw material for cyaniding with subsequent gold extraction into solution of 90–97%.
The desublimators proposed (Fig. 2, positions 2, 3) are double-screw reactor-mixers. The purpose of equipment 2 is to control arsenic sublimate in the range 200–300°C. The purpose of equipment 3 is to control sublimates of crystalline sulfur and to condense part of the moisture at 80–100°C.
It is proposed to use Weishaupt (Germany) multi-fuel units as burner devices.
Use as heat carrier of a carbon–air mixture requires both production and economic development taking account of local conditions. The specifications for this equipment are very rigid. With the prescribed productivity for one industrial unit (furnace) for sulfide flotation concentrate for Yuzhuralzoloto and Vysochaishii companies, the dwell time for sulfide concentrate within the working zone at 700–750°C comprises 2.5–3.0 tons/h, and 1–1.5 tons/h, respectively. These parameters are achieved in drum two-section furnaces with indirect heating with one of any of the following heat carriers: natural gas, diesel fuel, and fuel oil. The technical and economic characteristics of the furnaces planned for production and operation with different producers exceed the indices for furnaces with a central control system (CCS), cyclone and shaft.
The rotation frequency for the reaction tube is controlled smoothly, which it possible to control the dwell time for products in the isothermal zone. The furnace inclination is fixed.
Tube material for the first part of the unit is low-carbon steel, and for the second it is alloy steel. Heating units for the support sleeves, transmission gears, and pipes of the second section are made of special spring steel. The device is sealed. Seals are lobed, and leakage under rarefaction is not more than 300 mm of a water column per hour. The lobe material is special wear-resistant cermet. An arch-forming unit is used in the loading bunker, and a double-screw feeder excludes formation of “separations” of transported concentrate. The reaction tube (first section) from two sides is provided with inlets (direct flow and counterflow) for air, inert gas, heated steam, and pumping lines (evacuation). Preparation of these units and component assemblies for them are planned to be in domestic plants.
Conclusions. The research conducted for enrichment of lean ores, mining enrichment complex waste, and also development and use of units making it possible in practice to increase the degree extraction of precious metals from lean ores and waste of the mining industry, provide efficient processing for dumps of large mining and metallurgical combines.
This work was performed with the aim of increasing the profit and efficiency of gold mining enterprises in creative cooperation of scientists and specialist of MISiS and ZKM (Moscow) within the scope of combined work for undergraduate programs.
References
Amended Technical Report on the Mactung Property, Yukon, Canada, Wardrop, Vancouver (2009), www.natungsten.com/i/pdf/Tech-Report-Mactung.pdf, acc. 09.20.2016.
B. K. Mikhailov, G. V. Sedel’nikova, B. I. Benevol’skii, and A. I. Romanchuk, “Innovative technology for processing stubborn and lean gold ores based on rational usage,” Rudy Metally, No. 1, 5–8 (2014).
I. G. Antropova, Physicochemical Production Bases for Sulfide Roasting of Oxidized Lead-Zinc Ore in a Heated Steam Atmosphere: Dissert. Cand. Techn. Sci., RAN SOBNP, Ulan Ude (2005).
Yu. N. Mansurov, V. P. Reva, S. Yu. Mansurov, and M. V. Beloborodov, “Economic and social basis of material science development in the far east,” Tsvet. Metally, No. 11, 88–93 (2016).
J. E. Athey, L. K. Freeman, L. A. Harbo, et al., “Alaska’s mineral industry 2013,” Spec. Report 69 (2014), https://doi.org/10.14509/29128, acc. 09.20.2016.
A. N. Ertseva, Study of Solid-Phase Transformation Occurring during Heating of Sulfide Copper-Nickel Ore Raw Material and Development on the Basis of the Data Obtained of Improved Production Processes for Processing: Disert. Doc. Techn. Sci., Institute Gipronikel, St. Petersburg (2001).
R. A. Ayuso, K. D. Kelley, D. L. Leach, et al., “Evidence from regional Pb and Sr isotope sources Origin of the Red Dog Zn–Pb–Ag deposits, Brooks Range, Аlaska,” Econ. Geol., 99, No. 7, 1533–1553 (2004).
D. Gaun, Pebble Resource Estimate 2014, Northern Dynasty Minerals Ltd., Vancouver (2014), https://www.sec.gov/Archives/edgar/data/1164771/000106299315000646/exhibit99-1.htm, acc. 09.20.2016.
R. J. Goldfarb, R. Ayuso, M. L. Miller, et al., “The Late Cretaceous Donlin Creek gold deposit, Southwestern Alaska: Controls on epizonal ore formation,” Econ. Geol., 75, No. 4, 643–671 (2004).
Fort Knox Mine, Fairbanks North Star Borough, Alaska, USA: National Instrument 43-101 Technical Report, Kinross Gold, Vancouver (2015), http://fb.kinross.com/media/261547/2015%20fort%20knox%20tr.pdf, acc. 09.20.2016.
Kupol and Dvoinoye Mine, Russian Federation: National Instrument 43-101 Technical Report, Kinross Gold, Vancouver (2015), http://fb.kinross.com/media/261550/2015%20kupol-dvoinoye%20tr.pdf, acc. 09.20.2016.
N. L. Patison, G. Salamis, and V. J. Kortelainen, “The Suurikuusikko gold deposit: Project development summary of northern Europe’s largest gold resource,” Gold in the Central Lapland Greenstone Belt, Finland, V. Juhani Ojala (ed.), Geological Survey of Finland, Spec. Paper 44 (2007).
J. Chen, Y. Chen, Z. Wei, and F. Liu, “Bulk flotation of auriferous pyrite and arsenopyrite by using tertiary dodecyl mercaptan as collector in weak alkaline pulp,” Minerals Eng., No. 23, 1070–1072 (2010).
The Future of Mining in Canada’s North, The Conference Board, Inc., New York (2013), www.miningnorth.com/wp-content/uploads/2013/01/Final-13-201_FutureofMining_CFN.pdf, acc. 09.20.2016.
P. Weihed, P. Eilu, R. B. Larsen, et al., “Metallic mineral deposits in the Nordic countries,” Episodes, 31, 125–132 (2008).
Technical Report and Prefeasibility Study for the Back River Gold Property, Nunavut, Canada, Sabina, Vancouver (2014), www.miningdataonline.com/reports/BackRiver_FeasibilityStudy_2015.pdf, acc. 09.20.2016.
I. G. Trukhina, Yu. N. Mansurov, V. P. Reva, and V. A. Pimenov, “Structure of TiO2–ZrO2–SiO2 tubes,” Tsvet. Metally, No. 1, 61–65 (2016).
Z. V. Bocharov, V. A. Ighnakina, and D. V. Abryutin, Technology for Processing Gold-Containing Raw Material, ID MISiS, Moscow (2011).
Yu. N. Mansurov, V. P. Reva, S. Yu. Mansurov, and M. V. Beloborodov, “Economic and social basis of material science development in the far east,” Tsvet. Metally, No. 11, 88–93 (2016).
S. C. Kim, Yu. N. Mansurov, and S. H. Li, “The method for producing copper nanoparticles and analysis of their lubricating ability,” Solid St. Phenom., 265, 738–744 (2017).
E. E. Shaposhnikova and Yu. N. Mansurov, “Methods for improving gold extraction at the enterprise Pokrovskii Rudnik,” Mezhdunar. Zh. Eksperim. Obraz., No. 8-4, 45–49 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 2, pp. 71–76, February, 2018.
Rights and permissions
About this article
Cite this article
Mansurov, Y.N., Miklin, Y.A., Miklin, N.A. et al. Methods and Equipment for Breaking Down Gold-Containing Concentrates from Lean Ores and Mining Industry Waste. Metallurgist 62, 169–175 (2018). https://doi.org/10.1007/s11015-018-0640-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-018-0640-z