Features of nonmetallic inclusion formation and evolution in the course of treating spring steel of 54SiCr6 (60S2KhA) grade are studied. It is established that the majority of nonmetallic inclusions (NI) present within steel are complex oxides of the composition SiO2–MnO–Al2O3–CaO–MgO, at whose surface during steel crystallization and cooling as a rule there is a deposit of MnS. NI composition changes in the course of metal treatment. At the start of treatment, their base is MnO–SiO2–Al2O3, and subsequently there is an increase in content of CaO and MgO as a result of molten metal reaction with slag and lining. The average NI size and overall oxygen content decrease in the course of steel treatment, and this points to effective refining. The composition of NI present is close to that of the slag cover assimilating layer used in the intermediate ladle. A more significant increase is noted in MgO and Al2O3 content within it, and this points to effective assimilation of NI of the most unfavorable types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is well known (see, for example, [1, 2]) that the presence of nonmetallic inclusions (NI) affects the level of service properties and quality characteristics of spring steels and objects. Not only their overall content is important, but also mainly certain types of NIs, for example based on corundum, capable of becoming stress concentrators leading to metal failure during operation. In view of this, an important task is establishment of features of the change in properties (type, amount, size, morphology, distribution throughout the volume) of NIs during start-to-finish technology for manufacturing spring steel rolled product.
Steel of type 54SiCr6 (60S2KhA) produced by the developed technology [3] was used in the study. Its distinguishing features are: use of a metal charge pure with respect to nonferrous metals, and also a number of production methods aimed at increasing steel cleanliness with respect to NIs. In this case, it concerns: delivery of carbon-containing materials for output of metal semifinished product in a steel-pouring ladle (SL); use as a silicon-containing material of ferroalloy FeSi75−SHP, and also factional delivery of silicon- and manganese-containing ferroalloys for forming fluid silicate inclusions; use of a double-layer slag coating with an assimilation layer in the intermediate ladle (IL).
In order to establish features of the evolution of NI properties during treatment of molten metal, four samples were studied in the course of metal treatment in a furnace-ladle unit (FLU), and three samples from an IL during steel continuous casting (SCC) (Table 1).
Research was carried out by means of contemporary procedures of optical and electron microscopy, local x-ray spectral analysis in СarlZeissAxiovert 40 Mat and Jeol JSM 6610 LV instruments using and energy dispersion Inca X-act energy dispersion microanalyzer produced by Oxford Instruments.
The results obtained indicate that the majority of NIs present are complex oxide composites of the SiO2–MnO–Al2O3–CaO–MgO system at whose surface during steel crystallization and cooling there is MnS deposition. The composition of inclusions is subject a regular change in the course of metal treatment. Whereas at the start of treatment their base comprises the system MnO–SiO2–Al2O3, subsequently there is an increase CaO and MgO content. Analysis carried out on the basis of data in [4] shows that the majority of NIs recorded in the initial metal treatment stages of the MnO–SiO2–Al2O3, MnS–SiO2–MnO systems are in a liquid condition, which is achieved due to fractional addition of silicon- and manganese-containing materials in a required ratio [3]. Their typical external appearance is shown in Fig. 1. Since the established NI compositions are to a certain extent conditional due to the limited precision of their determination, therefore not in all cases are they actually in a homogeneous liquid condition. In some NIs, the presence of certain fractions of solid phases is possible (see Fig. 1a ). The chemical composition of NIs of the MnS–SiO2–MnO system is conditional to an even greater extent, since manganese sulfide is almost insoluble in liquid silicates and is present as a shell (see Fig. 1b ), and separation of manganese between oxide and sulfide components is connected with additional errors.
NIs (see Fig. 1) are typical for metal samples AKP1, AKP2, and AKP3, and not typical for metal samples of APK4 and PK1 collected during steel subsequent treatment. They have a globular shape, which points to a fluid condition. Due to this, NIs are subject to coalescence, which facilitates their removal in slag.
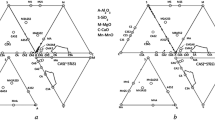
Analysis carried out by means of data in [4] indicates that NIs based on the CaO–SiO2–MgO system, in contrast to the majority of cases, are in a solid (crystalline) condition. It should also be noted that inclusions were detected in the first sample collected immediately on entry of metal into the FLU. The mechanism of their formation is connected with the modifying effect of lime on silicate inclusions [5], and the presence of magnesium in their composition may be due to washing of a FL magnesite lining by metal and slag [6]. Charging of a significant weight of feldspar (more than 0.5 ton) facilitates this. These inclusions (Fig. 2), recorded in samples of metals AKP 3, AKP 4, PK 1, PK 2, and PK 3 (see Table 1), are likely to contain alumomagnesium spinel. However, its formation is not connected with the steel deoxidation and alloying schemes used, since silicon-containing material with a low Al content was used.
Recorded typical NI external appearance and composition of the CaO–SiO2–MgO system in samples of AKP 3, AKP 4, PK 1, PK 2, and PK 3 metal (see Table 1).
Features established for the change in the proportion of liquid NIs and their average size on the basis of average data for their size and aggregate condition in the course of ladle treatment and SCC are shown in Fig. 3.
It is seen that at the start of ladle treatment fluid NIs predominate, whits their proportion subsequently decreasing due to removal into slag. The average NI size at the start of ladle treatment is about 15 μm and it increases to ≈25 μm as a result of coalescence (see Fig. 3b ). Attention is drawn to the presence of a minimum in Fig. 3a . To all appearances an increase in the proportion of liquid NI in the course of steel ladle treatment is connected with the efficiency of solid inclusion modification.
The actual NI composition is complex, not corresponding to the ternary composition diagram. Calculation and analysis of the average content of the main components has been carried out in order to establish features of the change in chemical composition (Table 2).
It follows from data in Table 2 that towards the end of ladle treatment and over the extent of the whole SCC the overall molar fraction of most favorable NI components (Al2O3, MgO) is not more than 0.20–0.25 of the overall content. It should be noted that these NIs are not recorded within metal sample AKP 1. After adding a slag cover using feldspar, NIs are detected within the metal with an insignificant molar fraction of Al2O3 and MgO. In a sample of APK 3 metal, a relative increase is observed in molar faction of MgO, which to all appearances is connected with delivery of a significant (of the order of 2 tons) weight of silicon-containing material. Before metal crystallization, the molar fraction of MgO within the inclusion composition is essentially unchanged. An important situation is that a considerable part of NI over the extent of the whole metal treatment has a silicate base. To all appearances, a marked recovery of the presence of aluminum within metal does not occur due to its low content and kinetic limitations. With a fixed aluminium content [Al] ≈ 0.005%, if it is in dissolved (not bonded into oxide) condition, there should be reduction of silica. With an aluminum concentration above 0.001%, in equilibrium with dissolved oxygen in metal there should be corundum [7], although analysis shows that the system even with [Al] ≈ 0.005% moves towards equilibrium extremely slowly.
The main purpose with formation of liquid SiO2−MnO inclusions is an increase in metal cleanliness as a result of their removal into slag. In order to evaluate the capacity of these inclusions for floating and assimilation by a slag cover, tests were carried out for selected metal specimens for overall oxygen content in a LECO-136 gas analyzer. The method of restorative melting was used in a stream of inert gas-carrier in accordance with GOST 17745−90. In order to guarantee obtaining reliable results, five specimens weighing about 0.5 g were prepared from each sample of cast metal and rolled product. It was established that metal refining from NI occurred over the extent of the whole steel treatment (Fig. 4). Consequently, a slag cover with steel ladle treatment exhibited good assimilating capacity. A favorable slag composition in the IL made it possible to carry out metal refining during billet continuous casting. It was also seen that the overall oxygen content in a metal sample collected from rolled product was less than in metals samples from the IL, which points to good assimilating capacity of the slag-forming mix (SFM) of grade Accutherm ST-SP512-21-3 used in a SCCU mold.
It follows from the features established that metal refining does not reach its maximum level due to limitations of treatment in the FLU over time. It is important that there is no increase in NI content in an AKP 3 sample collected after delivery of a significant weight of ferroalloys. The overall oxygen content in metal at the start of SCC is about equivalent to its content before melt delivery to the SCCU. To all appearances, this is connected with the fact that after transfer of the ladle to the SCCU there is no bottom blowing with argon, which considerably intensifies NI removal. Some increase in overall oxygen content in the sample of PK2 metal is apparently connected with the entry of exogenic NI. Analysis of a PK3 sample shows that metal refining in the IL occurs before the end of SCC. In order to evaluate the efficiency of using an SFM grade GShOS-10 as a refining layer in an IL slag coating chemical composition was analyzed at the start and end of SCC. It is seen from data obtained (Table 3) that the content of the main components within slag is quite similar to the average NI chemical composition.
This has a favorable effect on their assimilation by slag with retention of good functional properties. A protective slag covering in an IL was added at the start of melting, preceding testing, and therefore it is difficult to establish precisely the weight of assimilated NI (see Table 3). Nonetheless, an increase is seen in the content of MgO and Al2O3 within the composition of an assimilating slag coating in the IL. This points to the efficiency of assimilation of the most unfavorable types of NI by slag.
Analysis of rolled product of steel of the test melt for overall oxygen content (according to GOST 17745−90) showed that its content is 0.0014%, whereas for rolled product of steel of similar grade (60S2KhA), prepared by basic technology, this index was 0.0020%. If presence is considered of oxygen dissolved within metal, the increase in the degree of metal cleanliness with respect to NI is even more significant.
In studying metal specimens collected from springs prepared from rolled product by basic technology, it was recorded that a reason for occurrence of a “crack” defect is an increased content of readily melting nonferrous metal impurities [2]. On the basis of this, and carrying out a test melt, charge materials were used of improved quality with an increased proportion of iron in a charge. Nonetheless, in the course of studying metal samples collected at different steel treatment stages NI were detected with an increased content of nonferrous metal impurities (Fig. 5). These NIs have a considerable size and may be the reason for the occurrence of cracks in rolled product and finished objects. They were detected in samples of metal collected at the start of ladle treatment and at the end of SCC. Thus, the measures adopted for improving charge material quality do not entirely guarantee the absence of unfavorable nonferrous metal impurities localized within NI. To all appearances, metal contamination with nonferrous metal impurities could occur during melting, and also during ladle treatment as a result of their accumulation and transfer into metal from a lining, since in previous melts there were no special requirements for charge materials. In order to improve steel purity with respect to nonferrous material impurities it is expedient to carry out prior melting and treatment in a ladle with similar specifications for charge material quality.
Conclusions. Features have been established for the formation and evolution of nonmetallic inclusions (NI) in the course of treating spring steel of grade 54SiCr6 (60S2KhА). It has been revealed that the majority of NIs present within steel are complex oxide systems SiO2–MnO–Al2O3–CaO–MgO, at whose surface during steel crystallization and cooling as a rule there is deposition of MnS. Inclusion composition is subject to a regular change in the course of metal treatment. Whereas at the start of treatment their base is of the MnO–SiO2–Al2O3, subsequently there is an increase in the CaO and MgO content as a result of melt reaction with slag and lining.
Indices for the average NI size and overall oxygen content decrease steadily in the course of steel treatment, and this points to its efficient refining. Metal refining did not achieve its maximum degree due to limitations with respect to time, and the composition of NIs present is quite similar to that of the slag cover assimilating layer used in an intermediate ladle. The most significant increase MgO and Al2O3 content within it has been recorded, and this points to effective assimilation of the most unfavorable types NI by slag.
Use of the technology developed has made it possible to achieve a reduction by more than 25% in total oxygen content in rolled product from 0.0020 to 0.0014–0.0015% and even more significantly increase steel cleanliness with respect to NIs.
The research was carried out at Bardin TsNIIchermet and supported by a grant of the Russian Scientific Fund (project No. 14-19-01726).
References
A. I. Zaitsev, K. A. Kosyrev, and I. G. Rodionova, “Contemporary trends in development of metallurgical technology for achieving a good set of service properties and high quality steel indices,” Probl. Chern. Metal. Materialoved., No. 3, 5–15 (2012).
A. I. Zaitsev, A. B. Stepanov, V. A. Sarychev, and A. Yu. Dzyuba, “Study of types, reasons for occurrence of defects of continuously cast billet rolled product and finished objects of spring steels, including nonmetallic inclusion formation,” Probl. Chern. Metal. Materialoved., No. 1, 35–45 (2015).
A. I. Zaitsev, A. B. Stepanov, V. A. Sarychev, et al., “Study of the effect of content and form of impurities present, nonmetallic inclusions on qualitative properties of spring steels,” Metallurg, No. 3, 40–47 (2015).
Slag Atlas [Russian translation], Metallurgiya, Moscow (1985).
E. Kh. Shakhpazov, A. I. Zaitsev, I. G. Rodionova, and G. V. Semerkin, “Key areas of development of metallurgical technology for satisfying an increasing requirement for steel quality,” Elektrometallurgiya, No. 2, 2012 (2011).
A. I. Zaitsev, I. G. Rodionova, G. V. Semernin, et al., “New types of unfavorable nonmetallic inclusions based MgO−Al2O3 and metallurgical factors governing their content within metal. Pt. 1,” Metallurg, No. 2, 50–55 (2011).
T. Fujisawa and H. Sakao, “Equilibrium relations between the liquid iron alloys and deoxidation products resulting from Mn–Si–Al complex deoxidation,” Tetsu-to-Hagane, 63, No. 9, 1494–1503 (1977).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 10, pp. 45–49, October, 2015.
Rights and permissions
About this article
Cite this article
Stepanov, A.B., Zaitsev, A.I., Sarychev, B.A. et al. Evolution of Nonmetallic Inclusions During Spring Steel Treatment. Metallurgist 59, 917–922 (2016). https://doi.org/10.1007/s11015-016-0194-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-016-0194-x