The Magnitogorsk Metallurgical Combine (MMK) has collected data on the alkali content of the ironore-bearing charge materials for its blast furnaces, coke ash, top dust, and sludge during the course of production operations. The results obtained from determining the alkali contents of the charge materials and smelting products and furnace performance indices in the blast-furnace shop were used to calculate the alkali balance for the period 1997–2011 in order to monitor and evaluate changes in alkali input and output for the shop as a whole. The alkali load for the shop increased from 5.05 in 1998 to 7.61 kg/ton in 2008. Most of the alkalis enter the blast furnaces with the sinter (32–58%), the coke ash (21–33%), and pellets made at the SSGPO (16–37%). The contribution of the other components of the furnace charge is negligible (0.2–1.5% of the load) due to the small amounts of these materials that are used in the charge. A comparative analysis was made of the alkali load on blast furnaces at the MMK, the Severstal combine, and the Novolipetsk combine and the effects of the load (alkali input) on blast-furnace productivity and coke rate were determined. The results made it possible to conclude that each kilogram increase in alkali input increases fuel consumption by 3.2 kg/ton pig iron.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The alkalis that are introduced into blast furnaces together with the charge materials adversely affect the furnace’s operating condition and performance. They facilitate the encrustation of metal on the furnace wall, lead to hanging of the stock, and attack the refractory lining. Alkalis also result in the formation of excess slag crust. This weakens the crust and creates the possibility of its sudden slippage, which often leads to burn-through of the tuyeres. Swelling and weakening of the sinter and pellets and a reduction in the size of the coke lumps under the influence of the alkalis lead to changes in the gas permeability of the stock, redistribution of the gas flow, and a consequent decrease in the efficiency of thermal and reducing processes in the furnace. All of these and other effects stemming from the presence of alkalis result in a deterioration in the condition of the furnace, a decrease in its productivity, and an increase in coke rate [1–5].
According to data from the studies [6], the amount of excess coke consumption that can take place due to the entry of alkalis into a blast furnace can reach 33 kg for each additional kilogram of alkalis. Later studies performed at the Novolipetskii Metallurgical Combine (NLMK) [7] indicated that each additional kilogram of alkalis decreases furnace productivity 4.5 rel.% and increases the average coke rate 11.3 kg/ton pig iron.
An investigation of the operation of cooled blast furnaces in Japan established the character of distribution of the contents of potassium and alkalis for each charge component over the height of the furnace. The distribution of alkali metals in the stock corresponds to the shape of the isotherms, and alkali content reaches particularly high values below the plastic zone [8].
The above observations prove that a blast furnace contains zones with high concentrations of alkalis that are incommensurate with the concentrations in the initial charge. This can be attributed to the existence of a circulating mass of alkali metals and continuous cycles in which those metals undergo sublimation and precipitation. Alkali metals circulate constantly in the furnace, even under equilibrium conditions. During steady-state furnace operation, the rate of circulation – which is characterized by the circulating mass – depends entirely on the physicochemical conditions in the bosh and hearth.
The circulation of alkalis in the working space of a blast furnace can be described as follows [9]: the initial branch of the circulating system is comprised of carbonates of alkali metals that are deposited in pores inside lumps of the charge materials located in the upper part of the stock. The carbonates become liquid as they descend in the furnace (at 891°C for potassium and 854°C for sodium) and they react with carbon monoxide to form vapors which make up the ascending circulation branch. The ascending branch consists of cyanides formed when carbonates in the vapors of the alkalis are reduced along with their oxides after the cyanides react with ferrous oxide. The primary compounds – silicates – are not components of the descending circulation branch.
To alleviate the harmful effects on blast furnaces, some metallurgical plants have introduced limitations on the amount of alkalis allowed in the initial charge materials (see Table 1).
Blast furnaces differ from one another in their volume, the proportioning of the components of the charge, the thermal state of the top part of the furnace, and other characteristics. In other words, the conditions under which smelting of the iron-bearing charge materials takes place are different in different furnaces. The specific smelting conditions in a given furnace depend on the distribution of alkalis in the discharge channels and the overall accumulation of alkalis in the furnace.
Placing limits on the alkali content of a blast-furnace charge solves the problem to some extent but does not solve it completely.
Our goal here is to study the alkali content of the charge materials and smelting products in blast-furnace smelting, determine the alkali load (the quantity of alkalis that enters the furnace), and analyze its effect on furnace productivity and coke rate. In connection with this, the Ironmaking Laboratory at the Magnitogorsk Metallurgical Combine (MMK) analyzed the amounts of alkalis in the iron-ore-bearing charge materials in MMK blast furnaces, in the ash of the coke, and in the top dust and sludge from 1997 through September 2011. The dynamics of the change in the alkali contents of these materials is shown in Fig. 1.
The alkali content of the sinter changed very little during the period 1997–2005 and was 0.214–0.264%, while it increased to 0.344% after 2006 (the increase occurred in 2007) before decreasing to 0.295% in 2010. These changes are related to changes in the alkali content of the agglomerated iron-ore bearing materials. In particular, they reflect the fact that the alkali content of the concentrate at the Sokolovo-Sarbaiskii Mining-Beneficiation Association (SSGPO, located in Rudny, Kazakhstan) increased from 0.243% in 2005 to 0.362% in 2007, and the content of that concentrate in the sintering-machine charges increased from 3.5–2.8 million tons/yr in 2004–2005 to 6.0 million tons in 2007. The increase in the amount of alkalis in the blast-furnace charge after 2006 increased their content to 0.65% in the top dust and 0.51% in the sludge in 2008 (Fig. 2). The alkali content of the slags in the blast furnaces rose from 1.45% in 2005 to 2.12% in 2008 and 2.05–1.97% in 2011.
The results obtained from determining the quantity of alkalis in the charge materials and smelting products of the blast furnaces and the performance indices of the blast-furnace shop were used to determine the annual alkali balances in the period 1997–2010 (Fig. 3) in order to monitor and evaluate changes in the input and output of alkalis for the shop as a whole.
The results of calculations showed that the alkali load (see Fig. 3) for the shop changed from 5.05 (1998) to 7.61 kg/ton (2008). The largest quantity of alkalis enters the furnace with the sinter (32–58%), the coke ash (21–33%), and the pellets from the SSGPO (16–37%). The remaining components of the blast-furnace change contribute negligible (0.2–1.5%) amounts to the total alkali load, due to the small quantities of these components in the charge.
The alkali load changed by insignificant amounts prior to 2006 and was about 5.5 kg/ton pig. The increases in the load in 2007 and especially in 2008 were related to increases in the alkali contents of the sinter and the pellets.
The total output of alkalis from the furnace and the output of alkalis in just the slag were not calculated before 2005 due to a lack of data on the slag’s alkali content prior to that year. Alkali output changed from 4.79 kg/ton in 2005 to 7.16 kg/ton in 2008. It is apparent from the analysis that most (95.8–97.4%) of the total amount of alkalis discharged from the furnace leave with the slag. The quantity of alkalis leaving with the top dust and sludge totals just 4.4–2.4% of total alkali output. Total alkali output was 13.7–2.6% lower than total alkali input during the period 2005–2008. This imbalance includes the quantities of alkalis that went into increasing the circulating mass inside the furnace and that were deposited on the furnace walls when alkali input grew. The total accumulation of alkalis in the blast furnaces increased during the period 2006–2008, when total alkali input also increased.
Alkali input changed from 6.8 to 8.0 kg/ton pig after 2011. As in previous years, in 2011 most of the alkalis (97.4–96.2%) left the furnace with the slag. The quantity of alkalis that left in the top dust and the sludge was just 2.6–4.0% of the total alkali output.
Examination of the balances for the alkali metals showed that most of the alkalis leave the furnace with the slag.
A comparative analysis of the alkali load on blast furnaces at the MMK, the Severstal, and the NLMK revealed the following. Alkali input in the blast furnace shop is 3.1–3.3 kg/ton pig at Severstal and 2.0 kg/ton pig at the NLMK. Thus, compared to the MMK, the alkali load is less than half as large at Severstal and less than one-third as large at the NLMK. These results have to do with the low alkali content of the iron-ore-bearing raw materials and the coke at Severstal and the NLMK.
The high alkali input at the MMK compared to Severstal and the NLMK – a difference of 3–4 kg – has a number of consequences: an increase in the quantity of alkalis that are circulating in the blast furnaces; a substantial reduction in the degree of utilization of the gas in the furnaces due to the occurrence of reactions in which the alkalis are reduced by the carbon of the coke (in the lower part of the furnace) and carbon monoxide is formed; the formation of additional CO when the alkalis are again oxidized by carbon dioxide at higher levels in the furnace (inside the shaft) and the consequent release of heat. Use of the increased concentration of carbon monoxide is impossible under the existing thermodynamic conditions. The situation just described can cause the coke rate to increase to as much as 30–40 kg/ton pig, according to the data in [7].
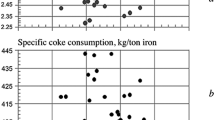
The following was determined from an analysis that was made of the effect of the alkali load (alkali input) on the productivity of blast furnaces and the coke rate during the period 1997–2011 in the blast-furnace shop at the MMK. No statistically reliable relationship was found to exist between the alkali load and smelting rate (unit productivity), but the load was determined to be directly proportional to fuel consumption. The data that were obtained (Fig. 4) lead to the conclusion that each kilogram increase in alkali input increases unit fuel consumption by 3.2 kg/ton pig.
Conclusion. Under conditions that make it impossible to decrease alkali input and eliminate the buildup of alkalis in the stock, the only realistic way of alleviating their harmful effect on furnace operating conditions and performance is to periodically remove the accumulated mass.
The approach presently being used to reduce the adverse effect of alkalis on blast-furnace operation and the associated technical-economic indices is to limit the amount of alkalis that enters the furnace with the charge materials. If limits of this type are put in place, then materials such as sludge and top dust should no longer be included in the charge and should instead be used in specially designed units.
References
“Circulation of alkalis and their effect on blast-furnace operations,” Ref. Zh. Metallurgiya, 3B170 (1981).
M. A. Pavlov, The Metallurgy of Pig Iron, Metallurgizdat, Moscow (1955), Vol. 1, pp. 87–88.
“Effect of alkalis on blast-furnace performance,” Ref. Zh. Metallurgiya, 4B216 (1981).
“Effect of alkalis and zinc on the destruction of a blast-furnace lining and burn-through of the tuyeres,” Ref. Zh. Metallurgiya, 2B173 (1980).
G. Vysotskii and D. Vitser, “Condition of the refractory lining of two blast furnaces after their blow-out,” Cher. Met., No. 13, 22–28 (1976).
E. I. Khrushchev, I. M. Peftiev, A. A. Malimon, et al., “Mechanism of the accumulation of alkalis in a blast furnace and ways of limiting their harmful effect,” Proc. 5th Int. Congr. Blast-Furnace Operators, Dnepropetrovsk–Krivoy Rog, June 7–12, 1999, Porogi, Dnepropetrovsk (1999), pp. 218–219.
I. F. Kurunov, V. N. Titov, V. L. Emel’yanov, et al., “Analysis of the behavior of alkalis in a blast furnace,” Metallurg, No. 9, 34–39 (2009).
M. Schneider, “Effect of alkali us on the operation of a blast furnace” [Russian translation, No. 129/84 from Revue Met., No. 10, 621–636 (1979)], MMK, Magnitogorsk (1984).
K. P. Abraham and L. J. Steffansson, “The alkali problem in the blast furnace,” Scand. J. Met., 4, No. 5, 193–204 (1975).
O. F. Koryakova et al., “Improving blast-furnace smelting to alleviate the adverse effect of alkalis and zinc,” Cher. Met.: Byull. NTiEI, No. 15, 13–33 (1980).
Alkalis in the Blast Furnace: Informstal Survey, Chermetinformatsiya, Moscow (1979).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 9, pp. 28–31, September, 2015.
Rights and permissions
About this article
Cite this article
Gridasov, V.P., Logachev, G.N., Pishnograev, S.N. et al. Behavior of Alkalis in Blast Furnaces. Metallurgist 59, 761–765 (2016). https://doi.org/10.1007/s11015-016-0171-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-016-0171-4