Abstract
Aging is a multifaceted and progressive physiological change of the organism categorized by the accumulation of deteriorating processes, which ultimately compromise the biological functions. The objective of this study was to investigate the anti-aging potential of berberine (BBR) in D-galactose (D-Gal) induced aging in rat models. In this study, male Wistar rats were divided into four groups: The control group was given only vehicle, the BBR group was treated with berberine orally, the D-Gal group was treated with D-galactose subcutaneously and the BBR + D-Gal group was treated with D-galactose and berberine simultaneously. D-galactose exposure elevated the pro-oxidants such as malondialdehyde (MDA) level, protein carbonyl and advanced oxidation protein products (AOPP) in the brain. It decreased the anti-oxidants such as reduced glutathione (GSH) and ferric reducing antioxidant potential (FRAP) in the brain. D-galactose treatment also reduced the mitochondrial complexes (I, II, III and IV) activities and elevated the inflammatory markers such as interleukine-6 (IL-6), tumor necrosis factor- α (TNF-α) and C-reactive protein (CRP). The mRNA expressions of IL-6 and TNF-α in the brain were upregulated following D-galactose exposure. Berberine co-treatment in D-galactose induced aging rat model prevented the alteration of pro-oxidant and anti-oxidant in the brain. Berberine treatment restored the mitochondrial complex activities in the brain and also normalized the inflammatory markers. Based on these findings we conclude that berberine treatment has the potential to mitigate brain aging in rats via stabilizing the redox equilibrium and neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is categorized by modifications of numerous physiological processes and biological pathways that leads to an increased susceptibility to age-related disorders and death (Tatullo 2023). Aging is associated with cognitive weakening and several neurodegenerative diseases (Munkhzul et al. 2023). It is exciting to see that a recent report has described twelve hallmarks of aging (López-Otín et al. 2023) which is a significant achievement from the earlier accepted nine hallmarks of aging (López-Otín et al. 2013). Persistently increased oxidative stress leads to several life-threatening pathological conditions besides playing a major role during aging (Tong et al. 2023; Valko et al. 2007). The oxidative stress theory of aging states that age-associated functional losses arise due to the accumulation of reactive oxygen species (ROS) -induced damages (Bonomini et al. 2015).
The immune system goes through abundant and intense changes during aging (Furman et al. 2019). During aging, there is a state of proinflammatory stimulation categorized by increased circulating proinflammatory cytokine levels such as IL-6 and TNF-α and localized tissue inflammation (Bektas et al. 2020). Oxidative stress and neuroinflammation have been linked to age-related neurodegeneration and neuro-behavioral changes (Garg et al. 2017). Elevated ROS and inflammatory markers - IL-6 and TNF-α in induced aged rat model have been reported recently (Kumar et al. 2021).
Mitochondrial dysfunction is prominent in aging which is characterized by diminished oxidative phosphorylation and elevated free radical and ROS generation. Aging is an important factor during which stress level increases in the endoplasmic reticulum which in turn leads to mitochondrial dysfunction (Glancy and Balaban 2012; Yang et al. 2024).
Numerous phytochemicals have been reported to interact with the human biochemical system thereby affecting health parameters. There are several phytochemicals that have tremendous health benefits such as polyphenols (flavonoids, tannins, stilbenes, coumarins, lignans), terpenoids (isoprenol, cornosic acid, β-caryophyllene, α-tocopherol) and alkaloids (reserpine, tomatidine, spermidine, berberine, calycosin). These phytochemicals are reported to show anti-inflammatory, anti-aging, anti-neurodegenerative, anti-cancer properties (Okoro et al. 2021). Berberine (BBR) is the main isoquinoline alkaloidal component of stems and roots of several Berberis species such as B. aristata, B. petiolaris and B. vulgaris (Singh et al. 2019). BBR can pass through blood brain barrier and elicit many pharmacological and therapeutic actions including being neuroprotective and neurotrophic (Ma et al. 2010). BBR has many pharmacological activities, such as being analgesic, anti-inflammatory, anticancer, antidiabetic, antioxidant (Samadi et al. 2020). It is also reported to cause memory enhancement and act as an antidepressant (Kulkarni and Dhir 2010). BBR inhibits mTOR/S6 signaling with decrease in the oxidants (Zhao et al. 2013). Reports also claim the use of berberine in the therapeutics of neurodegenerative diseases (Dadgostar et al. 2022; Singh and Sashidhara 2017). An in vitro study claimed that berberine prevented the axonal degeneration (Wang et al. 2023). Recently we reported that berberine treatment attenuates aging induced alterations in erythrocyte via stabilizing redox homeostasis in D-galactose induced senescent rats (Yadawa et al. 2023). Several studies have demonstrated that long-term administration of D-galactose induces natural aging mimicking effect in many tissues of rodents (Sadigh-Eteghad et al. 2017).
In view of the varied beneficial effects of berberine, the present study was designed to investigate the anti-aging effect of berberine in the brain of 2-D-Galactose (D-gal) induced accelerated aging rat model. In this study our main focus was on oxidative stress, neuroinflammation and mitochondrial dysfunction. We have undertaken this study to provide a scientific explanation to the pleiotropic biological activities of berberine.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide, 4, 7-diphenyl-1, 10-phenanthroline disulfonic acid disodium salt (DPI), 2, 4, 6-Tri (2-pyridyl)-s-triazine (TPTZ), 2, 4-dinitrophenylhydrazine (DNPH), reduced glutathione (GSH) and dithio-bis-nitrobenzoic acid (DTNB) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Berberine Chloride was purchased from TCI Chemicals (India) Pvt. Ltd. Other chemicals were procured from Merck, Germany, and HiMedia Labs, Mumbai, India.
Animals and experimental procedure
In this study male Wistar rats (200 g ± 15 g b.w.) were used. The rats were obtained from the Animal House facility of Indian Institute of Toxicological Research, CSIR, Lucknow, India. They were kept in the animal house facility of Department of Biochemistry, University of Allahabad in a controlled environment (25 ± 2oC temperature and 55 ± 15% relative humidity) with a 12-h light/dark cycle. Rats were acclimatised for a week and were given rodent diet of nutrient rich pellets and drinking water ad libitum. After one week of acclimatisation the rats were randomly divided into 4 groups of 6 rats in each. Group-1 (negative control) was given vehicle (0.9% saline); Group-2 (BBR) was given berberine suspended in 0.9% saline (200 mg/kg b.w. orally) (El-Horany et al. 2018; Yadawa et al. 2023); Group-3 (D-Gal- positive control) was given D-galactose dissolved in 0.9% saline (200 mg/kg b.w., subcutaneously) and Group-4 (BBR + D-Gal) was given berberine and D-galactose (as given in group-2 and 3 respectively) daily for 6 weeks (Yadawa et al. 2023). At the end of experimental protocols rats were sacrificed using standard procedure. Brains were dissected out and stored in -80oC for further experiments.
All animal care and experimental procedures were carried out with the approval of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Animal Ethics Committee (IAEC/AU/2019(1)/08), University of Allahabad, India.
Brain homogenate preparation
Brain was dissected out and washed in ice-cold phosphate buffered saline (PBS 0.02 M, pH 7.4). 10% brain homogenates (w/v) were prepared in ice cold PBS (0.02 M, pH 7.4). Brain homogenates were centrifuged at 12000xg at 4oC for 15 min, supernatant was stored for different biochemical estimations.
Lipid peroxidation assay
To measure the lipid peroxidation in brain homogenate malondialdehyde (MDA) level was assayed by the protocol of Esterbauer and Cheeseman (1990). Briefly, 200 µl brain homogenate was added in 3 ml of PBS. The brain homogenates were incubated in 1 ml of 10% trichloroacetic acid (TCA) and 2 ml of 0.67% thiobarbituric acid (TBA) for 20 min at 90oC-100oC in water bath and then cooled. The reaction mixture was centrifuged at 1000xg for 5 min. The absorbance of the supernatant was recorded at 532 nm. The concentration of MDA was calculated using extinction coefficient (Ɛ= 153,000) and expressed as nmol/mg protein.
Reduced glutathione (GSH) assay
GSH level was estimated using the protocol of Singh et al. (2017). GSH assay is based on the capability of –SH group to reduce 5,5’-dithiobis, 2- nitrobenzoic acid (DTNB) into a yellow coloured product and the absorbance was noted at 412 nm. The concentration of GSH was calculated from standard calibration curve, expressed as mg/mg protein.
Protein carbonyl (PCO) assay
Protein carbonyl content was measured by the protocol given by Levine et al. (1990). The protein carbonyl content was calculated using absorption coefficient of 22,000 M-1cm-1, and concentration was expressed in nmol/ mg protein.
Measurement of FRAP (ferric reducing antioxidant potential)
The protocol given by Benzie and Strain (1996) was used to determine the FRAP with slight modification. FRAP reaction mixture was made by mixing 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride and 10 mM 2,4,6-tripyridyl-s-triazine (prepared in 40 mM HCl) in the ratio of 10:1:1 respectively. Hundred microlitres of homogenate was added to 3 ml FRAP reaction mixture and the contents were vortexed. The absorbance was taken at 593 nm on UV-VIS spectrophotometer at the interval of 30 s for 4 min. FRAP value was extrapolated using the standard calibration curve and expressed as µmol Fe (II)/ g tissue.
Determination of AOPPs (advanced oxidation protein products)
The level of AOPPs was estimated by using the protocol given by Witko-Sarsat et al. (1996). Briefly, in 0.2 ml of brain homogenate 1.0 ml of 1.16 M potassium iodide was added. After two minutes 2.0 ml of glacial acetic acid was added. The absorbance was noted immediately at 340 nm. PBS was set as blank and chloramine-T solution (0–100 µmol/l) was used as standard. The AOPPs was expressed as µmol of chloramine-T equivalents/ mg protein (µmol/mg protein).
Reactive oxygen species (ROS) measurement
ROS was measured by flow cytometer (Accuri A C6 Stream cytometer, Becton Dickinson Biosciences, California, USA) in the brain homogenate using standard protocol. The green fluorescence of DCFH-DA was measured through the FL-1 channel and every tube noted minimum 10,000 events. C6 Sampler programming was used to analyse the data. Unstained sample was used as control.
Measurement of inflammatory markers- IL-6, TNF-α and C-reactive protein in plasma
IL-6, TNF-α and C-reactive protein (CRP) was measured in the plasma by commercially available ELISA kit (Krishgen BioSystem, India). IL-6, TNF-α and CRP were measured by the protocols provided with kit manuals.
RNA extraction, cDNA synthesis and PCR
The total RNA from the brain of all groups were extracted using Trizol-T Reagent (SRL, India) according to its user manual provided, dissolved in nuclease free water and quantified using the nanodrop SPECTRO Star Nano Spectrophotometer (BMG LABTECH, Germany). Two microgram RNA was reverse transcribed to synthesize cDNA by using commercially available cDNA synthesis kit (New England BioLabs MA, USA). cDNA was synthesised by using the protocol provided with cDNA synthesis kit manual. PCR was done by using specific primers for IL-6 (forward primer 5′- TCCTACCCCAACTTCCAATGCTC- 3′, Reverse primer 5′- TTGGATGGTCTTGGTCCTTAGCC- 3′), TNF-α (Forward primer 5′-AAATGGGCTCCCTCTCATCAGTTC- 3′, Reverse primer 5′-TCTGCTTGGTGGTTTGCT ACGAC − 3′) and β-actin (Forward primer 5′- TGTGATGGTGGGAATGGGTCAG − 3′, Reverse primer 5’-TTTGATGTCACGCACGATTTCC − 3′). The PCR were performed in 25 µl reaction mixture using PCR kit (New England BioLabs MA, USA). The reactions were carried out in Smart PCR Pro CYBERLAB Thermo cycler. The samples were denatured at 95 °C for 5 min and amplified using conditions (denaturation at 94 °C for 45 s, primer annealing at 62 °C for 30 s, elongation at 72 °C for 45 s and no. of cycles– 35).
The PCR products were electrophoresed on 2% agarose gel having ethidium bromide. The gel bands were visualized, images were captured using gel doc system (BioRad, USA). The mRNA expression of IL-6 and TNF-α were expressed as relative fold change of band intensity relative to control normalized by β-actin.
Mitochondrial complex activity assay
To isolate the mitochondria, brain was homogenized in medium having 230 mM mannitol, 70 mM sucrose, 1.0 mM EDTA, and 10 mM Tris–HCl, pH 7.40 in a homogenizer (Powergen-500 Fisher Scientific, USA). The homogenate was centrifuged at 700xg for 10 min and supernatant at 8000xg for 10 min to pellet mitochondria that were washed in the same conditions to obtain mitochondrial preparations (Navarro et al. 2005). The mitochondria obtained was stored in the same medium. The purity of the mitochondrial preparation was assessed by the activity of succinate dehydrogenase. Electron transport chain complexes (I–IV) activities have been measured according to the Srividhya et al. (2009).
Histological study
To assess the effect of aging on brain architecture, haematoxylin-eosin (HE) staining was done in the coronal brain sections. After perfusion brains were dissected out and fixed for 24 h in 10% formalin, dehydrated in graded ethanol and embedded in paraffin wax. Blocks were prepared and 5 μm thick coronal sections of brain were cut on a microtome and collected on albumin-coated slides. HE staining was performed in brain of rats using standard protocol. Sections were viewed and images were taken under Olympus microscope.
Statistical analysis
The results are expressed as mean ± SEM. Statistical analyses were done using Graph Pad Prism 5 software. The statistical significance of differences among different groups were calculated by ANOVA (one way analysis of variance) followed by Tukey’s post hoc test. Value of p˂0.05 was considered as statistically significant. The * indicates significant difference in comparison to control group and # denotes significant difference as compared to D-Gal group.
Result
Co-treatment of berberine mitigates the oxidative stress
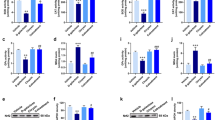
Lipid peroxidation was determined in the brain homogenate by estimating the MDA level. The MDA level in D-Gal treated rats was significantly high (52.74 nmol/mg protein; p < 0.01) in comparison to the control and BBR group (41.03 and 45.37 nmol/mg protein respectively). The MDA level reduced in the BBR + D-Gal group (21.00 nmol/mg protein; p < 0.001) than D-Gal group (Fig. 1A).
Effect of berberine on oxidative stress. A Represents the effect of D-galactose and berberine on the lipid peroxidation (MDA level) in the brain of rats. MDA level was significantly high in the brain of D-gal treated rats which was further decreased in D-gal + BBR treated rats. B represents the effect of D-gal and berberine on GSH level in the brain of rats. GSH levels were decreased in D-gal treated rats and elevated in D-gal + BBR treated rats. C shows the protein carbonylation in the different groups of rats. PCO levels was high in the brain of D-gal treated rats however, that was low in D-gal + BBR treated rats. D represents the ferric reducing antioxidant potential (FRAP) of brain in D-gal and berberine treated rats. FRAP decreased in the brain of D-gal treated rats while it was high in D-gal + BBR rats. E represents the effect of D-gal and berberine on AOPP in the brain. AOPP level significantly increased in D-gal treated rats in the brain. Berberine treatment in the D-gal treated rats prevented the elevation of AOPP. All the data are presented as mean ± SEM (n = 6); *p˂0.05, **p˂0.01, ***p˂0.001 when compared with control, #p˂0.05, ###p˂0.001 when compared with D-Gal
Our result shows the GSH level decreased in the brain of D-Gal exposed rats (0.204 mg/ mg protein; p < 0.01) compared to control and BBR rats (0.276 & 0.357 mg/ mg protein respectively). Co-treatment of BBR in D-Gal treated rats prevented the decrease in GSH level (0.239 mg/ mg protein; p < 0.05) (Fig. 1B).
Protein carbonyl (PCO) is the biomarker of oxidative stress. PCO content in the brain of D-Gal induced aging rats increased significantly (56.30 nmol/mg protein; p < 0.01) in comparison to control and BBR groups (50.05 & 50.70 nmol/mg protein). However, PCO content did not increase in BBR + D-Gal treated rats (49.49 nmol/mg protein; p < 0.01) when compared with D-Gal rats (Fig. 1C).
Biochemical estimation showed decreased FRAP in the brain of D-Gal treated rats (50.96 µmol Fe(II)/g of tissue; p < 0.001) in comparison to control and BBR groups (141.35 & 131.73 µmol Fe(II)/g of tissue respectively). In BBR + D-Gal group FRAP was elevated (94.23 µmol Fe(II)/g of tissue; p < 0.001) than that of D-Gal group (Fig. 1D).
AOPPs are also biomarker of oxidative stress. The level of AOPP in the brain of D-Gal treated rats increased (0.22 µmol/mg protein; p < 0.001) compared to control and BBR rats (0.10 & 0.11 µmol/mg protein respectively). BBR co-treatment in the D-Gal treated rats attenuated the AOPP (0.15 µmol/mg protein; p < 0.01) in comparison to D-Gal rats (Fig. 1E).
BBR prevents the ROS production
Flow cytometric data shows that ROS level was high in D-gal treated rats (288 AU, p < 0.001) when compared with control and BBR group (58 AU and 54.5 AU respectively). When BBR was given simultaneously with D-gal, the ROS level decreased (222 AU, p < 0.01) compared to D-Gal group (Fig. 2) (AU– arbitrary unit).
Represents the level of ROS in brain. The level of ROS was high in D-gal treated rat compared to control and BBR group. Berberine treatment in D-gal treated rat lowered the ROS level compared to D-gal group. All the data are presented as mean ± SEM (n = 6); *p˂0.05, **p˂0.01, ***p˂0.001 when compared with control, #p˂0.05, ##p˂0.01, ###p˂0.001 when compared with D-Gal
BBR reduces the inflammation in D-galactose induced aging
The level of inflammatory markers IL-6, TNF-α and C-reactive protein elevated in the plasma of D-galactose treated rats (67.56 pg/ml, 1450.13 pg/ml and 216.15 ng/ml plasma respectively; p < 0.01) compared to the control group (45.24 pg/ml, 1076.5 pg/ml and 107.37 ng/ml respectively). BBR co-treatment in the D-Gal treated rats lowered the level of IL-6, TNF-α and C-reactive protein (53.08 pg/ml, 1232.78 pg/ml, 144.36 ng/ml; p < 0.05) in comparison to D-gal treated rats (Fig. 3A, B and C).
Effect of berberine on inflammatory markers. A shows the plasma IL-6 level in the different groups. Plasma IL-6 level was high in the D-gal treated rats compared to that of control. Berberine co-treatment prevented the elevation of plasma IL-6 as compared to D-gal rats. B denotes the plasma TNF-α level. TNF-α level elevated in the D-gal exposed rat however, berberine treatment attenuated the TNF-α level. C represents the C-reactive protein (CRP) level in the serum. CRP level increased in the D-gal treated rats however; berberine treatment reduced the serum CRP level. D represents RT-PCR products of IL-6, TNF-α and β-actin in the different groups of rat brain analysed on 2% agarose gel. E & F show relative fold change in IL-6 and TNF-α mRNA normalised by β-actin. Expression of IL-6 and TNF-α mRNA increased in D-gal treated rats. Co-treatment of berberine decreased the IL-6 and TNF-α mRNA expression in the brain. All the data are presented as mean ± SEM (n = 6); **p˂0.01, ***p˂0.001 when compared with control; ##p˂0.01, ###p˂0.001 when compared with D-Gal
Inflammatory cytokines IL-6 and TNF-α mRNA expressions were checked by RT-PCR in the brain. IL-6 mRNA expression upregulated in the brain of D-gal treated rats (1.79 fold, p < 0.001) in comparison to control and BBR groups. Co-treatment of BBR with D-gal mitigated the upregulation of IL-6 mRNA (1.26 fold, p < 0.001) when compared to D-gal rats (Fig. 5A & B). TNF-α mRNA expression also upregulated in the brain of D-gal treated rats (1.47 fold, p < 0.01) in comparison to control and BBR groups. However, the treatment of BBR with D-gal downregulated the TNF-α mRNA (1.08 fold, p < 0.01) compared to D-gal rats (Fig. 3D, E and F).
BBR restores the mitochondrial function
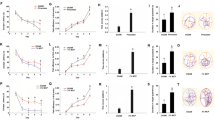
The mitochondrial function was measured by estimating the mitochondrial complexes NADH-ubiquinol oxidoreductase (Complex I), succinateubiquinol oxidoreductase (Complex II), ubiquinol cytochrome c oxidoreductase (Complex III) and cytochrome c oxidase (Complex IV) activities. Complexes I, II, III and IV activities decreased in D-gal treated rats (69.66, 13.33, 101.71 and 25.95 nmol/min/mg protein respectively, p < 0.001) in comparison to control (111, 28.71, 161.93 and 47.22 nmol/min/mg protein respectively). Co-treatment of berberine in D-gal treated rats restores the activities of Complex I, II, III (99.67, 21.91, 148.07 nmol/min/mg protein respectively, p < 0.001) and IV (38.29, nmol/min/mg protein respectively, p < 0.01) in comparison to D-gal group (Fig. 4).
Effect of berberine on mitochondrial complexes activities. A, B, C, and D show the mitochondrial complex I, II, III, IV activities respectively. D-gal treatment lowered the mitochondrial complexes activities in the brain. Co-treatment of berberine prevented the decrease in mitochondrial complexes activities in the brain. All the data are presented as mean ± SEM (n = 6); ***p˂0.001 when compared with control; ##p˂0.01, ###p˂0.001 when compared with D-Gal
Histological study
In the histological study of brain we mainly focused on hippocampal region of the brain. To examine the histological changes in the hippocampal region, HE staining was performed in the coronal sections of brain hippocampus. In the control and BBR group rats the pyramidal cell layer was intact and compactly arranged. In case of D-gal treated rats the pyramidal cell layer was broken and loosely arranged while co-treatment of berberine with D-gal did not show any deformity in the pyramidal cell layer of hippocampus (Fig. 5).
Coronal brain section microphotographs through hippocampus stained with haematoxylin and eosin. Intact and compactly arranged pyramidal cell layer can be seen in the control and BBR groups. In D-gal treated rats this layer is damaged and loosely arranged while in D-gal + BBR group pyramidal cell layer is not damaged. Upper lane of microphotograph is of 10X magnification and lower lane is of 40X magnification. Arrows show the pyramidal cell layers, whether those were intact and compactly arranged or damaged and loosely arranged
Discussion
D-galactose induced aging in organs of rodents show notable similarity to natural aging in terms of biochemical, morphological and behavioral data and is a well established animal model for aging research (Ji et al. 2014; Pantiya et al. 2023). Higher levels of D-galactose cause overproduction of ROS (Yanar et al. 2011). Increased lipid peroxidation could be involved in neuronal damage in the brain leading to brain aging. In this study we found that D-gal treatment (200 mg/kg b.w.) enhanced the lipid peroxidation in the brain, this finding is corroborated by previous finding (Liu et al. 2021). Our result showed that berberine co-treatment attenuated lipid peroxidation in brain of D-gal treated rats indicating the anti-oxidative potential of berberine. These results indicate that D-galactose triggered oxidative stress however BBR treatment diminished stress in rats. The increased lipid peroxidation could be the cause behind decreased antioxidative defence systems such as catalase and GSH (El-Shiekh et al. 2020). GSH sustains redox homeostasis in cells and inhibits oxidative damage. D-gal treatment decreases GSH level in rats and mice (Li et al. 2021) thus affecting the redox balance. In the present study we also found that D-gal administration for 6 weeks decreased GSH level in the brain of rats. Furthermore, BBR treatment enhanced the GSH level in the brain. Previous studies also report that BBR caused an increase in GSH level in rodents (Gendy et al. 2023; Seth et al. 2021). Protein carbonyl is a stable oxidative alteration in protein structure (Stadtman and Berlett 2008). Protein carbonylation increased in the D-gal treated rat brain while it was unaltered in the berberine treated rat brain. Allameh et al. (2020) reported that berberine lowers protein carbonylation in rodents. AOPPs are formed during oxidative stress, belong to the category of dityrosine-containing protein products (Witko-Sarsat et al. 1998).
FRAP is a reliable measure for oxidative injury (Benzie and Strain 1996). Berberine treatment prevented the decrease in FRAP in D-gal induced aging rats. Adefegha et al. (2021) also reported that berberine increases FRAP in vitro. D-gal exposure elevated the ROS level in the brain however, berberine treatment reduced the ROS production. This result also explains the driving force behind alteration of other oxidative and antioxidative markers. During aging AOPPs level elevated (Zhuang et al. 2021) which is established by oxidative stress. In this experiment we also found the higher level of AOPP in D-galactose treated aging rats. Berberine lowered the AOPP level in the brain of aging rat model. A recent study also claims that berberine diminishes AOPP level in diabetic rats (Zych et al. 2020). It is well established that berberine activates the anti-oxidant systems in disease models and thereby attenuates the oxidative stress (Gupta et al. 2023; Luo et al. 2023).
D-galactose increases the inflammatory markers and induces neuro-inflammation through the stimulation of NFκ-B signaling pathways, resulting in memory impairment (Hadzi-Petrushev et al. 2014; Shwe et al. 2018). In the present study we observed that inflammatory markers IL-6, TNF-α and CRP were high in the plasma of D-galactose treated rats. The mRNA expression of IL-6 and TNF-α upregulated in the D-gal treated rats which again supports the occurrence of inflammation in D-gal induced aging brain in rats. Berberine stabilizes the inflammatory factors: TNF-α, IL-1β and IL-6 (Hussien et al. 2018). In our study we also found the similar results where berberine co-treatment prevented the elevation of IL-6, TNF-α and CRP in plasma and also normalized the mRNA expression of IL-6 and TNF-α in the brain. In BV-2 microglia, berberine lowers the lipopolysaccharide- or interferon-γ-induced iNOS and cyclooxygenase-2 level, and inhibits inflammatory cytokines IL-6, IL-1β and TNF-α expression (Lu et al. 2010). Recently it is reported that berberine treatment prevents the neuroinflammation in the rodents (Liu et al. 2023).
Mitochondria have a vital role in aging process and are considered as contributing factor of cell survival and cell death which are fundamental markers of neurodegeneration (Li et al. 2017). Mitochondrial Complex I is the key site of ROS production and therefore potentially contribute to the changes in brain function throughout aging (Paradies et al. 2011). Increased ROS production is linked with mitochondrial dysfunction (Bonnard et al. 2008). It is reported that aging is linked with deterioration in mitochondrial function, characterized by a decline in oxidative phosphorylation and ATP synthesis and enhanced free radical production (Long et al. 2007). The present study indicates that D-galactose administration results in mitochondrial dysfunction as indicated by decrease in mitochondrial complexes I, II, III and IV activities. The co-treatment of berberine in D-gal administered rats restored the mitochondrial complex activities in comparison to D-gal treated rats. Due to imbalanced redox homeostasis in D-galactose induced aging, increased ROS was one of the main contributors to mitochondrial dysfunction. Berberine is an activator of energy regulators and mitochondrial function and thus promotes mitochondrial energy output (Fang et al. 2022; Qin et al. 2020). Antioxidative potential of berberine (Yadawa et al. 2023) may be the driving force to improve the mitochondrial function in D-galactose induced aging brain.
In the present experiment histological study showed that D-galactose treatment caused the pyramidal cell layer damage in the hippocampus region. The enhanced ROS production and increased inflammation might cause the distortion in hippocampus in D-gal induced aging brain. Berberine treatment reduces the neuronal damage and cerebral edema in traumatic brain injury mice model (Chen et al. 2014). Berberine treatment can diminish brain tissue injury and neurological deficits at early time points following stroke (Song et al. 2012). Similarly, berberine co-treatment protected the hippocampal pyramidal cell layer from damage in D-gal treated rats. This result once again suggests the neuroprotective potential of berberine in induced aging brain in rats. This effect of berberine may arise due to its anti-oxidative and anti-inflammatory effects.
Conclusion
In this study we found that D-galactose treatment in the rats resulted in the elevation in the pro-oxidants, inflammatory markers and decrease in the level of antioxidant and antioxidant markers and mitochondrial complexes activities in the brain. From our results we conclude that berberine administration attenuates the markers of D-galactose induced brain aging in rat. Outcome of this study suggests that berberine has the ability to mitigate the brain aging in rats via stabilising the redox homeostasis, inflammatory markers and mitochondrial function in the brain and has anti-aging potential. These findings provide an understanding into likely interventions which may be suggested for age dependent alterations especially neurodegeneration and cognition.
Data availability
Data generated or analysed during this study are provided in full within the published article.
References
Adefegha SA, Oboh G, Okeke BM (2021) Comparative effects of berberine and piperine on the neuroprotective potential of neostigmine. J Complement Integr Med 18:491–497
Allameh H, Fatemi I, Malayeri AR, Nesari A, Mehrzadi S, Goudarzi M (2020) Pretreatment with berberine protects against cisplatin-induced renal injury in male Wistar rats. Naunyn-Schmiedeberg’s Archives Pharmacol 393:1825–1833
Bektas A, Schurman SH, Franceschi C, Ferrucci L (2020) A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- and long-term inflammaging? Immun Ageing 17:23
Benzie IFF, Strain JJ (1996) The Ferric Reducing Ability of Plasma (FRAP) as a measure of antioxidant power: the FRAP Assay. Anal Biochem 239:70–76
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin M-A, Morio B, Vidal H, Rieusset J (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118(2):789–800
Bonomini F, Rodella LF, Rezzani R (2015) Metabolic syndrome, aging and involvement of oxidative stress. Aging Disease 6:109
Chen C-C, Hung T-H, Lee CY, Wang L-F, Wu C-H, Ke C-H, Chen S-F (2014) Berberine protects against Neuronal Damage via Suppression of Glia-Mediated Inflammation in traumatic brain Injury. PLoS ONE 9, e115694
Dadgostar E, Moghanlou M, Parvaresh M, Mohammadi S, Khandan M, Aschner M, Mirzaei H, Tamtaji OR (2022) Can Berberine Serve as a New Therapy for Parkinson’s Disease? Neurotox Res 40:1096–1102
El-Horany HE-S, Gaballah HH, Helal DS (2018) Berberine ameliorates renal injury in a rat model of D-galactose-induced aging through a PTEN/Akt-dependent mechanism. Arch Physiol Biochem 126:157–165
El-Shiekh RA, Ashour RM, El-Haleim A, Ahmed EA, Abdel-Sattar KA, E (2020) Hibiscus sabdariffa L.: a potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed Pharmacother 128:110303
Esterbauer H, Cheeseman KH (1990) [42] determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal, in: methods in Enzymology. Elsevier, pp 407–421
Fang X, Wu H, Wei J, Miao R, Zhang Y, Tian J (2022) Research progress on the pharmacological effects of berberine targeting mitochondria. Front Endocrinol 13:982145
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25:1822–1832
Garg G, Singh S, Singh AK, Rizvi SI (2017) Antiaging Effect of Metformin on Brain in naturally aged and accelerated Senescence Model of Rat. Rejuven Res 20:173–182
Gendy AM, Soubh A, Elnagar MR, Hamza E, Ahmed KA, Aglan A, El-Haddad AE, Farag MA, El-Sadek HM (2023) New insights into the role of berberine against 3-nitropropionic acid-induced striatal neurotoxicity: possible role of BDNF–TrkB–PI3K/Akt and NF-κB signaling. Food Chem Toxicol 175:113721
Glancy B, Balaban RS (2012) Role of mitochondrial Ca2+ in the regulation of Cellular energetics. Biochemistry 51:2959–2973
Gupta P, Gupta H, Tripathi S, Poluri KM (2023) Biochemical and metabolomic insights into antifungal mechanism of berberine against Candida Glabrata. Appl Microbiol Biotechnol 107:6085–6102
Hadzi-Petrushev N, Stojkovski V, Mitrov D, Mladenov M (2014) d-galactose induced inflammation lipid peroxidation and platelet activation in rats. Cytokine 69:150–153
Hussien HM, Abd-Elmegied A, Ghareeb DA, Hafez HS, Ahmed HEA, El-moneam NA (2018) Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem Toxicol 111:432–444
Ji Z-H, Liu C, Zhao H, Yu X-Y (2014) Neuroprotective effect of Biatractylenolide against Memory Impairment in d-Galactose-induced aging mice. J Mol Neurosci 55:678–683
Kulkarni SK, Dhir A (2010) Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res 24:317–324
Kumar R, Kumar M, Rizvi SI (2021) Chitosan displays a potent caloric restriction Mimetic Effect in senescent rats. Rejuven Res 24:390–396
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn B-W, Shaltiel S, Stadtman ER (1990) [49] determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. Elsevier, pp 464–478
Li PA, Hou X, Hao S (2017) Mitochondrial biogenesis in neurodegeneration. J Neurosci Res 95:2025–2029
Li F, Huang H, Wu Y, Lu Z, Zhou X, Tan F, Zhao X (2021) Lactobacillus fermentum HFY06 attenuates d-galactose-induced oxidative stress and inflammation in male Kunming mice. Food Funct 12:12479–12489
Liu B, Xie Y, Chen J, Xue J, Zhang X, Zhao M, Jia X, Wang Y, Qin S (2021) Protective Effect of Molecular Hydrogen Following Different Routes of Administration on D-Galactose-Induced Aging mice. J Inflamm Res Volume 14:5541–5550
Liu J, Chen H, Yu T, Fu X, Qian C, Feng X (2023) Berberine mitigates intracerebral hemorrhage-induced neuroinflammation in a gut microbiota-dependent manner in mice. Aging 15:2705–2720
Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, Cui X, Packer L, Liu J (2007) d-Galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology 8:373–381
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) Hallm Aging. Cell 153:1194–1217
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2023) Hallmarks of aging: an expanding universe. Cell 186:243–278
Lu D-Y, Tang C-H, Chen Y-H, Wei I-H (2010) Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem 110:697–705
Luo H, Kou T, Su Y, Shen Y, Yin L (2023) Experimental research on the evaluation of left ventricular systolic function by layered speckle tracking before and after berberine treatment in a cardiac hypertrophy rat model. Cardiovasc Diagnosis Therapy 13:367–383
Ma X, Jiang Y, Wu A, Chen X, Pi R, Liu M, Liu Y (2010) Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS ONE 5:13489
Munkhzul C, Yi SS, Kim J, Lee S, Kim H, Moon J-S, Lee M (2023) The microRNA-mediated gene regulatory network in the hippocampus and hypothalamus of the aging mouse. PLoS ONE 18, 0291943
Navarro A, Gómez C, Sánchez-Pino M-J, González H, Bández MJ, Boveris AD, Boveris A (2005) Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol-Regul Integr Comp Physiol 289:R1392–R1399
Okoro NO, Odiba AS, Osadebe PO, Omeje EO, Liao G, Fang W, Jin C, Wang B (2021) Bioactive phytochemicals with anti-aging and Lifespan extending potentials in Caenorhabditis elegans. Molecules 26:7323
Pantiya P, Thonusin C, Ongnok B, Chunchai T, Kongkaew A, Nawara W, Arunsak B, Chattipakorn N, Chattipakorn SC (2023) Chronic D-galactose administration induces natural aging characteristics, in rat’s brain and heart. Toxicology 492:153553
Paradies G, Petrosillo G, Paradies V, Ruggiero FM (2011) Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int 58:447–457
Qin X, Jiang M, Zhao Y, Gong J, Su H, Yuan F, Fang K, Yuan X, Yu X, Dong H, Lu F (2020) Berberine protects against diabetic kidney disease via promoting PGC-1α‐regulated mitochondrial energy homeostasis. Br J Pharmacol 177:3646–3661
Sadigh-Eteghad S, Majdi A, McCann SK, Mahmoudi J, Vafaee MS, Macleod MR (2017) Correction: D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS ONE 12, e0190328
Samadi P, Sarvarian P, Gholipour E, Asenjan KS, Aghebati-Maleki L, Motavalli R, Hojjat‐Farsangi M, Yousefi M (2020) Berberine: a novel therapeutic strategy for cancer. IUBMB Life 72:2065–2079
Seth E, Ahsan AU, Kaushal S, Mehra S, Chopra M (2021) Berberine affords protection against oxidative stress and apoptotic damage in F1 generation of Wistar rats following lactational exposure to chlorpyrifos. Pestic Biochem Physiol 179:104977
Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2018) Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol 101:13–36
Singh SP, Sashidhara KV (2017) Lipid lowering agents of natural origin: an account of some promising chemotypes. Eur J Med Chem 140:331–348
Singh AK, Singh S, Garg G, Rizvi SI (2017) Rapamycin mitigates erythrocyte membrane transport functions and oxidative stress during aging in rats. Arch Physiol Biochem 124:45–53
Singh AK, Singh SK, Nandi MK, Mishra G, Maurya A, Rai A, Rai GK, Awasthi R, Sharma B, Kulkarni GT (2019) Berberine: a plant-derived alkaloid with therapeutic potential to Combat Alzheimer’s disease. Cent Nerv Syst Agents Med Chem 19:154–170
Song B, Tang X, Wang X, Huang X, Ye Y, Lu X, Wei X, Zeng Y (2012) Bererine induces peripheral lymphocytes immune regulations to realize its neuroprotective effects in the cerebral ischemia/reperfusion mice. Cell Immunol 276:91–100
Srividhya R, Zarkovic K, Stroser M, Waeg G, Zarkovic N, Kalaiselvi P (2009) Mitochondrial alterations in aging rat brain: effective role of (–)-epigallo catechin gallate. Int J Dev Neurosci 27:223–231
Stadtman ER, Berlett BS (2008) Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev 30:225–243
Tatullo M (2023) Entropy meets physiology: should we translate Ageing as Disorder? Stem cells. https://doi.org/10.1093/stmcls/sxad084
Tong T, Guo J, Wu Y, Sharma D, Sangar M, Sangpreecha N, Song D, Unno T, Ham K, Kang S (2023) Dietary supplementation of ark clams protects gut health and modifies gut microbiota in D-galactose‐induced aging rats. J Sci Food Agric 104(2):675–685
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Wang P, Wang X, Wang Q, Jiao Y, Wang X, Chen C, Chen H, Song T (2023) Cognitive improvement via a modulated rhythmic pulsed magnetic field in D-galactose-induced accelerated aging mice. Brain Res 1810:148372
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313
Witko-Sarsat V, Friedlander M, Khoa TN, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer J-M, Jungers P, Drüeke T, Descamps-Latscha B (1998) Advanced oxidation protein products as Novel mediators of inflammation and monocyte activation in chronic renal Failure1, 2. J Immunol 161:2524–2532
Yadawa AK, Srivastava P, Singh A, Kesherwani R, Bhoumik S, Kumar R, Arya JK, Rizvi SI (2023) Berberine may provide redox homeostasis during aging in rats. Z für Naturforschung C 78:307–315
Yanar K, Aydın S, Çakatay U, Mengi M, Buyukpınarbaşılı N, Atukeren P, Sitar ME, Sönmez A, Uslu E (2011) Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin Pharmacol Toxicol 109:423–433
Yang M, Liu Y, Luo S, Liu C, Jiang N, Li C, Zhao H, Han Y, Chen W, Li L, Sun L (2024) DsbA-L ameliorates renal aging and renal fibrosis by maintaining mitochondrial homeostasis. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-023-01216-1
Zhao H, Halicka HD, Li J, Darzynkiewicz Z (2013) Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging 5:623–636
Zhuang J, Chen X, Cai G, Wu D, Tu C, Zhu S, Huang Y, Xu P, Zhong Z (2021) Age-related accumulation of advanced oxidation protein products promotes osteoclastogenesis through disruption of redox homeostasis. Cell Death Dis 12:1160
Zych M, Wojnar W, Kielanowska M, Folwarczna J, Kaczmarczyk-Sedlak I (2020) Effect of Berberine on Glycation, Aldose reductase activity, and oxidative stress in the lenses of Streptozotocin-Induced Diabetic rats in Vivo—A preliminary study. Int J Mol Sci 21:4278
Acknowledgements
Dr. Arun Kumar Yadawa is grateful to University Grant Commission, India for providing Dr. DS Kothari Postdoctoral Fellowship. Authors also acknowledge the Department of Biotechnology, Government of India for providing support under the “Research Resources, Service Facilities, and Platforms” program.
Funding
This experimental work was financially supported by University Grant Commission, India in the form of Dr. DS Kothari fellowship (No.F.4 − 2/2006 (BSR)/BL/19–20/0240; dated 23.06.2020) to Dr. Arun Kumar Yadawa.
Author information
Authors and Affiliations
Contributions
SIR designed the experiments, interpreted the results and critically reviewed the manuscript. AKY designed and performed the experiments, analysed the data, interpreted the results and wrote the manuscript. AS, PS, RK and JKA performed the experiments.
Corresponding author
Ethics declarations
Ethics approval
All animal care and experimental procedures were carried out with the approval of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Animal Ethics Committee (IAEC/AU/2019(1)/08), University of Allahabad, India.
Consent for publication
(include appropriate statements): All authors agree to publish this manuscript in Metabolic Brain Disease.
Consent to participate
(include appropriate statements): Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadawa, A.K., Srivastava, P., Singh, A. et al. Berberine attenuates brain aging via stabilizing redox homeostasis and inflammation in an accelerated senescence model of Wistar rats. Metab Brain Dis 39, 649–659 (2024). https://doi.org/10.1007/s11011-024-01350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-024-01350-7