Abstract
Epilepsy significantly reduces the patient’s quality of life, and we still need to develop new therapeutic approaches to control it. Transplantation of cells such as Sertoli cells (SCs), having a potent ability to release a variety of growth and immunoprotective substances, have made them a potential candidate to deal with neurological diseases like epilepsy. Hence, this study aims to evaluate whether SCs transplant effectively protects the hippocampus astrocytes and neurons to oppose seizure damage. For this purpose, the effects of bilateral intrahippocampal transplantation of SCs were investigated on the rats with the pentylenetetrazol (PTZ) induced seizure. After one-month, post-graft analysis was performed regarding behavior, immunohistopathology, and the distribution of the hippocampal cells. Our findings showed SCs transplantation reduced astrogliosis, astrocytes process length, the number of branches, and intersections distal to the soma of the hippocampus in the seizure group. In rats with grafted SCs, there was a drop in the hippocampal caspase-3 expression. Moreover, the SCs showed another protective impact, as shown by an improvement in pyramidal neurons’ number and spatial distribution. The findings suggested that SCs transplantation can potently modify astrocytes’ reactivation and inflammatory responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a prevalent neurological disorder that significantly reduces the life quality of patients. The world health organization (WHO) estimates that 50 million people suffer from it globally nowadays (WHO (n.d.) para. 1). This disorder is predisposed to aberrant recurring neuronal activity within the brain. Cognitive and behavioral impairments after seizures have been proven to be prevalent comorbidities in people with epilepsy (Jokeit and Ebner 2002; Fisher et al. 2005). The primary etiology of epilepsy is an imbalance between activation and inhibition within the central nervous system (CNS) (Scharfman 2007). The CNS possesses auto-protective and repairing mechanisms to defend itself from neuronal damage brought on by epilepsy, according to the evidence (Mori et al. 1991; McCloskey et al. 2005).
The seminiferous tubules of testis tissue are home to Sertoli cells (SCs), which partially resemble mesenchymal stem cells (MSCs). Creating the blood-testis barrier within the seminiferous epithelium is critical in developing an immunological defense for the growing germ cells. Additionally, SCs can release a variety of growth and immunoprotective substances, which create an immune-privileged environment when combined. These immunoprotective qualities are essential for the co-implantation SCs with other tissues and the survival of spermatogenic cells (Gong et al. 2017). For instance, co-grafting rat or mouse ventral mesencephalon neurons with SCs resulted in the co-grafted cells being neuroprotective (Shamekh et al. 2005). Moreover, previous studies showed improved striatal neuronal atrophy and the reversal of locomotor abnormalities after transplanting SCs into the rat striatum of a 3-nitropropionic acid model of Huntington’s disease (Rodriguez et al. 2003). In light of this, co-grafting neural cells with SCs, which release several trophic and immunosuppressive chemicals, might be a successful strategy for treating neurodegenerative illnesses (Willing et al. 1998).
In the PTZ-kindling mouse model of epilepsy, it is shown that astrocytes are activated in particular cortical areas (Ueno et al. 2020). PTZ-kindling in rats resulted in a moderate loss of neurons in the CA1, CA3, CA4, and dentate gyrus hippocampal regions. Most of the injured cells were cycline B1 positive. It is suggested that PTZ-kindling may be a suitable model to investigate the mechanisms of seizure-induced neuronal death because cycline B1 expression has been described in hippocampal neurons of patients with temporal lobe epilepsy (Nagy and Esiri 1998; Pavlova et al. 2006). Findings of Zhu et al. (Zhu et al. 2015) demonstrated that PTZ-kindled mice exhibited considerable hippocampal neuronal loss and astrocytosis and suggested that NMDA receptor NR2B subunits are involved in clinical and physiological processes that are connected to epilepsy, such as hippocampus astrocytosis, oxidative stress, and neuronal degeneration (Zhu et al. 2015). In the current study, we aim to investigate the neuroprotective effects of SCs transplant in the PTZ-rat models of epilepsy.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Media (DMEM/F12) was obtained from Invitrogen (Invitrogen, Carlsbad, CA, USA). Pentylenetetrazol (PTZ) (P6500) was purchased from Sigma Aldrich (St. Louis, Mo, USA). Antibodies against GFAP (cat number ab7260, 1:300), GDNF (cat number ab18956, 1:300), caspase-3 (ab32351, 1:500), and Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC kit (cat number ab64264) were bought from Abcam (Cambridge, Massachusetts). Other materials were purchased from Sigma Alderich (St. Louis, Mo, USA).
Isolation and culture of SCs
After receiving approval from the animal care committee of Shahid Beheshti University of Medical Sciences, male albino Wistar rats (20–30 days old) were killed under deep anesthesia, and the testes were removed. The tunica albuginea was taken from the individual testis, which was sequentially digested first with trypsin (0.25%) for 15 min and then with collagenase (0.1%) for 15 min at 37 °C. Afterward, fetal bovine serum (FBS) was added. Following centrifugation of the solution, the pellet was transferred to a culture media containing DMEM/ F12 and FBS (10%). Forty-eight hours later, the culture media was changed to discard the debris and red blood cells.
Immunocytofluorescence
SCs were cultured in a 24-well plate and fixed by 4% paraformaldehyde (PFA). After washing with PBS, cells were permeabilized with Triton Χ-100. Then, cells were incubated with a normal serum of goat followed by overnight incubation with the primary antibodies against GDNF and FasL at 4 °C. The fluorescent secondary antibodies were applied after washing with PBS. For visualization of the nuclei, cells were stained by DAPI. Preparations were examined under a fluorescent microscope (Olympus IX71, Japan).
Kindling procedure
The current paper obtained 36 adult male rats (Sprague-Dawley, 200–220 g) from the Laboratory Animal Center of Shahid Beheshti University of Medical Sciences, Tehran, Iran. The University Ethics Committee approved this animal experiment (IR.SBMU.RETECH.REC.1399.534). The rats were housed in four per cage at 22◦C, under a 12 h light/dark cycle with ad libitum access to water and food. For kindling, rats received a single dose of 40 mg/kg PTZ dissolved in 500 µl of normal saline intraperitoneally (i.p.) every 48 h. A total of 12–15 doses of PTZ were given to each rat. Following each PTZ injection, we observed convulsive behavior for 20 min (Rajabzadeh et al. 2012). The seizures were classified according to the Racine score (Racine 1972) as follows: Stage 0: no response, Stage 1: ear and facial twitching, Stage 2: myoclonic jerks without upright position, Stage 3: myoclonic jerks and upright position with bilateral forelimb clonus, Stage 4: tonic-clonic seizures, and Stage 5: generalized tonic-clonic seizures, loss of postural control.
SCs transplantation
Bilateral Sertoli cell transplantation was performed one day after the last injection of PTZ. The animals were assigned to one of three experimental/transplant groups: control or intact groups (n = 12), PTZ (n = 12), and PTZ + Sertoli cells (500,000 cells; n = 12). The Sertoli cells were maintained alive in suspension using a 2 µl DMEM aliquot stored on ice during surgery. After being anesthetized (i.p.) with xylazine (10 mg/kg) /ketamine (70 mg/kg), the animals were bilaterally transplanted with SCs in each hippocampus using a 5µL Hamilton microsyringe placed at the following coordinates, relative to (Bregma = -3.6, Lat = 2, DV = 3.2). In the PTZ group, rats received media as a vehicle. Rats were sacrificed at 30 days post-transplantation.
Measurement of short-term spatial memory by T-maze test
T-maze (each arm 30 × 15 × 7 cm and 7 × 7 cm centerpiece) was constructed using black acrylic plastic. After 30 min of the habitation, the trial was started by placing mice facing away from the goal arms into the start arm. The rat was allowed to freely explore and choose the left or right goal arm. The tail cleared the goal arm and then closed the goal arm using the centerpiece. After 30 s, the centerpiece was eliminated, and the rat was moved back into the start arm, letting it choose again between the two open-goal arms. The T-maze test was performed through two daily trials at one hour for three consecutive days. According to the scoring, we performed analyses. If the rat repeatedly chose the same goal arm in the same trial, the score would be 0. If the rat chose different goal arms in the same trial, it would be 1.
Passive avoidance tests
A two-compartment box system was used for passive avoidance training (Shuttle box) to evaluate long-term memory. The irradiated chamber (35 × 20 × 15 cm3), made of transparent plastic, was linked using an 8 × 8 cm2 guillotine. Baseline evaluations of rats were carried out on day 0. First, all groups became habituated to the system. Then, the rat was located in the irradiated zone. After 5 s, the guillotine door opened. The rat was taken from the dark area into the home cage as soon as entering it. After 30 min, the habituation test was repeated and continued at the same interval as the acquisition test with the closed-door guillotine. In the following, we applied a 50 Hz, 1.2 mA constant current shock for 1.5 s immediately after its entrance to the dark zone. In the next step, the rat was removed from the dark region and placed in the home cage 20 s later. It got a foot shock and entered the dark zone another time. The training stopped when the rat remained in the light zone. After twenty-four hours, a retention test was conducted to evaluate long-term memory. For this purpose, each animal was put in the illuminated chamber for 10 s. In this stage, the door opened, and the latency of entering the dark zone (step-through latency (STLa)) and the time spent in the dark compartment/zone (TDC) were recorded, representing the retention performance. The maximum score was 300 s, and these sessions had no electric shock.
Immunohistochemistry
During the first step of the IHC process, the rat was deeply anesthetized by 100 mg/kg ketamine and 10 mg/kg xylazine. Then, it was transcardially perfused with chilled saline and fixed by a fixator containing 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). In this stage, its brain was extracted and placed in formalin before being prepared and placed on the slides. Formalin-fixed tissues were embedded in paraffin wax, and three samples were randomly selected in each group. Then, the paraffin-embedded tissue section was sliced typically by a rotary microtome (Thermo Fisher Scientific, Waltham, MA, USA) with a thickness of 6 μm. Next, 8–10 coronal sections were randomly chosen in each sample. Subsequently, five fields were randomly captured at high magnification. The slide was immersed in xylene to remove the paraffin and then transferred to the water through a series of diluted alcohols (100%, 95%, 70%, and 50%) before staining. Afterward, antigen retrieval was carried out before immunohistochemistry (0.01 M citrate buffer solution (pH 6.0), 0.01 M PBS buffer (pH7.0), 0.05 M EDTA (pH 8.0), 0.05 M Tris-EDTA (pH 9.0), and 0.05 M Tris-HCl). The primary antibodies were anti-GFAP antibody, anti-caspase-3, and FasL diluted in the PBS solution containing 0.3% Triton X-100 and 1% bovine serum albumin (BSA). Thus, the sections were incubated in them overnight at 4 °C. Consequently, they were incubated with secondary antibodies of an avidin-biotin complex substrate in 0.05 M Tris buffer (pH 7.6) containing 0.05% 3,3-diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide or secondary fluorescent conjugated antibodies. After the immunohistochemical reaction, the sections were mounted by Mounting Medium for IHC (Abcam. Cambridge, UK). Also, Fluorescence microphotographs were captured by fluorescent microscopy (E200, Nikon, Japan). All the steps were performed by a researcher blinded to experimental conditions.
Astrocyte morphology and distribution
Astrocyte cells were stained specifically with GFAP antibody (specific astrocyte marker). Thirty individual GFAP+ cells were chosen and captured by the 40X objective lens for reconstruction (Langhammer et al. 2010; Li et al. 2011). The figure was imported to ImageJ (Java. NIH, USA). The nucleus of each astrocyte was marked as the center. We performed the Sholl analysis based on previous instructions to quantify the total process length (Boroujeni et al. 2021; Moghaddam et al. 2021). Each image was imported to ImageJ to measure the soma size. After setting the image scale with the Wand tools, the border of each soma was selected to determine the nearest neighbor distance (NND). We used a script for Fiji (ImageJ) developed by Y.Mao (Mao 2016) as described in previous studies (Davis et al. 2017). Finally, the regularity index (RI) can be calculated as follows:
where \({X}_{NND}\) is the average NND of a population and \({\delta }_{NND}\) is the standard deviation of that population (Wäussle et al. 1993; Davis et al. 2017). For the calculation of the arbor area, a polygon was drawn manually by connecting the endpoints of the appendages using the ImageJ Polygon tools (Davis et al. 2017).
Spatial distribution of hippocampal neurons
In addition, we investigated the spatial distribution of neurons in the pyramidal layer of the hippocampus employing the Voronoi tessellation method. Each polygon represents a space occupied by a cell (Torquato and Haslach Jr 2002). The neurons were mapped by the ImageJ Voronoi Plugin (Java. NIH, USA). Each polygon was drawn around the cell body of neurons. For this purpose, brain section images were captured using the 40X objective lens (Nikon Eclipse, E-200). After setting the scale, each image was imported to ImageJ by the Voronoi plugin, and then Polygons were drawn by clicking on the nuclei. Next, we calculated the polygon area by running analysis → Measure (Safaeian and David 2013; van Horssen et al. 2014), and also the percentage coefficient of variation (CV) (standard deviation of the polygon areas/mean×100) to determine the spatial distribution of neurons. The CV of approximately 33–64% was associated with a random distribution of the neurons. The CVs of 33% have a regular pattern, and those above 64% are considered a clustered distribution (Duyckaerts and Godefroy 2000).
Data analysis
Results were analyzed using the Graph Pad Prism 8 software (Graph Pad Software Inc., La Jolla, CA, USA). We assessed normality and variance homogeneity data using Kolmogorov–Smirnov and Brown-Forsythe tests. For data with a normal distribution and variance homogeneity, statistical analysis was performed by the one-way analysis of variance (ANOVA) protocols followed by multiple comparison tests utilizing Tukey’s method to analyze the differences. Otherwise, the statistical analysis of the data was carried out by a non-parametric test. Moreover, a P-value of less than 0.05 was considered statistically significant. Statistical outputs were shown in APA style.
Results
SCs exhibited fibroblast-kike morphology and expressed GDNF and FasL
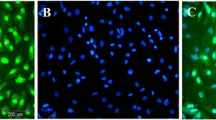
Fibroblast-like cells appeared in culture dishes one week after Sertoli cells were cultured. The cells grew rapidly and covered the surface (Fig. 1a). In the current paper, we evaluated SCs for the expression of GDNF and FasL after their isolation from the testicular tissue of immature rats. The former is a neurotrophic marker that SCs secrete, and the latter is an immunological marker that is a specific marker of SCs. Immunocytochemistry analysis indicated that Sertoli cells were immunopositive to the neurotrophic factor of GDNF (Fig. 1b). FasL is a tumor necrosis factor-related type II transmembrane protein. Our results indicated that SCs were immunopositive for FasL (Fig. 1c). Following the injection of SCs into the hippocampus of rats, the FasL-positive SCs transplant can be seen in the hippocampus after 30 days (Fig. 1d).
In vitro characterization of SCs. a A spindle-shaped morphology of SCs after 1 week of isolation. b Immunocytochemical staining revealed these cells were immunopositive for GDNF and c FasL. d Hippocampal sections were immunostained with anti FasL. The FasL positive SCs transplant could clearly be seen in the hippocampus
Implantation of SCs augmented memory functions
We performed the T maze to measure the effect of SCs transplant on short-term spatial memory (Fig. 2a). Our results showed that the alternation rate decreased significantly in the PTZ group as opposed to the control group (P < 0.001). Also, following the SCs transplantation, the alternation rate increased compared to the PTZ group (P < 0.001) (Fig. 2b). On the other hand, after PTZ injection, latency showed a considerable increment compared to the control (P < 0.001). However, latency represented a noticeable decline in the SCs receiving group compared to the PTZ group (P < 0.001) (Fig. 2c). Furthermore, one-month post-injection of SCs into the rat brains, there was a significant difference in time spent in the dark compartment between PTZ injected rats and PTZ + Sertoli cells group (P < 0.001) (Fig. 2e). According to results of STLa, the difference between PTZ group and PTZ + Sertoli cells group was statistically significant (P < 0.001) (Fig. 2f).
The effect of SCs implantation on short term memory and long-term memory at 30th day. a Sample movement traces of a rat in T-maze. b Two important variables of T-maze, the alternation rate (%) (c) and latency as indicators of short-term memory. e Time spent in dark compartment and f step through latency. (*P < 0.05; ***P < 0.001). The values were expressed as means ± SD
SCs implantation diminished apoptosis in the hippocampus after PTZ injection
The protein expression level related to apoptosis (caspase-3) was determined in the hippocampus 30 days after SCs transplantation. The immunohistochemistry results revealed that the upregulation of caspase-3 in the hippocampus was higher in the PTZ group than in the control (P < 0.001). However, the expression level of caspase-3 dropped noticeably in the PTZ + Sertoli cells group compared to the PTZ group (P < 0.001) (Fig. 3).
Effect of SCs transplant on the expression of the caspase-3 in hippocampus, and analyze of caspase-3 expression in different groups. An increase in caspase-3 expression relative to the control group is clearly detectable in the PTZ one. Caspase-3 expression reduced significantly in PTZ + Sertoli cells rats compared to PTZ ones. (**P < 0.01; ***P < 0.001). The values were expressed as means ± SD
Grafted SCs in PTZ-lesioned rats declined gliosis
For the detection of astrogliosis, immunohistochemistry against GFAP was done (Fig. 4a). Consequently, astrogliosis of the PTZ group was statistically higher than that of the control (P < 0.001). In the group receiving SCs, the number of astrocytes exhibited a significant drop compared with the PTZ group (P < 0.001) (Fig. 4b.). Also, GFAP-positive cells were subjected to the Sholl analysis to study the morphology of astrocytes. The findings demonstrated a significant reduction in the NND and regularity index of the PTZ group in contrast to the control group (P < 0.001). However, SCs transplant increased the NND and regularity index compared to the PTZ group (P < 0.001) (Fig. 4c, d).
Effect of seizure and SCs grafted on the astrogliosis in hippocampus of rats. a Immunohistochemical staining for astrocyte marker (GFAP). As indicated in graph (b), SCs implantation reduced the number of GFAP positive cells in PTZ + Sertoli cells compared to the PTZ group. Two quantitative indicators of spatial distribution of astrocytes, NND and regularity index were showed in (c) and (d), respectively. (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SD
Grafted SCs prevented astrocyte reactivity in the hippocampus of PTZ-injected rats
The Sholl analysis showed that injection of PTZ significantly reduced the complexity of astrocytes compared to the control group (P < 0.001). On the other hand, the SCs transplant increased the complexity of astrocytes in PTZ + Sertoli cells compared to the PTZ group (P < 0.001) (Fig. 5a). Our results showed that the total astrocyte process length was increased in the PTZ + Sertoli cells group in comparison with the PTZ group (P < 0.001) (Fig. 5b). Moreover, the grafted SCs reduced the soma size of astrocytes in PTZ + Sertoli cells compared to the PTZ group (P < 0.001) (Fig. 5c). The transplant of SCs increased the astrocyte arbors area and the total number of branches in PTZ + Sertoli cells compared to the PTZ group (P < 0.001) (Fig. 5d, e). However, the percentage of the primary branch of astrocytes in the control group, PTZ group, and PTZ + Sertoli cells group were 60.6%, 77.7%, and 48%, respectively. Also, the percentage of the secondary branch of astrocytes in control, PTZ, and PTZ + Sertoli cells groups were 32.7%, 20.5%, and 42.2%, respectively. The tertiary arbores of astrocytes in control, PTZ, and PTZ + Sertoli cell groups were 6.7%, 1.8%, and 9.8%, respectively (Fig. 5f).
Sholl analysis findings. a The injection of PTZ reduced the complexity of astrocyte processes while the SCs transplant prevented it. b SCs graft increased the total astrocyte process length compared to the PTZ group. c Astrocyte soma size reduced in PTZ + Sertoli cells group compared to the PTZ one. d Transplant of SCs increased astrocyte arbors area. e Total number of branches was increased in PTZ + Sertoli cells compared to the PTZ group. f Percentage of primary, secondary and tertiary arbors of astrocyte in subjects. (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SD
Transplant of Sertoli cells protected hippocampal neurons against PTZ toxicity
Stereological analysis showed that the number of pyramidal hippocampus neurons decreased significantly in the PTZ group compared to the control group (P < 0.001). Our results demonstrated that the number of pyramidal neurons was protected in the PTZ + Sertoli cells as opposed to the PTZ group (P < 0.001) (Fig. 6a). In this study, we showed that the mean number of dark neurons increased in the hippocampus of PTZ injected group compared to the control group (P < 0.001). Grafted SCs decreased the mean number of dark neurons in the PTZ + Sertoli cells group compared to the PTZ group (P < 0.001) (Fig. 6b).
The estimation of the number of hippocampal neurons. b The effects of the SCs transplant on the number of the neurons c and dark neurons. As shown in the graphs, although SCs transplant significantly improved the number of the neurons and it was failed to increase the number of dark neurons. Black arrows marked healthy neurons and black triangle marked dark neurons (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SD
Grafted Sertoli cells improved the spatial distribution of hippocampal neurons in the Aβ injected rats
Figure 7a displays the Voronoi tessellation ls in control, PTZ, and PTZ + Sertoli cell groups. According to our data, 30.33% of the polygon’s area of cells was in the range of 60–100 µm2 in the control group compared to 38.1% in the PTZ group and 28% in the PTZ + DPSC group (Fig. 7b). Due to the decrease in the number of cells, the dimensions of the polygons were increased. In the PTZ group, CV classification was significantly higher than in other groups, while the mean CV of polygon areas in all groups was in a random range (33–64%) (Fig. 7c).
Voronoi analysis. a A micrograph of cells and schematic of Voronoi tessellation in the hippocampus of study groups. b Mean ± Standard deviation of Voronoi polygon area (%) and c Coefficient of Variation (CV) within the pyramidal layer of hippocampus. (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SD
Discussion
In the current paper, we evaluated SCs for the expression of GDNF and FasL after their isolation from the testicular tissue of immature rats. The former is a neurotrophic marker that SCs secrete, and the latter is an immunological marker that is a specific marker of SCs. Also, its expression indicates the absence of graft rejection in the tissue. Our results showed that the injection of SCs improved memory function prevented astrocyte cell proliferation, stopped hippocampal cell death, and generally led to neuroprotection. This effect of SCs mainly occurred through the secretion of neurotrophic markers such as GDNF.
In addition, the immunocytochemical image of SCs after isolation in the culture medium revealed that these cells could express the neurotrophic marker GDNF and the FasL marker. Therefore, they can play a role in neuroprotection by secreting GDNF. On the other hand, SCs in the culture medium could express FasL, which has a role in transplant rejection. FasL is also a key mediator of the apoptosis of immune cells through its interaction with the Fas receptor. It regulates immunological responses and maintains T-cell tolerance (Askenasy et al. 2005).
Consequently, FasL is used as an immunomodulatory agent to promote immune tolerance. Indeed, the immune responses of allogenic T cells were suppressed in immature dendritic cells with the modified FasL gene. Allograft tolerance of transplanted solid organs, such as the lung, liver, heart, kidney, and pancreatic islet, was promoted. The longevity of allograft recipients increased by stimulating the differentiation of T helper one cells into T helper two ones (Naderi et al. 2011; Qian et al. 2013; Chen et al. 2014). In a study, Chen et al. (Chen et al. 2019) indicated that IL-10 and FasL co-transduction of immature dendritic cells induced immune tolerance and survival of liver allograft in rats. The L-10-FasL/immature dendritic cells caused an increased reduction in the expression of CD86, CD80, and major histocompatibility complex class II (MHC II) and T-cell proliferation (Chen et al. 2019). Therefore, the expression of the FasL marker by SCs could show that SCs would not undergo graft rejection after injection.
Our immunohistochemical analysis of the FasL marker of SCs injected into the hippocampus showed that these injected cells were seen in the hippocampal tissue one month after the injection. Moreover, it was proposed that SCs may display their immunoprotective activity by producing FasL. The immune response was downregulated because of the apoptosis of lymphocytes caused by FasL binding to Fas on activated lymphocytes (Nagata and Golstein 1995). FasL-transfected myoblasts survived and defended the transplanted islets when co-transplanted with islets after being transfected with FasL mRNA (Lau et al. 1996). However, a study demonstrated that SCs from gld mice, which lack FasL, did not survive following transplantation under the kidney capsule. By contrast, the result differed in SCs from mouse models expressing FasL constitutively. When a FasL antibody was injected intraperitoneally, the defense, offered by wild-type testicular tissue, was removed (Sanberg et al. 1997). Thus, the capacity of the Sertoli cell to confine immunosuppression to the graft’s environment may be extremely vital. It may eliminate the need for long-term immunosuppression, reducing this treatment’s health hazards, such as neurotoxicity, hepatotoxicity, nephrotoxicity, and hypertension (Shaw et al. 1996; Willing et al. 1998).
Furthermore, our findings revealed improved long-term and short-spatial memory after the injection of SCs into the epileptic rats’ models. A study demonstrated that SCs could produce neurotrophic substances, including GDNF and vascular endothelial growth factor (VEGF) (Ahmadi et al. 2018). The conditioned media, derived from SCs, facilitated neurite outgrowth and prevented oxidative stress-related cell death. The pro-inflammatory cytokines’ expression levels decreased after SC grafting in a rat model of Huntington’s disease (Ahmadi et al. 2018). VEGF was also suggested to promote angiogenesis, facilitating neuroprotection (Duffy et al. 2004). In addition to VEGF’s protective role in the CNS, which stimulates self-renewal, the survival of microglia and neurons increased (Duffy et al. 2004). In another study, astrocyte migration significantly decreased after transplanting SCs into hippocampi that had been lesioned (Aliaghaei et al. 2019). This result can be related to the SCs’ anti-inflammatory activity, mediated by specific cytokines and prostanoid molecules (Doyle et al. 2012). It can be attributed to endogenous neural stem cells, which facilitate the regeneration of a functional neural network (Ebrahimi et al. 2018). In another way, transplanted SCs might trigger innate hippocampus neurogenesis by secreting neurotrophic substances like GDNF.
Similarly, a study by Nivet et al. (Nivet et al. 2011) demonstrated that grafting human olfactory ecto-mesenchymal stem cells into damaged mouse hippocampi might induce local neural stem cells to differentiate into neurons and reduce learning and memory impairment. In another search, Cui et al. (Cui et al. 2017) showed that intravenous human umbilical cord mesenchymal stem cells improved cognitive impairment by increasing hippocampus neurogenesis and reducing oxidative stress in the Tg2576 CE mouse model. Thus, SCs might improve memory by producing neurotrophic factors, which can help alleviate neuroinflammation and prevent apoptosis within the hippocampus.
Our immunohistochemical results against the GFAP marker, an astrocytic marker, showed a decrease in the amount of astrogliosis in the group receiving Sertoli cells compared to the epilepsy group. Also, NND and regularity index demonstrated a rise in rats that received Sertoli cells compared to the epilepsy group. Neuroinflammation is among the critical processes in the pathogenesis of epilepsy. Microglia and astrocytes of the brain are activated in response to several brain injuries, including persistent seizure activity. Moreover, these cells start to produce pro-inflammatory cytokines, and different adverse outcomes are brought on by the inflammatory response to the assault, such as long-term plastic alterations, altered neuronal excitability, dysfunctional astrocytes, impaired blood-brain barrier permeability, and neurodegeneration. These outcomes potentially play a role in the emergence of chronic spontaneous seizures (Suleymanova 2021). Previous studies reported astrogliosis in the pathogenesis of epilepsy and seizure aggravation (Fedele et al. 2005; Dariani et al. 2013; Becker 2018). They demonstrated that GDNF, the neurotrophic factor produced by SCs, could alleviate astrogliosis (Iannotti et al. 2003; Deng et al. 2011).
In addition, our immunohistochemical analysis of the caspase-3 marker revealed that the injection of SCs prevented apoptosis in the hippocampus of epilepsy model rats. Furthermore, our stereological analysis of hippocampal pyramidal layer neuron counts displayed prevention of pyramidal neuron death in the SCs receiving group compared to the epilepsy group. In a previous investigation, rats injected with Aβ1- 42) showed decreased expression levels of cleaved caspase 3 after cell implantation (Aliaghaei et al. 2019). Consistently, a study by Aslani et al. presented caspase 3 inactivation and elevated gene expression levels for BCL2 family members in SCs exposed to cytotoxic substances (Aslani et al. 2017). Similarly, it was discovered that SCs produced additional complement and apoptosis inhibitors such as protease inhibitor-9 and serpina3n (Mital et al. 2010). Therefore, it would seem that these cells might control cell death pathways by creating an immune-privileged microenvironment within engraftment sites.
In the current paper, examining the effects of astrocyte morphology with Sholl software showed that the injection of SCs transformed the morphology of astrocytes into resting astrocytes. A change in astrocyte morphology to a reactive state was evident in the epilepsy group. These active astrocytes can cause the release of inflammatory factors and the death of neural cells. Also, the injection of SCs caused the transformation of active astrocytes into resting astrocytes. Alterations in morphology, transcription, and function were all astrocyte reactivity parts. As hypertrophy was an essential characteristic of astrocyte reactivity, cell body and processes were expanded in reactive astrocytes (Wilhelmsson et al. 2006). The primary processes changed in number or became polarized toward the injury site since astrocyte arborization was rearranged with reactivity (Wilhelmsson et al. 2006; Bardehle et al. 2013). In this regard, Lannotti et al. discovered that GDNF reduced astroglial reactivity and altered the morphological characteristics of reactive astrocytes (Iannotti et al. 2003). Concerning morphology, Deng et al. represented that GDNF treatment decreased astrocyte hypertrophy, which caused these cells to have elongated processes, as seen in vivo. Furthermore, in vivo and in vitro GDNF therapy significantly diminished the production of chondroitin sulfate proteoglycans (CSPGs) and GFAP, two markers of astrogliosis (Deng et al. 2011).
Moreover, we showed that the injection of SCs prevented changes in the spatial arrangement of neurons in the pyramidal layer. Neuronal density, volume variations, and how the cells are arranged spatially in different brain regions are essential for connection formation, function, and development. This represented how the brain’s connectivity and assembly of the neurons were accomplished (Linker et al. 2011; Keeley et al. 2020). Recent studies suggested several diseases or situations affecting development may alter the cells’ spatial distribution and density (Duyckaerts and Godefroy 2000; Thom et al. 2009). For example, cerebral ischemia changed the CA1 pyramidal spatial arrangement of neurons in the hippocampus, transforming it into a random pattern (Sarkala et al. 2019). In concordance with our result, a previous study demonstrated that SCs transplantation did not change the spatial arrangement of Purkinje cells (Mohammadi et al. 2018).
A study by Shamekh et al. (Shamekh et al. 2008) on Parkinson’s disease investigated whether SC pre-conditioned medium could promote the differentiation of the 796 MB cell line into a dopaminergic phenotype. According to the results, secretory products produced from SC-conditioned media boosted the 796RMB cell line’s cell proliferation and dopaminergic neuronal development. They resulted that SC pre-conditioned medium has a mitogenic effect on the 796RMB cell line during cell proliferation, SC pre-conditioned medium has a selective effect on the 796RMB cell line during neuronal differentiation, and SC pre-conditioned medium increases the percentage of dopaminergic neurons in those 796RMB cells committed to a neuronal phenotype during differentiation.
In addition, the number of dark neurons in the cell recipient group was less than in the epilepsy group in our study. Unprogrammed inhibition of a phase transition among structures—likely the transformation of hyaloplasm from a sol to a gel—led to the emergence of dark neurons, which might be reversible or irreversible. This process caused cell death through a mechanism apart from apoptosis or necrosis (Gallyas et al. 2006; Gallyas 2007; Zimatkin 2018). According to microscopic findings in animal studies, “dark” neurons produced in epilepsy models (Atillo et al. 1983) or a significant amount of metabolic or physical noxae were able to recover (Csordas et al. 2003). Therefore, SCs transplantation can inhibit cell death.
Conclusion
The SCs transplantation in epileptic rat models could cause neuroprotective effects, mainly through the production of neurotrophic factors by SCs. Generally, SCs transplantation resulted in memory improvement and inhibition of neuroinflammation and astrogliosis within the epileptic rat models. Also, a lower rate of graft rejection was demonstrated using SCs, mainly through the expression of FasL. These results showed that SCs could potentially be used in the co-transplantation setting. Further research is required to comprehensively find out the positive and negative features of SCs transplantation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmadi H, Boroujeni ME, Sadeghi Y, Abdollahifar MA, Khodagholi F, Meftahi GH, Hadipour M, Bayat A-H, Shaerzadeh F, Aliaghaei A (2018) Sertoli cells avert neuroinflammation-induced cell death and improve motor function and striatal atrophy in rat model of Huntington disease. J Mol Neurosci 65:17–27. https://doi.org/10.1007/s12031-018-1062-x
Aliaghaei A, Meymand AZ, Boroujeni ME, Khodagoli F, Meftahi GH, Hadipour MM, Abdollahifar MA, Mesgar S, Ahmadi H, Danyali S (2019) Neuro-restorative effect of sertoli cell transplants in a rat model of amyloid beta toxicity. Behav Brain Res 367:158–165. https://doi.org/10.1016/j.bbr.2019.03.030
Askenasy N, Yolcu ES, Yaniv I, Shirwan H (2005) Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood 105:1396–1404. https://doi.org/10.1182/blood-2004-06-2364
Aslani F, Sebastian T, Keidel M, Fröhlich S, Elsässer H-P, Schuppe H-C, Klug J, Mahavadi P, Fijak M, Bergmann M (2017) Resistance to apoptosis and autophagy leads to enhanced survival in sertoli cells. MHR: Basic Sci Reprod Med 23:370–380. https://doi.org/10.1093/molehr/gax022
Atillo A, Söderfeldt B, Kalimo H, Olsson Y, Siesjö B (1983) Pathogenesis of brain lesions caused by experimental epilepsy. Acta Neuropathol 59:11–24. https://doi.org/10.1007/bf00690312
Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580–586. https://doi.org/10.1038/nn.3371
Becker AJ (2018) Review: animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol Appl Neurobiol 44:112–129. https://doi.org/10.1111/nan.12451
Boroujeni ME, Simani L, Bluyssen HA, Samadikhah HR, Zamanlui Benisi S, Hassani S, Akbari Dilmaghani N, Fathi M, Vakili K, Mahmoudiasl G-R (2021) Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem Neurosci. https://doi.org/10.1021/acschemneuro.1c00111
Chen C, Akiyama K, Yamaza T, You YO, Xu X, Li B, Zhao Y, Shi S (2014) Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med 6:322–334
Chen L, Zhang L, Zhu Z, He W, Gao L, Zhang W, Liu J, Huang A (2019) Effects of IL-10‐and FasL‐overexpressing dendritic cells on liver transplantation tolerance in a heterotopic liver transplantation rat model. Immunol Cell Biol 97:714–725. https://doi.org/10.1111/imcb.12252
Csordas A, Mazlo M, Gallyas F (2003) Recovery versus death of dark(compacted) neurons in non-impaired parenchymal environment: light and electron microscopic observations. Acta Neuropathol 106:37–49. https://doi.org/10.1007/s00401-003-0694-1
Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, Lv P, Xing Q, Qu R, Yao N (2017) Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res 320:291–301. https://doi.org/10.1016/j.bbr.2016.12.021
Dariani S, Baluchnejadmojarad T, Roghani M (2013) Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J Mol Neurosci 51:679–686. https://doi.org/10.1007/s12031-013-0043-3
Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De Groef L (2017) Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-01747-8
Deng LX, Hu J, Liu N, Wang X, Smith GM, Wen X, Xu XM (2011) GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol 229:238–250. https://doi.org/10.1016/j.expneurol.2011.02.001
Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, Pasham MR, Kim KH, Dufour JM (2012) Immunoprotective properties of primary sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod 86(6):1–14. https://doi.org/10.1095/biolreprod.110.089425
Duffy AM, Bouchier-Hayes DJ, Harmey JH (2004) Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. VEGF Cancer 2:133–144
Duyckaerts C, Godefroy G (2000) Voronoi tessellation to study the numerical density and the spatial distribution of neurones. J Chem Neuroanat 20:83–92. https://doi.org/10.1016/S0891-0618(00)00064-8
Ebrahimi MJ, Aliaghaei A, Boroujeni ME, Khodagholi F, Meftahi G, Abdollahifar MA, Ahmadi H, Danyali S, Daftari M, Sadeghi Y (2018) Human umbilical cord matrix stem cells reverse oxidative stress-induced cell death and ameliorate motor function and striatal atrophy in rat model of Huntington disease. Neurotox Res 34:273–284. https://doi.org/10.1007/s12640-018-9884-4
Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rülicke T, Crestani F, Boison D (2005) Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain 128:2383–2395. https://doi.org/10.1093/brain/awh555
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472. https://doi.org/10.1111/j.0013-9580.2005.66104.x
Gallyas F (2007) Novel cell-biological ideas deducible from morphological observations on dark neurons revisited. Ideggyogyaszati Sz 60:212–222
Gallyas F, Gasz B, Szigeti A, Mázló M (2006) Pathological circumstances impair the ability of dark neurons to undergo spontaneous recovery. Brain Res 1110:211–220. https://doi.org/10.1016/j.brainres.2006.06.078
Gong D, Zhang C, Li T, Zhang J, Zhang N, Tao Z, Zhu W, Sun X (2017) Are sertoli cells a kind of mesenchymal stem cells? Am J Transl Res 9:1067
Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM (2003) Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol 183:379–393. https://doi.org/10.1016/s0014-4886(03)00188-2
Jokeit H, Ebner A (2002) Effects of chronic epilepsy on intellectual functions. Prog Brain Res 135:455–463. https://doi.org/10.1016/s0079-6123(02)35042-8
Keeley PW, Eglen SJ, Reese BE (2020) From random to regular: variation in the patterning of retinal mosaics. J Comp Neurol 528:2135–2160. https://doi.org/10.1002/cne.24880
Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL (2010) Automated Sholl analysis of digitized neuronal morphology at multiple scales: whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A 77:1160–1168. https://doi.org/10.1002/cyto.a.20954
Lau HT, Yu M, Fontana A, Stoeckert CJ Jr (1996) Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science 273:109–112. https://doi.org/10.1126/science.273.5271.109
Li C, Zhao R, Gao K, Wei Z, Yaoyao Yin M, Ting Lau L, Chui D, Cheung Hoi Yu A (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 8:67–80. https://doi.org/10.2174/156720511794604543
Linker RA, Lee D-H, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134:678–692. https://doi.org/10.1093/brain/awq386
Mao Y (2016) Nearest neighbor distances calculation with ImageJ
McCloskey DP, Croll SD, Scharfman HE (2005) Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci 25:8889–8897. https://doi.org/10.1523/jneurosci.2577-05.2005
Mital P, Kaur G, Dufour JM (2010) Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 139:495–504. https://doi.org/10.1530/rep-09-0384
Moghaddam MH, Bayat A-H, Eskandari N, Abdollahifar M-a, Fotouhi F, Forouzannia A, Rafiei R, Hatari S, Seraj A, Shahidi AMEJ (2021) Elderberry diet ameliorates motor function and prevents oxidative stress-induced cell death in rat models of Huntington disease. Brain Res 1762:147444. https://doi.org/10.1016/j.brainres.2021.147444
Mohammadi R, Heidari MH, Sadeghi Y, Abdollahifar MA, Aghaei A (2018) Evaluation of the spatial arrangement of Purkinje cells in ataxic rat’s cerebellum after sertoli cells transplantation. Folia Morphol (Warsz) 77:194–200. https://doi.org/10.5603/FM.a2017.0091
Mori N, Wada JA, Watanabe M, Kumashiro H (1991) Increased activity of superoxide dismutase in kindled brain and suppression of kindled seizure following intra-amygdaloid injection of superoxide dismutase in rats. Brain Res 557:313–315. https://doi.org/10.1016/0006-8993(91)90151-k
Naderi N, Moazzeni S, Pourfathollah A, Alimoghaddam K (2011) High expression of Fas ligand on cord blood dendritic cells: a possible immunoregulatory mechanism after cord blood transplantation. Transplantation proceedings, Elsevier, Amsterdam
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456. https://doi.org/10.1126/science.7533326
Nagy Z, Esiri MM (1998) Neuronal cyclin expression in the hippocampus in temporal lobe epilepsy. Exp Neurol 150:240–247. https://doi.org/10.1006/exnr.1997.6753
Nivet E, Vignes M, Girard SD, Pierrisnard C, Baril N, Devèze A, Magnan J, Lanté F, Khrestchatisky M, Féron F (2011) Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Investig 121:2808–2820. https://doi.org/10.1172/jci44489
Pavlova T, Stepanichev M, Gulyaeva N (2006) Pentylenetetrazole kindling induces neuronal cyclin B1 expression in rat hippocampus. Neurosci Lett 392:154–158. https://doi.org/10.1016/j.neulet.2005.09.021
Qian C, Qian L, Yu Y, An H, Guo Z, Han Y, Chen Y, Bai Y, Wang Q, Cao X (2013) Fas signal promotes the immunosuppressive function of regulatory dendritic cells via the ERK/β-catenin pathway. J Biol Chem 288:27825–27835. https://doi.org/10.1074/jbc.m112.425751
Racine RJ (1972) Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr clin Neurophysiol 32:281–294. https://doi.org/10.1016/0013-4694(72)90177-0
Rajabzadeh A, Bideskan AE, Fazel A, Sankian M, Rafatpanah H, Haghir H (2012) The effect of PTZ-induced epileptic seizures on hippocampal expression of PSA-NCAM in offspring born to kindled rats. J Biomed Sci 19:56. https://doi.org/10.1186/1423-0127-19-56
Rodriguez AI, Willing AE, Saporta S, Cameron DF, Sanberg PR (2003) Effects of sertoli cell transplants in a 3-nitropropionic acid model of early Huntington’s disease: a preliminary study. Neurotox Res 5:443–450. https://doi.org/10.1007/bf03033174
Safaeian N, David T (2013) A computational model of oxygen transport in the cerebrocapillary levels for normal and pathologic brain function. J Cereb Blood Flow Metab 33:1633–1641. https://doi.org/10.1038/jcbfm.2013.119
Sanberg PR, Saporta S, Borlongan CV, Othberg AI, Allen RC, Cameron DF (1997) Article commentary: the Testis-Derived cultured sertoli cell as a Natural Fas-L secreting cell for Immunosuppressive Cellular Therapy. Cell Transplant 6:191–193. https://doi.org/10.1177/096368979700600213
Sarkala HB, Jahanshahi M, Dolatabadi LK, Namavar MR (2019) Effect of G-CSF on the spatial arrangement of CA1 hippocampal pyramidal neurons after brain ischemia in the male rats. J Chem Neuroanat 98:80–86. https://doi.org/10.1016/j.jchemneu.2019.04.007
Scharfman HE (2007) The neurobiology of epilepsy. Curr Neurol Neurosci Rep 7:348–354
Shamekh R, Newcomb J, Mallery J, Cassady C, Saporta S, Cameron D, Sanberg P, Willing A (2005) Survival of rat or mouse ventral mesencephalon neurons after cotransplantation with rat sertoli cells in the mouse striatum. Cell Transplant 14:551–564. https://doi.org/10.3727/000000005783982747
Shamekh R, Saporta S, Cameron DF, Willing AE, Sanberg CD, Johe K, Sanberg PR (2008) Effects of sertoli cell-conditioned medium on ventral midbrain neural stem cells: a preliminary report. Neurotox Res 13:241–246. https://doi.org/10.1007/bf03033507
Shaw L, Kaplan B, Kaufman D (1996) Toxic effects of immunosuppressive drugs: mechanisms and strategies for controlling them. Clin Chem 42:1316–1321
Suleymanova EM (2021) Behavioral comorbidities of epilepsy and neuroinflammation: evidence from experimental and clinical studies. Epilepsy Behav 117:107869. https://doi.org/10.1016/j.yebeh.2021.107869
Thom M, Eriksson S, Martinian L, Caboclo LO, McEvoy AW, Duncan JS, Sisodiya SM (2009) Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol 68:928–938
Torquato S, Haslach H Jr (2002) Random heterogeneous materials: microstructure and macroscopic properties. Appl Mech Rev 55:B62–B63. https://doi.org/10.1007/978-1-4757-6355-3
Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Takahashi Y, Matsumoto Y, Okamoto M, Ishihara T (2020) Pentylenetetrazol kindling induces cortical astrocytosis and increased expression of extracellular matrix molecules in mice. Brain Res Bull 163:120–134. https://doi.org/10.1016/j.brainresbull.2020.07.019
van Horssen P, van den Wijngaard JP, Brandt M, Hoefer IE, Spaan JA, Siebes M (2014) Perfusion territories subtended by penetrating coronary arteries increase in size and decrease in number toward the subendocardium. Am J Physiol Heart Circ Physiol 306:H496–H504. https://doi.org/10.1152/ajpheart.00584.2013
Wäussle H, Grüunert U, Röhrenbeck J (1993) Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol 332:407–420. https://doi.org/10.1002/cne.903320403
Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci 103:17513–17518. https://doi.org/10.1073/pnas.0602841103
Willing AE, Cameron DF, Sanberg PR (1998) Sertoli cell transplants: their use in the treatment of neurodegenerative disease. Mol Med Today 4:471–477. https://doi.org/10.1016/s1357-4310(98)01355-0
World Health Organization (n.d.). https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed 9 Feb 2023
Zhu X, Dong J, Shen K, Bai Y, Zhang Y, Lv X, Chao J, Yao H (2015) NMDA receptor NR2B subunits contribute to PTZ-kindling-induced hippocampal astrocytosis and oxidative stress. Brain Res Bull 114:70–78. https://doi.org/10.1016/j.brainresbull.2015.04.002
Zimatkin S (2018) Dark neurons of the brain. Neurosci Behav Physiol 1–5. https://doi.org/10.1007/s11055-018-0648-7
Acknowledgments
We are grateful for the funding provided by Hearing Disorders Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
AA and MHH designed and conceived the study, analyzed and interpreted the data, and revised the manuscript for intellectual contents; MT, MB, MHM, MF and KV wrote the manuscript; AB and MH revised the manuscript; SY, NE, MAA and AHB performed the experiments; MM and MS had a crucial role in data collection and revised the manuscript and drafted the manuscript for the intellectual content.
Corresponding authors
Ethics declarations
Ethics approval
Ethical approval was obtained by the ethics committee of the Shahid Beheshti University of Medical Sciences, Tehran Iran (IR.SBMU.RETECH.REC.1399.534).
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflicts of interest/Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehranpour, M., Sani, M., Beirami, A. et al. Grafted Sertoli cells prevent neuronal cell death and memory loss induced by seizures. Metab Brain Dis 38, 2735–2750 (2023). https://doi.org/10.1007/s11011-023-01309-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01309-0