Abstract
The NLR family, pyrin domain containing 3 (NLRP3) inflammasome drives the progression of Alzheimer’s disease (AD). Ginkgolide B (GB) is a potential anti-inflammatory compound that controls neuro-inflammation. The aim of this study was to evaluate the effect of GB on the NLRP3 inflammasome in AD. The effect of GB on the conversion between the M1 and M2 microglial phenotype was examined using quantitative real-time PCR and immunostaining. Western blotting assays and ELISA were used to detect changes in neuro-inflammation following GB treatment, including the NLRP3 inflammasome pathway and autophagy. In order to evaluate the cognitive function of male senescence-accelerated mouse prone 8 (SAMP8) mice, behavioral tests, including the Morris water maze and novel object recognition tests, were performed. GB significantly decreased the intracellular pro-inflammatory cytokine levels in lipopolysaccharide-treated BV2 cells and improved cognitive behavior in SAMP8 mice. Moreover, GB deactivated the NLRP3 inflammasome, and this effect was dependent on autophagy. Ubiquitination was associated with GB-induced autophagic NLRP3 degradation. These results were further validated in the hippocampus of SAMP8 mice. Thus, GB exerted a neuroprotective effect on the cognitive function of SAMP8 mice by suppressing the activation of NLRP3 inflammasome via autophagic degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a major medical and social issue worldwide (Ding et al. 2020). However, effective therapeutic strategies that can improve the symptoms of AD remain limited. Several hypotheses, such as the amyloid, the tau, the neurotrophic factor and the inflammation hypothesis, have been proposed in order to account for the etiology of AD (Zhang et al. 2020). The inflammation hypothesis posits that the activation of microglia and astrocytes releases toxic substances and pro-inflammatory cytokines, resulting in the neuronal dysfunction and apoptosis that lead to the pathological process of AD (Wang et al. 2015). The inflammasome is defined as a cytoplasmic polymeric protein complex that functions as the platform for Caspase-1 activation and for the maturation of the pro-inflammatory cytokine interleukin-1β (IL-1β) (Heneka et al. 2013). Mounting evidence suggests that the NLR family, pyrin domain containing 3 (NLRP3) inflammasome is closely associated with the pathogenesis of AD (Feng et al. 2020). As a pivotal player in inflammatory progression, the NLRP3 inflammasome may represent a possible therapeutic target for AD via the suppression of neuroinflammation. It has been observed that hyperphosphorylation of tau protein is inhibited and cognitive impairment is restrained in tau22/Asc−/− and tau22/Nlrp3−/− mice (Ising et al. 2019).
Several compounds extracted from Chinese herbal medicines, including baicalin, schisandrin, nootkatone and resveratrol, may prove beneficial for AD treatment by inhibiting the NLRP3 inflammasome pathway (Jin et al. 2019; Qi et al. 2019; Yan et al. 2020). For instance, resveratrol, a natural polyphenolic compound, may represent a potential candidate for AD treatment due to its ability to improve cognitive function by inhibiting the inflammation and mitochondrial dysfunction caused by amyloid β (Aβ), as evidenced by downregulated expression levels of IL-1β, NLRP3 and NF-κB (Yan et al. 2020). In recent years, molecules targeting NLRP3 for the treatment of AD have attracted increased attention. Thus, the aim of this study was to evaluate a compound known as ginkgolide B (GB) for its potential effects on the NLRP3 inflammasome signaling pathway in order to ameliorate AD.
The senescence-accelerated mouse prone 8 (SAMP8) strain is a mouse model that was established using phenotypic selection from the AKR/J strain (Butterfield and Poon 2005). SAMP8 mice spontaneously develop age-related learning and memory deficits, as well as key pathological features similar to those observed in patients with AD, including Aβ deposition, tau hyperphosphorylation, and increased inflammation and oxidative stress; thus, these mice have been widely used as an animal model for AD (Diaz-Perdigon et al. 2020; Jia et al. 2020).
Ginkgo biloba is a medicinal plant, whose extracts have been used to improve memory in Chinese Traditional Medicine for centuries. Moreover, several studies have been conducted to investigate the efficacy of these extracts and their effects on neurodegenerative disorders, such as AD (Vellas et al. 2012; Hashiguchi et al. 2015; Tan et al. 2015). It has been reported that the spatial memory of amyloid precursor protein (APP) transgenic mice can be improved following treatment with Ginkgo (Stackman et al. 2003). The Ginkgo extracts, EGb and EGb-761, display antioxidant activity in the treatment of AD (Vellas et al. 2006; Mohamed and Abd El-Moneim 2017). Several studies have demonstrated that EGb exerted its anti-inflammatory effect by inhibiting microglial production of pro-inflammatory mediators, such as prostaglandin E2, nitric oxide (NO), TNF-α, IL-6 and IL-1β (Gargouri et al. 2018).
Terpene trilactones, ginkgolides and bilobalide are the active compounds in Ginkgo biloba. Ginkgolide J inhibits the detrimental effects of Aβ, reverses the inhibition of long-term potentiation in the CA1 hippocampal region and inhibits Aβ-induced cell death (Vitolo et al. 2009). GB, one of the active terpene lactones isolated from Ginkgo biloba, has been found to exert an anti-inflammatory effect in several neurological diseases (Birkenhager and van Diermen 2020). It has been reported that GB alleviates hypoxia-induced neuronal injury in the rat hippocampus by preventing NLRP3 inflammasome activation. In addition, GB reduced the levels of IL-1β in an ischemic stroke model and displayed a therapeutic effect on depression by regulating the STAT3 pathway (Zhang et al. 2018). It also plays an essential role in myelin sheath regeneration by upregulating astrocyte-derived neurotrophic factors and reducing microglia-modulated neuroinflammation (Yin et al. 2019). However, the effect of GB on neuroinflammation in AD is not fully understood. Autophagy is an intracellular reaction driving the clearance of impaired organelles and misfolded proteins. A number of studies have suggested that NLRP3 inflammasome activity is often negatively regulated by autophagy (Salminen et al. 2012). Moreover, the autophagy-lysosomal pathway governs protein clearance in tau-transgenic mice (Qin et al. 2018). The role of GB in autophagy and the degradation of NLRP3 in the pathogenesis of AD were investigated in this study. The current findings may provide experimental evidence for clinical decisions on GB application, not only in AD but also in other aging- and inflammation-related disorders.
Materials and methods
Chemicals and reagents
ATP (A9130; Beijing Solarbio Science & Technology Co., Ltd.), lipopolysaccharide (LPS; BS904; Biosharp Life Sciences), 3-methyladenine (3-MA; HY-19,312; MedChemExpress), nigericin (HY-100,381; MCE), MG-132 (HY-13,259; MCE) and monosodium urate (MSU; U-2875; Sigma-Aldrich, Merck KGaA) were obtained from Sigma-Aldrich (Merck KGaA). Fetal bovine serum (FBS; 10,100,147) was obtained from Gibco (Thermo Fisher Scientific, Inc.). Penicillin-streptomycin (V900929) was purchased from Sigma-Aldrich (Merck KGaA). ELISA kits for IL-1β, IL-6 and TNF-α were purchased from Beijing Solarbio Science & Technology Co., Ltd.
Experimental design
The LPS-induced in vitro inflammation model and the SAMP8 mouse model of AD were used to investigate the effect of GB on AD-related cognitive impairment and the underlying mechanism.
In vitro study
The murine microglial cell line BV2 was purchased from the American Type Culture Collection and cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% FBS, 100 units/ml penicillin G and 100 µg/ml streptomycin. The cells were maintained at 37℃ in a 5% CO2 atmosphere. To stimulate an inflammatory response, BV2 cells were treated with ATP (5 mM; MilliporeSigma) for 3 h, then stimulated with LPS (1 µg/mL; MilliporeSigma) for 24 h. An equal volume of saline was added in the culture medium of the control group (Dai et al. 2019) (Fig. 1). For treatment, ATP- and LPS-treated BV-2 cells were treated with 10, 20 or 40 µM with GB (94,970; Sigma-Aldrich, Merck KGaA; 95% purity) for 6 h. MG-132 (10 µM) or 3-MA (5 mM) were added 1 h before GB administration to block proteasome and autophagy, respectively.
In vivo study
Seven-month-old male SAMP8 mice and senescence-accelerated mouse resistant 1 (SAMR1) mice, obtained from The Experimental Animal Center of Nanjing University (Nanjing, China), were kept in specific-pathogen-free room with a 12-h day/night cycle. The animals had free access to sterile feed and autoclaved water ad libitum. After a 1-week adaptation period, the mice were administered with GB by gavage at a dosage of 25, 50 or 100 mg/kg for 21 days (Fig. 2). The Morris water maze and novel object recognition tests were performed to evaluate the cognitive functions of mice. After behavioral tests, mice were sacrificed by cervical dislocation for tissue collection. The procedures used in the study were approved by The Laboratory Animal Ethics Committee of Xuzhou Medical University (No. 202010W006).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from brain tissue samples or cells using TRIzol® reagent (Takara Bio, Inc.). RNA purity was verified using a NanoDrop 2000 spectrophotometer. cDNA was synthesized using a reverse transcriptase (Takara Bio, Inc.). SYBR (Takara Bio, Inc.) was used to conduct the qRT-PCR analysis. The sequences of the primers used were as follows: IL-1β forward, 5’-TGCCACC-TTTTGACAGTGATG-3’ and reverse, 5’-AAGGTCCACGGGAAAGACAC-3’; CD68 forward, 5’-G-GGGCTCTTGGGAACTACAC-3’ and reverse, 5’-GTACCGTCACAACCTCCCTG-3’; IL-10 forward, 5’-ACTTGGGTTGCCAAGCCTTA-3’ and reverse, 5’-GACACCTTGGTCTTGGAGCTTA-3’; YM-1 forward, 5’-CAGGTCTGGCAATTCTTCTGAA-3’ and reverse, 5’-GTCTTGCTCATGTGTGTAAGTGA-3’; TGF-β forward, 5’-CATCCATGACATGAACCGGC-3’ and reverse, 5’-GAAGTTGGCATGGTAGC-CCT-3’; GAPDH forward, 5’-TGTGTCCGTCGTGGATCTGA-3’ and reverse, 5’-TTGCTGTTGAAGTCGCAGGAG-3’. The relative expression of the genes of interest were normalized to the internal control, GAPDH, according to a previous protocol (Schmittgen and Livak 2008).
ELISA
The levels of IL-1β, IL-2 and TNF-α were determined in each sample using ELISA. The samples were separately added to the wells of a 96-well plate. The ELISA procedure was carried out using the Mouse IL-1β ELISA (SEKM-0002), Mouse IL-6 ELISA kit (SEKM-0007) and Mouse TNF-α ELISA (SEKM-0034) kits, according to the manufacturer’s protocol (all from Beijing Solarbio Science & Technology Co., Ltd.). The optical density value was measured using a microplate reader (450 nm). The levels of these pro-inflammatory cytokines were calculated using a standard curve.
Immunofluorescence analysis
For immunofluorescence assays, the cells or brain sections were fixed as described previously(Al Mamun et al. 2020). The sections were then incubated with the following primary antibodies overnight at 4℃: Anti- ionized calcium-binding adapter molecule 1 (Iba-1; Wako Chemicals GmbH; 019-19741; 1:1,000), anti-CD16/32 (Invitrogen, Thermo Fisher Scientific, Inc.; PA5-47230,1:100) and anti-CD206 (ProteinTech Group, Inc.; 60143-1-Ig; 1:1,000). Subsequently, the cells and brain sections were incubated with Alexa Fluor 488 anti-mouse IgG (Invitrogen, Thermo Fisher Scientific, Inc.; A21202) and Alexa Fluor 555-conjugated goat anti-rabbit IgG (Invitrogen, Thermo Fisher Scientific, Inc.; A21432) at room temperature for another 1 h. The samples were then washed in PBS, then mounted onto glass slides. Protein expression was semi-quantified using ImageJ software (version 1.52; National Institutes of Health).
Western blotting and co-immunoprecipitation
Protein was extracted from cells or frozen mouse hippocampal tissue lysates using a commercial protein extraction kit (Elabscience Biotechnology, Inc.) according to the manufacturer’s protocol. A total of 50 µg protein from each group was transferred to PVDF membranes (MilliporeSigma), which were then blocked with 3% BSA (Beijing Solarbio Science & Technology Co., Ltd.) at 4 °C overnight. The membranes were then incubated with the following primary antibodies (all from Cell Signaling Technology, Inc.; rabbit polyclonal; 1:1,000): Anti-NLRP3 (15,101); anti-Pro-Caspase-1 (24,232); anti-Caspase-1 (89,332); anti-Pro-IL-1β (31,202); anti-IL-1β (63,124); anti-autophagy-related (Atg) 5 (12,994); anti-Atg7 (8558); anti-LC3b (2775); and anti-Sequestosome 1 (SQSTM1; 39,749). The membranes were then incubated with the anti-rabbit IgG–horseradish peroxidase (7074; Cell Signaling Technology, Inc.; 1:2000). An ECL kit (Beyotime Institute of Biotechnology) was used for visualization using an imaging system (Tanon Science and Technology Co., Ltd.).
For co-immunoprecipitation, different groups of BV2 cells or animal brain tissue samples were lysed with lysis buffer. The obtained supernatant was treated with 1 µg antibody overnight at 4 °C, then precipitated with A/G-agarose beads. Primary antibodies against ubiquitin (Ub; 3936; Cell Signaling Technology, Inc.; 1:1,000) and NLRP3 (15,101; Cell Signaling Technology, Inc.; 1:200) were used. Subsequently, the beads were further washed in lysis buffer three times by centrifugation at 3000 x g at 4 °C. Western blot analysis was then performed as aforementioned.
Morris water maze (MWM) and novel object recognition (NOR) tests
Following GB treatment, a MWM test was carried out using a 150-cm-diameter pool filled with 23±1 °C water, with a platform (13 cm in diameter) positioned 1 cm below water surface (Liu et al. 2020). The animals were trained with space-learning tasks (within 60 s) 4 times a day for 4 consecutive days. On the last day of the test, a probe test was performed for 60 s without the hidden platform. The escape latency and swimming path were recorded.
The NOR test was conducted using a 50 cm × 25 cm × 50 cm black open-field box according to a previous study, with minor amendments (Karasawa et al. 2008). The mice were placed in the test box for 5 min, together with two objects placed in the box 30-cm apart. The mice were then taken out of the box and left for 120 min in their housing cage, then placed in the test box once again for another 5 min. One familiar object and one novel object were introduced. The time spent exploring the novel object within a 5-min period was recorded. ‘Exploration behavior’ was defined as sniffing, facing or biting the object. The apparatus was cleaned with 75% ethanol between each test.
Statistical analysis
GraphPad Prism 8.0.1 software (GraphPad Software, Inc.) was used for statistical analysis and data presentation. All data are presented as the mean ± SD and were analyzed using one-way or two-way ANOVA followed by Tukey’s post hoc test. P<0.05 indicates statistical significance.
Results
Effects of GB on inflammation in LPS-challenged BV2 cells
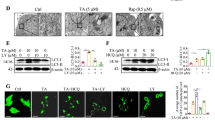
As shown in Fig. 3A, IL-1β, IL-6 and TNF-α expression levels significantly increased following LPS + ATP stimulation, compared with the control group (p < 0.01). Moreover, GB treatment (20 or 40 µM) significantly reduced the release of IL-1β, IL-6 and TNF-α in a dose-dependent manner (p < 0.01). As shown in Fig. 3B, the mRNA expression levels of the M1 microglial markers IL-1β and CD86 were significantly decreased in the GB-treated group (p < 0.01). By contrast, TGF-β, YM-1 and IL-10 mRNA expression levels were significantly increased following GB treatment (p < 0.01). Consistent with the results of qRT-PCR, the findings of immunofluorescence assays suggested that GB promoted microglia polarization from the M1 to the M2 phenotype, as GB increased the number of CD206+ cells while reducing that of CD16/32+ microglia (Fig. 3C). Altogether, these results suggested that GB treatment inactivated BV2 microglial cells and promoted a shift from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype.
Effect of ginkgolide B on inflammation in LPS-challenged BV2 cells. (A) The release of IL-1β, IL-6 and TNF-α was measured using ELISA (n=6). (B) The mRNA expression levels of M1-phenotype (IL-1β and CD68) and M2-phenotype (TGF-β, YM-1 and IL-10) microglia markers were determined using quantitative real-time PCR (n=3). (C) Immunofluorescence double-stained images showing the distribution and expression of Iba-1 (red), together with CD16/32 or CD206 (green). The fluorescence intensities of Iba-1 + CD16/32 and Iba-1 + CD206 co-staining were quantified (n=3). ##p < 0.01 vs. control; *p < 0.05, ** p < 0.01 vs. LPS + ATP group. The data are presented as the mean ± SD. Scale bar, 50 μm. IL, interleukin; Iba-1; ionized calcium-binding adapter molecule 1; LPS, lipopolysaccharide
NLRP3 inflammasome signaling is inactivated by GB in LPS-challenged BV2 cells
The expression levels of components of the NLRP3 inflammasome were then examined using western blot analysis. Following LPS and ATP stimulation, the protein levels of NLRP3 significantly increased compared with those of the control group (Fig. 4A, p < 0.01). LPS exposure also induced significant Caspase-1 activation (p < 0.01) and enhanced mature IL-1β expression (p < 0.01), whereas GB significantly decreased the protein levels of NLRP3, Caspase-1 and IL-1β relative to those of the LPS-treated group (Fig. 4A, p < 0.01).
Ginkgolide B inhibits NLRP3 inflammasome signaling in BV2 cells. (A) Representative western blot images and semi-quantification of the relative protein levels of NLRP3, Pro-Caspase-1, Caspase-1, Pro-IL-1β and IL-1β in BV2 cells. (B) Immunoblotting analysis of ubiquitin protein levels in cell lysates immunoprecipitated with NLRP3. (C) Representative images and relative protein levels of Pro-Caspase-1, Caspase-1, Pro-IL-1β and IL-1β in BV2 cells. ##p < 0.01 vs. control; *p < 0.05, ** p < 0.01 vs. LPS + ATP group. The data are presented as the mean ± SD (n=3). IL, interleukin; NLRP3, NLR family, pyrin domain containing 3; LPS, lipopolysaccharide
Ubiquitination has been reported to inhibit NLRP3 inflammasome activation (Han et al. 2019). NLRP3 polyubiquitination was measured in BV2 cell lysates using immunoprecipitation. The results suggested that GB induced NLRP3 polyubiquitination in BV2 cells (Fig. 4B). It has been documented that a variety of stimuli including ATP, nigericin, and MSU can activate the NLRP3 inflammasome (Sharif et al. 2019). To verify whether GB exclusively affected LPS-induced NLRP3 inflammasome activation, NLRP3 agonists were used in this study. As seen in Fig. 4C, GB inhibited the Caspase-1 cleavage and IL-1β secretion induced by all examined agonists, including MSU, ATP and nigericin, illustrating that GB was a broad inhibitor against NLRP3 inflammasomes. Taken together, these results indicated that GB might inhibit Caspase-1 activation and IL-1β maturation by promoting NLRP3 inflammasome degradation in vitro.
GB inhibits NLRP3 inflammasome activation via promoting NLRP3 autophagic degradation
GB-induced NLRP3 ubiquitination was inhibited by 3-MA, an autophagy inhibitor. By contrast, NLRP3 ubiquitination in GB-treated cells did not further alter with the co-administration of MG-132, a proteasome inhibitor (Fig. 5A, p < 0.01), indicating that GB triggered NLRP3 degradation via autophagy rather than ubiquitination/proteasomal degradation. Consistent with this finding, the downregulation of NLRP3, Caspase-1 and IL-1β protein expression levels in BV2 cells were abolished by 3-MA but not by MG-132 treatment (Fig. 5B, p < 0.01). Furthermore, the protein expression levels of autophagy-associated proteins were evaluated using immunoblotting. The expression levels of Atg5, Atg7 and LC3II/I were increased in BV2 cells following GB treatment, whereas those of SQSTM1 were significantly reduced, compared with the untreated groups (Fig. 5C, p < 0.05, p < 0.01). This indicated that the NLRP3 inflammasome was inactivated by GB-induced NLRP3 autophagic degradation.
GB inhibits NLRP3 inflammasome activation by promoting NLRP3 autophagic degradation. (A) Immunoblotting analysis of Ub protein levels in cell lysates immunoprecipitated with NLRP3. ##p < 0.01 vs. LPS group; **p < 0.01 vs. LPS + GB group. (B) The expression levels of NLRP3, Caspase-1 and IL-1β in cell supernatants and lysates were analyzed using immunoblotting. (C) Expression levels of Atg5, Atg7, SQSTM1 and LC3II/I following GB treatment in BV2 mouse microglia. ##p < 0.01 vs. control; * p < 0.05, **p < 0.01 vs. LPS + ATP + GB group. The data are presented as the mean ± SD (n=3). GB, ginkgolide B; IL, interleukin; NLRP3, NLR family, pyrin domain containing 3; LPS, lipopolysaccharide; Atg, autophagy-related; SQSTM1, sequestosome 1; Ub, ubiquitin
Effect of GB on learning and memory behavior in SAMP8 mice
In order to examine the effects of GB on learning and memory, a MWM test was performed. The representative swimming trajectories suggested that mice in the SAMP8 + GB group spent significantly more time on swimming in the target quadrant, compared with the SAMP8 group (Fig. 6A, p < 0.01). As shown in Fig. 6B, compared with the SAMP8 group, GB treatment (especially 100 mg/kg) significantly reduced the escape latency from the platform zone, whilst increasing the time spent in the target quadrant and the number of crossings over the platform zone (p < 0.01). These results indicated that GB significantly ameliorated the memory deficits of SAMP8 mice.
Gingkolide B improves learning and memory behavioral deficits in SAMP8 mice. (A) Representative swimming track on day 5 of the Morris water maze experiment. (B) Latency to cross the platform zone, time spent in target quadrant, and the target platform crossing number on day 5 of the Morris water maze experiment. (C) Escape latency on day 1-4 of the Morris water maze experiment. The ratio of the time spent exploring the novel object and the total time spent exploring both objects is shown as chance performance in the novel object recognition experiment. ##p < 0.01 vs. SAMR1 group, * p < 0.05, **p < 0.01 vs. SAMP8 group. The data are presented as the mean ± SD (n=10 mice/group). SAMP8, senescence-accelerated mouse prone 8; SAMR1, senescence-accelerated mouse resistant 1
Moreover, during the NOR test, SAMP8 mice treated with GB (50, 100 mg/kg) showed more preference towards the novel object than untreated SAMP8 mice, indicating that the deficit of SAMP8 mice in short-term habituation was rescued by GB (Fig. 6C, p < 0.05, p < 0.01).
GB ameliorates neuroinflammation in SAMP8 mice
The levels of pro-inflammatory cytokines in the hippocampus, including IL-1β, L-6 and TNF-α, were significantly decreased in the SAMP8 + GB group compared with the SAMP8 group (Fig. 7A, p < 0.01). Moreover, GB reduced the mRNA expression levels of M1-phenotype markers (IL-1β and CD68), while increasing those of M2 microglial markers (TGF-β, YM-1 and IL-10) in the hippocampus, suggesting that GB induced a shift from the M1 to the M2 phenotype (Fig. 7B). To confirm the neuroprotective effect of GB on hippocampal microglial cells, immunofluorescence assays were performed to assess microglial activation using Iba-1 staining (Fig. 7C). The microglial cells were strongly activated in the hippocampus of mice in the SAMP8 group. The administration of GB significantly inhibited this activation (p < 0.01).
Gingkolide B ameliorates neuroinflammation in SAMP8 mice. (A) The levels of the pro-inflammatory factors, IL-1β, IL-6 and TNF-α, in the hippocampal tissue of SAMP8 mice were examined using ELISA (n=6). (B) The mRNA expression levels of M1-phenotype (IL-1β, CD68) and M2-phenotype (TGF-β, YM-1 and IL-10) markers were determined using quantitative real-time PCR (n=3). (C) Representative images of immunofluorescence assays. Microglial activation was evaluated using Iba-1 staining (n=3). Scale bar, 50 μm. ##p < 0.01 vs. SAMR1 group, * p < 0.05, **p < 0.01 vs. SAMP8 group. The data are presented as the mean ± SD. IL, interleukin; Iba-1, ionized calcium-binding adapter molecular 1; SAMP8, senescence-accelerated mouse prone 8; SAMR1, senescence-accelerated mouse resistant 1
GB attenuates the NLRP3 inflammasome activation and induces autophagy in the brains of SAMP8 mice
The protein expression levels of markers of the NLRP3 inflammasome signaling and autophagy were examined in brain tissue using western blot analysis (Fig. 8A). The results showed that the expression levels of NLRP3, IL-1β and Caspase-1 increased in the hippocampal tissue of SAMP8 mice, but were reduced following GB treatment (p < 0.01). In addition, the levels of autophagy-associated proteins Atg5, Atg7 and LC3II/I were increased, while those of SQSTM1 decreased in SAMP8 mice following GB treatment. The effect of GB on NLRP3 ubiquitination was further examined. Consistent with the in vitro experiments, GB treatment induced NLRP3 polyubiquitination in the brain of SAMP8 mice, as determined using immunoprecipitation (Fig. 8B, p < 0.01). These results suggested that GB treatment promoted autophagy and attenuated the NLRP3 inflammasome activation via ubiquitination in hippocampus of SAMP8 mice.
GB attenuates the NLRP3 inflammasome activation and induced autophagy in the brains of SAMP8 mice. Hippocampal tissue samples from SAMP8 mice treated with GB (25, 50, 100 mg/kg/day) were used for immunoblotting. SAMR1 mice were used as control. (A) Protein expression levels of inflammatory markers and autophagy-associated proteins. (B) Immunoblotting analysis of ubiquitin protein levels in hippocampal tissue lysates immunoprecipitated with NLRP3. The data are presented as the mean ± SD (n=3). *p < 0.05, **p < 0.01 vs. SAMP8 group. GB, ginkgolide B; SAMP8, senescence-accelerated mouse prone 8; SAMR1, senescence-accelerated mouse resistant 1
Discussion
SAMP8, as well as its normal control SAMR1, is a frequently used murine model for spontaneous AD. Typically, SAMP8 mice develop cognition and behavior alterations at 4-8 months of age (Duan et al. 2020; Yang et al. 2020). Increased oxidative stress, excessive inflammation and activated microglia are found in the hippocampi of SAMP8 mice (Wang et al. 2019; Xie et al. 2020). Our in vivo experiments confirmed that GB enhanced the learning and memory abilities of SAMP8 mice.
Chronic neuroinflammation induces to the initiation and development of AD (Mamik and Power 2017; Chen et al. 2021). Excessive inflammation leads to neuronal necrosis and apoptosis, which consequently deteriorates cognition. Aβ activates microglia to generate inflammatory mediators that can trigger an inflammatory cascade. The inflammatory storm causes APP metabolism to further accelerate the progression of AD(Jiang et al. 2018). It is universally considered that activated microglia can be divided into two phenotypes: pro-inflammatory microglia (M1 phenotype) and anti-inflammatory microglia (M2 phenotype) (Chen et al. 2020). Classical M1 microglia are induced by inflammatory stimuli and signaling, which consequently produces a variety of pro-inflammatory cytokines, including reactive oxygen species, NO, TNF-α, IL-1β and IL-6 (Tang and Le 2016). By contrast, the M2 phenotype is governed by various anti-inflammatory factors. M2 microglia release anti-inflammatory cytokines, including IL-4 and IL-10, thereby contributing to immunosuppression and cognitive function improvement (Du et al. 2021). In addition, M2 microglia promote the cleavage of misfolded proteins and Aβ plaque (Tang and Le 2016). The suppression of M1 phenotype and induction of the M2 phenotype ameliorates cognitive impairment in 5 × FAD mice (Wang et al. 2020). The present study demonstrated that GB inactivated microglial cells by inhibiting the release of IL-1β, IL-6 and TNF-α. Furthermore, GB treatment could promote the shift from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype in BV2 cells.
The NLRP3 inflammasome is a macromolecular protein complex that consists of an NLRP3 scaffold, ASC and Caspase-1 precursors. The activation of the NLRP3 inflammasome controls the maturation of IL-1β and IL-18(Huang et al. 2021). NLRP3 is highly expressed in microglia and associated with multiple chronic inflammatory disorders induced by aggregated proteins, including Aβ (Yin et al. 2018). Moreover, the NLRP3 inflammasome is upregulated in post-mortem brains of patients with AD (Saresella et al. 2016). Thus, the NLRP3 inflammasome cascade functions as an essential modulator in AD pathology, suggesting that NLRP3 inflammasome may represent a therapeutic target for AD intervention. The activation of the NLRP3 inflammasome converts Pro-Caspase-1 into active Caspase-1, which induces the generation of the pro-inflammatory cytokines IL-18 and IL-1β (Sun et al. 2019). Consequently, the inflammatory downstream cascade is activated to accelerate inflammation and induce neuronal injury (Hou et al. 2020). In the present study, GB markedly inhibited the expression of the NLRP3 inflammasome, Caspase-1 and IL-1β in LPS-challenged BV2 cells and SAMP8 mice.
Autophagy and ubiquitination/proteasome degradation are crucial events in the cleavage of damaged proteins. E3 ubiquitin ligases recognize inflammatory substrates and cause poly-ubiquitination. A previous study has demonstrated that autophagy negatively modulated NLRP3 inflammasome activity (Demishtein et al. 2017). As GB downregulated NLRP3, is was assumed that NLRP3 might be degraded by autophagy or ubiquitination. Therefore, the autophagy inhibitor 3-MA and proteasome inhibitor MG-132 were both used in order to determine the mechanism underlying GB triggered NLRP3 degradation. The results demonstrated that NLRP3 inactivation effect was reduced following treatment with the autophagy inhibitor 3-MA, which suggested that GB-driven NLRP3 inflammasome inhibition and NLRP3 degradation was dependent on autophagy activation.
To conclude, GB significantly improved the learning and memory defects in SAMP8 mice by reducing the Caspase 1 activation and IL-1β maturation via the NLRP3 pathway. In addition, autophagy might be involved in the inactivation of the NLRP3 inflammasome. GB promoted autophagy and ubiquitination of NLRP3, both in the hippocampus of SAMP8 mice and LPS-induced BV2 cells. Moreover, GB regulated the balance between the M1 and M2 phenotypes of microglia. Therefore, GB might alleviate neuroinflammation and improve cognitive function in patients with AD. However, additional studies must be conducted to determine whether GB could be used in clinical practice.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R et al (2020) Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci USA 117(3):1742–1752. https://doi.org/10.1073/pnas.1914742117
Birkenhager TK, van Diermen L (2020) Electroconvulsive therapy: we are hesitant to use the most effective treatment for severe depression. Acta Psychiatr Scand 141(4):301–303. https://doi.org/10.1111/acps.13171
Butterfield DA, Poon HF (2005) The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol 40(10):774–783. https://doi.org/10.1016/j.exger.2005.05.007
Chen T, Zheng M, Li Y, Liu S, He L (2020) The role of CCR5 in the protective effect of Esculin on lipopolysaccharide-induced depressive symptom in mice. J Affect Disord 277:755–764. https://doi.org/10.1016/j.jad.2020.08.065
Chen T, Liu S, Zheng M, Li Y, He L (2021) The effect of geniposide on chronic unpredictable mild stress-induced depressive mice through BTK/TLR4/NF-κB and BDNF/TrkB signaling pathways. Phytother Res 35(2):932–945. https://doi.org/10.1002/ptr.6846
Dai W, Wang X, Teng H, Li C, Wang B, Wang J (2019) Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int Immunopharmacol 66:215–223. https://doi.org/10.1016/j.intimp.2018.11.029
Demishtein A, Fraiberg M, Berko D, Tirosh B, Elazar Z, Navon A (2017) SQSTM1/p62-mediated autophagy compensates for loss of proteasome polyubiquitin recruiting capacity. Autophagy 13(10):1697–1708. https://doi.org/10.1080/15548627.2017.1356549
Diaz-Perdigon T, Belloch FB, Ricobaraza A, Elboray EE, Suzuki T, Tordera RM et al (2020) Early sirtuin 2 inhibition prevents age-related cognitive decline in a senescence-accelerated mouse model. Neuropsychopharmacology 45(2):347–357. https://doi.org/10.1038/s41386-019-0503-8
Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C et al (2020) Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol 19(1):61–70. https://doi.org/10.1016/s1474-4422(19)30393-x
Du Y, Luo M, Du Y, Xu M, Yao Q, Wang K et al (2021) Liquiritigenin decreases Aβ levels and ameliorates cognitive decline by regulating microglia M1/M2 transformation in AD mice. Neurotox Res 39(2):349–358. https://doi.org/10.1007/s12640-020-00284-z
Duan R, Xue X, Zhang QQ, Wang SY, Gong PY, E Y et al (2020) ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging 12(14):14819–14829. https://doi.org/10.18632/aging.103544
Feng YS, Tan ZX, Wu LY, Dong F, Zhang F (2020) The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res Rev 64:101192. https://doi.org/10.1016/j.arr.2020.101192
Gargouri B, Carstensen J, Bhatia HS, Huell M, Dietz GPH, Fiebich BL (2018) Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 44:45–55. https://doi.org/10.1016/j.phymed.2018.04.009
Han X, Sun S, Sun Y, Song Q, Zhu J, Song N et al (2019) Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy 15(11):1860–1881. https://doi.org/10.1080/15548627.2019.1596481
Hashiguchi M, Ohta Y, Shimizu M, Maruyama J, Mochizuki M (2015) Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J Pharm Health Care Sci 1:14. https://doi.org/10.1186/s40780-015-0014-7
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678. https://doi.org/10.1038/nature11729
Hou Z, Qiu R, Wei Q, Liu Y, Wang M, Mei T et al (2020) Electroacupuncture improves cognitive function in senescence-accelerated P8 (SAMP8) mice via the NLRP3/Caspase-1 pathway. Neural Plast 2020:8853720. https://doi.org/10.1155/2020/8853720
Huang X, Shen H, Liu Y, Qiu S, Guo Y (2021) Fisetin attenuates periodontitis through FGFR1/TLR4/NLRP3 inflammasome pathway. Int Immunopharmacol 95:107505. https://doi.org/10.1016/j.intimp.2021.107505
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A et al (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575(7784):669–673. https://doi.org/10.1038/s41586-019-1769-z
Jia Y, Cao N, Zhai J, Zeng Q, Zheng P, Su R et al (2020) HGF mediates clinical-grade human umbilical cord-derived mesenchymal stem cells improved functional recovery in a senescence-accelerated mouse model of Alzheimer’s disease. Adv Sci (Weinh) 7(17):1903809. https://doi.org/10.1002/advs.201903809
Jiang J, Ding N, Wang K, Li Z (2018) Electroacupuncture could influence the expression of IL-1β and NLRP3 inflammasome in hippocampus of Alzheimer’s disease animal model. Evid Based Complement Alternat Med 2018:8296824-8296824. https://doi.org/10.1155/2018/8296824
Jin X, Liu MY, Zhang DF, Zhong X, Du K, Qian P et al (2019) Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci Ther 25(5):575–590. https://doi.org/10.1111/cns.13086
Karasawa J, Hashimoto K, Chaki S (2008) D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res 186(1):78–83. https://doi.org/10.1016/j.bbr.2007.07.033
Liu S, Zheng M, Li Y, He L, Chen T (2020) The protective effect of Geniposide on diabetic cognitive impairment through BTK/TLR4/NF-kappaB pathway. Psychopharmacology 237(2):465–477. https://doi.org/10.1007/s00213-019-05379-w
Mamik MK, Power C (2017) Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain 140(9):2273–2285. https://doi.org/10.1093/brain/awx133
Mohamed NE, Abd El-Moneim AE (2017) Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition 35:93–99. https://doi.org/10.1016/j.nut.2016.10.012
Qi Y, Cheng X, Jing H, Yan T, Xiao F, Wu B et al (2019) Combination of schisandrin and nootkatone exerts neuroprotective effect in Alzheimer’s disease mice model. Metab Brain Dis 34(6):1689–1703. https://doi.org/10.1007/s11011-019-00475-4
Qin Y, Zhang Y, Tomic I, Hao W, Menger MD, Liu C et al (2018) Ginkgo biloba extract EGb 761 and its specific components elicit protective protein clearance through the autophagy-lysosomal pathway in tau-transgenic mice and cultured neurons. J Alzheimers Dis 65(1):243–263. https://doi.org/10.3233/jad-180426
Salminen A, Kaarniranta K, Kauppinen A (2012) Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging 4(3):166–175. https://doi.org/10.18632/aging.100444
Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E et al (2016) The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener 11:23. https://doi.org/10.1186/s13024-016-0088-1
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q et al (2019) Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570(7761):338–343. https://doi.org/10.1038/s41586-019-1295-z
Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF (2003) Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol 184(1):510–520. https://doi.org/10.1016/s0014-4886(03)00399-6
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z et al (2019) Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis 10(8):542. https://doi.org/10.1038/s41419-019-1761-4
Tan MS, Yu JT, Tan CC, Wang HF, Meng XF, Wang C et al (2015) Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis 43(2):589–603. https://doi.org/10.3233/JAD-140837
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H (2006) The GuidAge study: methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761 for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology 67(9 Suppl 3):S6–11. https://doi.org/10.1212/wnl.67.9_suppl_3.s6
Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B et al (2012) Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol 11(10):851–859. https://doi.org/10.1016/S1474-4422(12)70206-5
Vitolo O, Gong B, Cao Z, Ishii H, Jaracz S, Nakanishi K et al (2009) Protection against beta-amyloid induced abnormal synaptic function and cell death by Ginkgolide J. Neurobiol Aging 30(2):257–265. https://doi.org/10.1016/j.neurobiolaging.2007.05.025
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Wang Y, Wang Q, Li J, Lu G, Liu Z (2019) Glutamine improves oxidative stress through the Wnt3a/beta-catenin signaling pathway in Alzheimer’s disease in vitro and in vivo. Biomed Res Int 2019:4690280. https://doi.org/10.1155/2019/4690280
Wang Y, Lin Y, Wang L, Zhan H, Luo X, Zeng Y et al (2020) TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging 12(20):20862–20879. https://doi.org/10.18632/aging.104104
Xie Z, Lu H, Yang S, Zeng Y, Li W, Wang L et al (2020) Salidroside attenuates cognitive dysfunction in senescence-accelerated mouse prone 8 (SAMP8) mice and modulates inflammation of the gut-brain axis. Front Pharmacol 11:568423. https://doi.org/10.3389/fphar.2020.568423
Yan Y, Yang H, Xie Y, Ding Y, Kong D, Yu H (2020) Research progress on Alzheimer’s disease and resveratrol. Neurochem Res 45(5):989–1006. https://doi.org/10.1007/s11064-020-03007-0
Yang Y, Li S, Huang H, Lv J, Chen S, Dias P et al (2020) Comparison of the protective effects of ginsenosides Rb1 and Rg1 on improving cognitive deficits in SAMP8 mice based on anti-neuroinflammation mechanism. Front Pharmacol 11:834. https://doi.org/10.3389/fphar.2020.00834
Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S et al (2018) NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol Neurobiol 55(3):1977–1987. https://doi.org/10.1007/s12035-017-0467-9
Yin JJ, He Y, An J, Miao Q, Sui RX, Wang Q et al (2019) Dynamic balance of microglia and astrocytes involved in the remyelinating effect of ginkgolide B. Front Cell Neurosci 13:572. https://doi.org/10.3389/fncel.2019.00572
Zhang Y, Liu J, Yang B, Zheng Y, Yao M, Sun M et al (2018) Ginkgo biloba extract inhibits astrocytic lipocalin-2 expression and alleviates neuroinflammatory injury via the JAK2/STAT3 pathway after ischemic brain stroke. Front Pharmacol 9:518. https://doi.org/10.3389/fphar.2018.00518
Zhang Y, Dong Z, Song W (2020) NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct Target Ther 5(1):37. https://doi.org/10.1038/s41392-020-0145-7
Funding
This work was supported by the fifth phase of “Project 333” Scientific Research Project Funding Plan of Jiangsu Province (BRA2019238), Medical Key Youth Talents Training Project of Jiangsu Province (QNRC2016367), Xuzhou Science and Technology Plan Project (KC19020) and Science and Technology Development Fund Project of Xuzhou Medical University Affiliated Hospital (XYFM2020024).
Author information
Authors and Affiliations
Contributions
LX and CD were involved with the experimental design and analyzed data; QH and YS performed the animal research; LX performed the other research and wrote the manuscript; DG provided technical support, experimental supervision, and the final revision of the manuscript. All authors have read and approved final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
All animal procedures were in accordance with the Medical Ethics Committee of Xuzhou Medical University (Jiangsu, China).
Consent for publication
We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, L., Dong, C., Geng, D. et al. Ginkgolide B inactivates the NLRP3 inflammasome by promoting autophagic degradation to improve learning and memory impairment in Alzheimer’s disease. Metab Brain Dis 37, 329–341 (2022). https://doi.org/10.1007/s11011-021-00886-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00886-2