Abstract
The night shift paradigm induces a state of chronic partial sleep deprivation (CPSD) and enhances the vulnerability to neuronal dysfunction. However, the specific neuronal impact of CPSD has not been thoroughly explored to date. In the current study, the night shift condition was mimicked in female Swiss albino mice. The classical sleep deprivation model, i.e., Modified Multiple Platform (MMP) method, was used for 8 h/day from Monday to Friday with Saturday and Sunday as a weekend off for nine weeks. Following nine weeks of night shift schedule, their neurobehavioral profile and physiological parameters were assessed along with the activity of the mitochondrial complexes, oxidative stress, serotonin levels, and inflammatory markers in the brain. Mice showed an overall hyperactive behavioral profile including hyperlocomotion, aggression, and stereotyped behavior accompanied by decreased activity of mitochondrial enzymes and serotonin levels, increased oxidative stress and inflammatory markers in whole brain homogenates. Collectively, the study points towards the occurrence of a hyperactive behavioral profile akin to mania and psychosis as a potential consequence of CPSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep, an evolutionarily conserved behavior throughout the animal kingdom, is fundamental for healthy physiological functions. The altered lifestyle of professionals such as nurses, doctors, security personnel, and call center workers have disturbed sleep cycles resulting in chronic partial sleep deprivation (CPSD) (Carey et al. 2011). These shift workers are prone to bad health and diseases, including cardiovascular complications, metabolic syndrome, gastrointestinal dysfunction, increased risk of cancer and hormonal imbalance (James et al. 2017). They are also more likely to suffer from more mental health complications such as depression, anxiety, alcohol abuse, poorer psychosocial life (Brown et al. 2020). Due to poorer neurocognition, shift workers are reported to have impaired vigilance and attention at the workplace and outside, resulting in increased road and workplace accidents (Ftouni et al. 2013; Rajaratnam et al. 2013). These not only affect the shift worker but also increases the health burden on the government. In comparison to the increasing number of people subjected to CPSD, relatively few clinical and preclinical studies have been carried out to delineate its neurological impact. Thus, more studies are needed to increase our understanding of how CPSD affects human health and disease.
The clinical studies have reported that chronic sleep deprivation (CSD) results in disordered cognitive functioning (Reynolds and Banks 2010), impaired alertness and trigger mania in bipolar patients (Lewis et al. 2017) and increases the incidence of major depression in young women (Conklin et al. 2018).
On the contrary, a large pool of studies exist depicting the neuronal effects of acute sleep deprivation (ASD) (Villafuerte et al. 2015). Acute sleep deprivation results in anxiety in humans (Pires et al. 2016), impaired memory (Graves 2003; Vecsey et al. 2009) and mania-like behavior (Kanazawa et al. 2016; Valvassori et al. 2017). Another study reported that ASD induces anxiety-like behavior and depression-like behavior in female mice (Gonzalez-Castañeda et al. 2016).
The existing CPSD studies included different approaches such as sleep-depriving the animals for 4 h for four weeks by using environmental noise and reported that CPSD resulted in increased plasma inflammatory biomarkers and altered clock genes expression (Zou et al. 2017). Using a forced locomotion approach, Manchanda et al. (2018) reported that CPSD resulted in anxiogenic behavior along with impaired recognition memory as assessed by elevated plus maze (EPM) and novel object recognition (NOR), respectively (Manchanda et al. 2018). The protocol employed 18 h of CPSD for 21 days. Further, CPSD-induced neuroinflammation was reported to underlie the observed behavioral deficits (Manchanda et al. 2018). Studies conducted by Mhaidat et al. (2015) and Alzoubi et al. (2012) reported that CPSD resulted in impaired spatial learning and memory assessed by using radial arm water maze along with increased oxidative stress (Alzoubi et al. 2012; Mhaidat et al. 2015). In these studies, CPSD was induced using the modified multiple platform (MMP) method for 8 h per day for six weeks.
However, these studies have not included a sleep recovery period, which has been suggested to play a significant role in combating the effects of accumulated sleep debt (Belenky et al. 2003). Clinically, night shift workers and other professionals get weekends or certain other days off, which acts as a sleep recovery period (Malmberg et al. 2010). The inclusion of a sleep recovery period is thus necessary to increase the translational potential of CPSD studies. As per the literature, there are four approaches to mimic human shiftwork in rodents by altering activity, sleep, food, and light timing (Opperhuizen et al. 2015). The current study design employed the altered “Timing of sleep” approach. As mice are nocturnal animals, the sleep deprivation period of 8 h in the light period during the weekdays was chosen.
Moreover, the weekend off was given as a sleep recovery period to mimic a weekly night shift schedule. We chose the MMP method for sleep deprivation because it offers benefits over the traditional flowerpot method of sleep deprivation. MMP excludes the effects of social isolation and immobility stress, as a group of animals together can be sleep deprived and the connecting rods between the platforms allow the animals to ambulate (Suchecki and Tufik 2000). Furthermore, the reported electroencephalographic results showed that this model is capable of disrupting rapid eye movement (REM) sleep along with slow-wave sleep (SWS) (Machado et al. 2004). Using the MMP method for sleep deprivation for 96 h, Machado et al. reported that the model abolished the REM sleep completely and reduced the SWS by 31 %. In another study, animals were sleep deprived for 20 h per day for 10 days using the MMP method. The EEG data showed that REM sleep was completely abolished, while non-REM sleep was significantly decreased in sleep-deprived animals (Gomez-Gonzalez et al. 2013). For the control group, homecage condition was preferred over wide platforms as previous studies have reported that animals on wide platforms in MMP had similar behavioral and neurobiological observations as homecage control animals (Alzoubi et al. 2012). The experiment duration of nine weeks was decided based on a pilot titration study that we carried out in our lab where the longer period of sleep deprivation resulted in mortality due to a drastic decrease in the body weight. Holistically, the model generated an environmental condition of CPSD with an intermittent recovery period to which the night shift workers are exposed.
According to epidemiological studies, females are at higher risk of developing neuronal disorders such as depression, psychiatric complications due to sleep deprivation as compared to males (King 1967; Stranges et al. 2012; Santhi et al. 2016; Salim 2017). Sleep deprivation disturbs the estrous cycle and hormonal milieu in females (Lindseth et al. 1980; Santhi et al. 2016). Conversely, hormonal alterations during the estrous cycle impact sleep regulation in females, depending upon the animal strain (Koehl et al. 2003). Further, the reported impact of the estrous cycle on cognition is not clear. It has been reported that the estrous cycle affect learning and memory in various tasks. Monfort et al. (2015) reported that male rats performed better than females in spatial learning and memory tasks, radial arm maze and MWM (Monfort et al. 2015). In an another study, mice in proestrous and estrous phases performed better in acquisition and recognition phases of the object recognition task than mice in metaestrous and diestrous phases (Cordeira et al. 2018). On the contrary, other studies reported that estrous cycle had little to no impact on learning and memory in spatial tasks. Healy et al. (1999) reported no difference in the performance of male and females rats in MWM (Healy et al. 1999). Similarly, Berry et al. (1997) reported that estrous and proestrous rats performed similarly in a modified version of MWM (Berry et al. 1997). These findings are further supported by data from Rodriguez et al (2011), which found that rats in different phases of the estrous cycle had similar performance in a modified version of the MWM (Rodríguez et al. 2011). Due to the inconsistencies in data reported in above mentioned studies, females are underrepresented in circadian and sleep deprivation studies. Therefore, female mice were used in the current study.
Due to the reported effects of sleep deprivation, including anxiety, mania, depression and impaired memory in above mentioned clinical and preclinical studies, we conducted a battery of behavioral tests to explore the effects of CPSD protocol on these behavioral aspects. Since the complexity of depression cannot be assessed by a single test, such as the forced swim test, we preferred using the sucrose preference test along with it. For the assessment of locomotor activity, an open field test and actophotometer were used. Along with it, aggressive and stereotyped behavior were also assessed. Anxiety-like behavior was assessed using an elevated zero maze. Due to the lack of consistency in the existing literature on the effect of sleep deprivation on memory in females, as discussed above, the current study employed three paradigms for assessment of learning and memory: MWM for spatial learning, NOR for recognition memory and EPM for spatial long term memory.
The mechanisms underlying the reported behavioral and physiological alterations due to ASD and CPSD include mitochondrial dysfunction (Andreazza et al. 2010a), increased oxidative stress (Alzoubi et al. 2012; Mhaidat et al. 2015; Villafuerte et al. 2015) accompanied by increased neuro-inflammation (Valvassori et al. 2017; Manchanda et al. 2018) and altered regulation of the serotonergic system (Bjorvatn et al. 2002). Due to the importance of these mechanisms in sleep deprivation-induced behavioral and physiological alterations, we included the above parameters along with a battery of behavioral tests in our current study.
Therefore, the present study was designed to mimic the CPSD condition of night shift work with weekends off as an intermittent sleep recovery period in mice. The mice were further analyzed for their behavioral profile, physiological parameters, brain mitochondrial functioning, oxidative stress, inflammatory markers, andserotonin levels.
Experimental procedures
Chemicals
All the chemicals and reagents used in the preparation of buffers for biochemical assays were of analytical grade. Ethylenediaminetetraacetic acid, Ethylene glycol-bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, Ellman’s reagent were procured from Himedia (Mumbai, India), Nitro blue tetrazolium chloride, Hydroxylamine hydrochloride, Trichloroacetic acid, and Thiobarbituric acid were obtained from Sigma-Aldrich (St. Louis, USA). Octylphenoxypolyethoxyethanol (IGEPAL CA 360) was purchased from MP Biomedicals (California, USA).
Animals
Female Swiss albino mice (n = 40) of age 12 weeks were procured from the Central Animal House of Panjab University, Chandigarh. The animals were housed under standard laboratory conditions (21 ± 2 °C temperature; 55 % relative humidity) 12:12 h light-dark cycle (lights on at 0600 h and off at 1800 h), with ad libitum access to normal chow diet and water. The experimental protocol was approved by the Institutional Animal Ethics Committee of Panjab University (PU/IAEC/S/16/16) and conducted by following the guidelines of the Committee for Control and Supervision of Experiments on Animals, India. All experiments were carried out between 0900 h and 1700 h during the light period. Mice were randomly divided into two groups and were assigned to CPSD (n = 20) and control conditions (n = 20). CPSD animals were exposed to the sleep deprivation paradigm and control animals were housed in their homecages under standard laboratory conditions.
Sleep deprivation paradigm
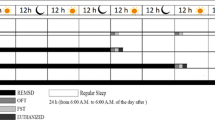
The Modified Multiple Platform (MMP) method of sleep deprivation was used in the study (Patti et al. 2010). In MMP, animals were kept in a water tank (90 × 90 × 30 cm) having 36 multiple platforms (3 cm diameter), surrounded by water up to 1 cm beneath the platforms, which were connected through stainless steel rods (0.5 cm diameter). Animals of the CPSD group were kept in the water tank for eight hours from 0900 h to 1700 h during the light period from Monday to Friday, with the weekend as the recovery period for nine weeks. The mice underwent a battery of behavioral tests in the ninth week. To facilitate behavioral testing in the last week, the study protocol was modified and included four hours of SD (0900 h to 1300 h) and four hours of behavioral tests (1300 h to 1700 h) for the first five days. Over the weekend, only behavioral assessment was continued and then animals were returned to their homecages for sleep recovery. The tests were planned and conducted to avoid the confounding effects of one task to another, as given in Fig. 1. Therefore, animals of each group (n = 20) were further divided into two groups having n = 10 and were assessed for respective behavioral tests. Ad libitum food and water were made available through a grid placed on top of the water tank and available in their homecages. Animals of the control group were kept in their home cages under 12:12 h light-dark standard conditions.
a Diagrammatic representation of the experimental protocol. SPT: sucrose preference test; ZM: zero maze, Acto: actophotometer; FST: forced swim test; OFT: open field test; CBBT: cotton bud biting test; NOR: novel object recognition; EPM: elevated plus maze; MWM: Morris water maze; GSH: reduced glutathione; SOD: superoxide dismutase; LPO: lipid peroxidation. b Layout of the modified multiple platform apparatus for sleep deprivation. C. Side view of the modified multiple platform apparatus
Neurobehavioral assessments
Assessment of spontaneous locomotor activity
Actophotometer
Animals were assessed for their spontaneous locomotor activity in a digital actophotometer (IMCORP, Ambala, India), which consisted of a dark painted square arena (35 × 35 × 18 cm), equipped with 12 infrared light-sensitive photocells arranged in two rows (six each) to assess ambulatory and rearing counts. Mice were individually placed in the chamber for initial 2 minutes of familiarization followed by digital scoring for 10 min. Locomotor activity was expressed in terms of number of total photo beam breaks for 10 min per mouse as ambulatory and rearing counts (Rabbani et al. 1994).
Open field test
The circular open field arena (40 cm diameter and 35 cm height) made up of wood and painted in black was used. The mice were individually recorded for their horizontal locomotor activity with a video tracking system (EthoVision Program, Version 3.1, Noldus, Netherlands) for 10 minutes (Bansal et al. 2018). The mice were assessed for the pathlength covered,number of line crossings, and rearing.
Assessment of anxiety
Elevated zero maze
Zero maze offers the benefit of uninterrupted exploration of the maze (Singh et al. 2007). The maze consisted of an elevated black ring platform (35 cm diameter) with adjacent two open and two closed compartments (5.5 cm runway width) of similar length (30 cm). Animals were placed in one of the closed compartments. The total number of entries and time spent in the open and closed compartments were assessed with a video tracking system (EthoVision Program, Version 3.1, Noldus, Netherlands) for 10 minutes.
Assessment of stereotyped behavior
Mice were observed for their stereotyped behavior for 30 minutes immediately after returning to the home cages at the end of the eighth week. The stereotypic behaviors such as jumping, popping, rearing, licking, and grooming were considered for scoring by following the previously described method (Dhir et al. 2010) with modification of scoring criteria, as discussed below. Here, popping refers to sudden jerky movements of the whole animal and not just its limbs. Licking refers to the licking of the cage walls by the animal. A modified way of scoring was used and the magnitude of behavioral phenotype was assessed while counting the frequency of behavioral phenotype and scored as: 0 = absence of behavioral phenotype; 1 = mice exhibited behavioral phenotype 1–5 times, considered as the presence of behavior; 2 = mice exhibited behavioral phenotype 6–10 times, considered as mild; 3 = mice exhibited behavioral phenotype 11–15 times, considered as moderate; 4 = mice exhibited behavioral phenotype 16–20 times, considered as severe; 5 = mice exhibited behavioral phenotype ≥ 21, considered as intense.
Assessment of depression
Forced swim test
It is also known as Porsolt’s forced swim test. The mice were individually placed and trained in the cylindrical glass jar of diameter 16.5 cm containing water up to 7 inches, and temperature maintained at 25 °C a day before the test period. The immobility period was calculated from the last 4 minutes of the 6-minute video recording (Bansal et al. 2018).
Sucrose preference test
For assessing anhedonia-like behavior, mice were given free access to two bottles containing 1 % sucrose solution and drinking water respectively for 12 h during the dark period. Animals were acclimatized to two bottles a day before the test period. The water consumption, sucrose solution and total liquid intake were calculated by measuring the volume before and after the test period. Percentage preference for sucrose was calculated. A sucrose preference at or lower than 65 % was taken as an anhedonia criterion (Strekalova and Steinbusch 2010).
Assessment of memory
Morris water maze
Spatial memory was assessed in the last week of the study using the morris water maze (MWM) (Sachdeva et al. 2018). MWM consisted of a circular pool (100 cm diameter) filled with water (24–25 °C), having an invisible escape platform submerged in water in the target quadrant. The maze was surrounded by visual cues for spatial orientation whose location was fixed throughout the experiment. The mean escape latency (time taken to reach the platform) was measured during the four days of MWM. During the acquisition, the animals received four trials per day of 60 seconds to locate the hidden platform.
Elevated plus-maze
The EPM consisted of two open and two closed arms and was used to evaluate memory acquisition and retention (Sachdeva et al. 2018). Mice were placed in the open arm facing outwards, and then the time taken by the animal to reach either of the closed arms was recorded as the initial transfer latency, a measure of spatial learning. A maximum of 90 s threshold period was given; otherwise, the mice were guided to reach the closed arm and were allowed to explore the closed arm for 30 seconds. The retention of this task was performed 24 h after the acquisition trial. Transfer latency recorded during the retention trial was recorded as retention latency, a measure of spatial memory.
Novel object recognition
The circular open field arena (40 cm diameter and 35 cm height) made of wood and painted black was used (Leger et al. 2013). The mice were given the habituation of 10 min in the open field arena. Subsequently, after 24 h, the mice were given the familiarization to similar objects for 10 min. Two plastic flowers were chosen as similar objects. After 24 h, one of the similar objects was replaced with a novel object, i.e., a mini tape dispenser. The position of both objects was kept the same as during the familiarization to similar objects and the mice were allowed to explore the objects for 5 min. The objects used in the study were of the same size and material but had different shapes and colors. The object exploration time, defined as the duration of time spent by the animal while directing its nose towards the object at a distance less than or equal to 2 cm, or sniffing or touching the object excluding standing or sitting on the object, were assessed using a video tracking system (EthoVision Program, Version 3.1, Noldus, Netherlands). The percent recognition index was calculated as follows:
Assessment of aggression
Cotton bud biting
Mice were tested for their aggressive behavior using a sterile cotton bud held against the face of the animal while holding the animal in other hand. The cut off time was set to 5 seconds. The number of violent bud biting attacks were counted as a marker of aggressive behavior (Park et al. 2015).
Physiological parameters
Body weight, food intake, water intake, and rectal temperature were observed once every week throughout the experiment. Rectal temperature was measured every Friday at 1800 h. The food intake, water intake and body weight were assessed every Saturday at 1800 h.
Biochemical estimations
Blood sample collection and brain samples preparation
The next day following the behavioral battery of tests, mice were anesthetized using thiopental sodium (50 mg/kg; i.p.). The serum and brain samples were collected from 0900 h to 1200 h. The conditions and timings for sample collection were kept the same for the control group. Blood samples were collected from retro-orbital plexus before euthanasia and centrifuged at 800 x g/10 min at 4 °C for the separation of serum. Following decapitation, the whole brains were isolated. The collected serum and brain samples were stored at -80 °C prior to their use in biochemical assays. The isolated brains were homogenized in the homogenization buffer containing 10 mM Tris, 1 mM EDTA, 1 mM EGTA, 150 mM MgCl2, 1 % IGEPAL CA 630. The homogenates were centrifuged (4000 g/20 min at 4 °C) and the supernatant was collected for the assessment of oxidative stress parameters.
Estimation of protein
The protein concentration of each sample was estimated as per the Lowry method using bovine serum albumin to prepare a standard plot (Lowry et al. 1951).
For biochemical estimations, the Beer-Lambert law was followed to calculate the concentration from the optical density using molar extinction coefficient values.
Superoxide dismutase
The cytosolic superoxide dismutase (SOD) assay was performed described by Kono (Kono 1978). This enzymatic inhibition assay involved the addition of the brain homogenate (0.005 ml) and hydroxylamine hydrochloride having pH 6.0 (50 µl) to the 2 ml of reagent mixture (96 mM Nitro blue tetrazolium + 50 mM sodium carbonate + 0.1 mM EDTA). The activity of SOD was observed as auto-oxidation of hydroxylamine at 560 nm for 2 min at the interval of 30 seconds and expressed as SOD units per mg protein.
Catalase
The assay of catalase was performed following a modified Claiborne method (Sachdeva et al. 2018). 1.95 ml of phosphate buffer (0.05 M, pH 7.0), 1.0 ml of hydrogen peroxide (0.019 M) was added to 0.05 ml of supernatant. The change in optical density was measured at 240 nm for 2 min at an interval of 30 seconds. Using extinction coefficient 43.1 M− 1 cm− 1, the activity of catalase was calculated and expressed as K min− 1 per mg of protein.
Reduced glutathione
Reduced glutathione (GSH) was assessed as per the method of Jollow et al. (1974). Briefly, 1 % sulphosalicylic acid was added to precipitate the brain homogenate (1 ml) followed by 60 min incubation at 4 °C. The precipitated samples were centrifuged at 1200 g for 15 min at 4 °C to obtain a clear supernatant. The assay mixture consisted of 0.1 ml of supernatant, 0.2 ml of 0.01 M Ellman’s reagent, and 2.7 ml of phosphate buffer (0.1 M, pH 7.4) and the reaction mixture developed yellow color. The optical density of the mixture was measured at 412 nm (Jollow et al. 1974). The concentration was calculated from observed optical density using extinction coefficient 1.36 × 104 M−1 cm−1 and results were expressed as µ moles of GSH per mg of protein.
Lipid peroxidation
Lipid peroxidation was assessed by measuring the levels of malondialdehyde in the brain homogenates according to the Wills method (Wills 1966). Briefly, the assay procedure included the addition of 0.5 ml of tris hydrochloride to the same volume of brain homogenate followed by incubation at 37 °C for 2 h. 1 ml of trichloroacetic acid was added to the reaction mixture and centrifuged at 1000 g for 10 min. Equal volumes of supernatant (1 ml) and 0.67 % thiobarbituric acid were mixed and kept in boiling water for 10 min. The samples were allowed to cool, and 1 ml of double-distilled water was added, and optical density was measured at 532 nm. Thiobarbituric acid reactive species were measured using extinction coefficient of 1.56 × 105 M− 1 cm− 1 and expressed as nanomoles per mg of protein.
Mitochondrial enzyme complex estimations
The whole brain was homogenized for mitochondrial complex estimations according to the method of Berman and Hastings in isolation buffer containing 215 mM mannitol, 75 mM sucrose, 0.1 % w/v bovine serum albumin, 20 mM HEPES buffer and 1 mM of ethylene glycol tetraacetic acid (EGTA) in 100 ml of distilled water and pH was adjusted to 7.2 with potassium hydroxide. The brain homogenates were centrifuged at 13,000 g for 5 minutes at 4 °C to obtain a pellet which was resuspended in isolation buffer with EGTA and centrifuged again at 13,000 g for 5 minutes at 4 °C. The supernatant was transferred to microcentrifuge tubes and topped off with isolation buffer with EGTA and centrifuged at 13,000 g for 10 minutes at 4 °C. The obtained pellet was resuspended in buffer without EGTA and further centrifuged at 10,000 g for 10 minutes at 4 °C. Finally, pellet free of contamination was dissolved in 0.2 ml of isolation buffer without EGTA and used for further analysis (Berman and Hastings 1999).
Complex I (NADH dehydrogenase activity)
NADH dehydrogenase was estimated by the spectrophotometric method of King and Howard (1967). The method involves the determination of the rate of NADH dependent reduction of cytochrome-c. The reaction was initiated by addition of mitochondrial sample in a mixture containing 0.2 M glycylglycine buffer with pH 8.0, 6 mM NADH in 2 mM glycylglycine buffer and 10.5 mM cytochrome-c. The change in absorbance for cytochrome-c was measured at 550 nm for 2 min. The complex activity was assessed using extinction coefficient, 19.6 mM− 1 cm− 1 and results were expressed as nanomoles of NADH oxidized/min/mg protein (King and Howard 1967).
Complex II (succinate dehydrogenase activity)
Complex II activity was estimated by the method of King (1967). The reaction was initiated by the addition of isolated mitochondrial sample in the mixture containing sodium phosphate buffer pH 7.8, 1 % bovine serum albumin, 0.6 M succinic acid, 0.03 M potassium ferricyanide. The change in absorbance for ferricyanide was measured at 420 nm for 180 s. The enzyme activity was assessed using extinction coefficient, 1040 M− 1 cm− 1, and results were expressed as nanomoles of succinate oxidized/min/mg protein.
Complex III (mitochondrial redox activity)
This is an indirect method to evaluate the mitochondrial redox activity via the conversion of Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) to formazan crystals by mitochondrial respiratory chain reactions in isolated mitochondria. The formed formazan crystals were dissolved in DMSO, and the developed color was measured at 580 nm (Liu et al. 1997).
Complex IV (Cytochrome-c oxidase)
The activity of cytochrome-c oxidase was assayed following the method developed by Sottocasa and his coworkers (Sottocasa et al. 1967). The change in absorbance was observed by the addition of isolated mitochondrial sample due to oxidation of previously reduced cytochrome C (by the addition of a few crystals of sodium borohydride) at 550 nm for 180 seconds. The complex activity was assessed using extinction coefficient, 29.5 mM− 1 cm− 1, and expressed as nanomoles per minute per mg of protein.
Estimation of serotonin using HPLC
The whole-brain homogenates were used to estimate serotonin levels using an electrochemical detector (Singh et al. 2017). The whole brain was homogenized in ice-cold methanol and acetonitrile to precipitate the proteins, followed by addition of the mobile phase (0.1 % Formic acid in water and 0.1 % Formic acid in Acetonitrile) to the supernatant. The samples were filtered through a 0.22 µm syringe filter before injecting into the HPLC column (Inertsil® ODS-4; 5 µm; 4.6 *250 mm). The mobile phase consisted of 0.1 % formic acid in water and 0.1 % formic acid in acetonitrile (75:25) with a 0.8 mL/min flow rate. The obtained data were analyzed using the Empower 3 chromatography data software by Waters (M/s Waters Corporation, Ilford, USA).
Estimation of neuro‐inflammatory cytokines and serum cortisol levels
Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were estimated in whole brain homogenates. The cortisol levels were estimated in serum. The IL-6, TNF-α, and cortisol estimations were done as per the instructions provided with the ELISA kit procured from Peprotech, INC. (New Jersey, USA) and R&D systems® (Minneapolis, USA), respectively.
Statistical procedures
All results were expressed as mean ± S.E.M. Data were analyzed with statistical software (Graph Pad Prism version 5). Most of the statistical analysis was done using Student’s t-tests. Two-way repeated measures ANOVA with Bonferroni tests were used to analyze mean escape latency data from MWM, physiological measurements of body weight, rectal temperature, and food and water intake. In this analysis, the group was the between-subject variable and the week was the within-subject variable. The confidence limit was set at 95 % (p < 0.05) and considered significant.
Results
Chronic partial sleep deprivation‐induced hyperactivity
Animals subjected to CPSD were found to have significantly increased exploratory behavior in the actophotometer and open field maze as compared to the control group. CPSD animals exhibited increased ambulatory counts (p < 0.001) in the actophotometer (Fig. 2a) and also displayed hyperlocomotion as assessed through the number of line crossings (p < 0.001), rearing counts (p < 0.001) and pathlength (p < 0.05) in the open field test (Fig. 2b). In the elevated zero maze, animals of the CPSD group showed a significant increase in the number of entries into the open and closed compartment (p < 0.01, p < 0.05, respectively). Although the time spent in the open compartment was increased yet not significant as compared to the control group (p = 0.09) (Fig. 2c). Stereotyped behavior demonstrated by repetitive grooming, popping, licking and jumping activities were observed in CPSD animals as the mean cumulative stereotyped scoring was increased significantly (p < 0.01) as compared to the control group (Fig. 2d). CPSD animals were found to be aggressive as the number of attacks were significantly increased (p < 0.01) in the cotton bud biting test (Fig. 3a). Holistically, the CPSD animals showed hyperactivity owing to increased exploration, hyperlocomotion, stereotypic, and aggressive behavior.
Effect of chronic partial sleep deprivation on locomotor activity, anxiety and stereotypic behavior (n = 10). a Ambulatory counts recorded by actophotometer. b Number of line crossings, pathlength, rearing counts analyzed by open field test. c Number of entries in open arms, Number of entries in closed arms, Time spent in open arms, Time spent in closed arms analyzed by elevated zero maze. d Stereotypic behavior including popping, grooming, licking, jumping and mean cumulative scoring. CPSD denotes chronic partial sleep-deprived animals. Values plotted are mean ± S.E.M. *p < 0.05, $p < 0.01, #p < 0.001, vs. respective control group
Effect of chronic partial sleep deprivation on aggression, depression, memory and physiological parameters (n = 10). a Cotton bud biting test b Sucrose preference test c Forced swim test d Initial transfer latency and retention latency analyzed by elevated plus maze e Object exploration time and recognition index analyzed by novel object recognition test f Mean escape latency G. Body weight H. Rectal temperature. CPSD denotes chronic partial sleep-deprived animals. Values plotted are mean ± S.E.M. *p < 0.05, $p < 0.01, #p < 0.001 vs. respective control group
CPSD animals did not show signs of anhedonia, as the percentage preference for the sucrose was not significantly altered as compared to the control group (p = 0.43) (Fig. 3b). In the forced swim test, the immobility period was not changed in the CPSD group as compared to the control group (p = 0.60) (Fig. 3c). Hence, CPSD animals did not display depression-like behavior. In the EPM test, the initial transfer latency, a measure of learning, did not differ in both groups (p = 0.13) (Fig. 3d). However, the retention latency, a measure of memory, was significantly decreased in the CPSD group (p < 0.001) (Fig. 3d). Furthermore, in the NOR test, the object exploration time and percent recognition index were also not found to be different in both the groups (p = 0.64); (Fig. 3e). Similarly, no statistically significant difference was found in mean escape latency assessed through MWM (F (1, 18) = 1.690; p = 0.2100) (Fig. 3f), thus indicating intact memory of CPSD animals.
Chronic partial sleep deprivation altered the physiological and neurobiological parameters
The food and water intake in CPSD animals did not differ compared to the control group, (F (9, 162) = 1.199; p = 0.2992) and (F (9, 162) = 0.8091; p = 0.6084) respectively. However, there was a significant decrease in the bodyweight of CPSD animals (F (9, 162) = 7.264; p < 0.001) along with increased rectal temperature (F (9, 162) = 4.971; p < 0.001). Multiple comparisons followed by Bonferroni’s post-hoc test revealed a significant decrease in body weight from the 2nd week till week 9 except weeks 4, 5 and 8 (Fig. 3g). Similarly, a significant increase in rectal temperature was observed at weeks 6 and 9 (Fig. 3h).
To assess theeffect of CPSD on biochemicals, whole-brain homogenates were used. Catalase activity and reduced glutathione were significantly reduced (p < 0.05) along with an increase in lipid peroxidation (p < 0.05) as compared to the control group (Fig. 4b, c, d). However, no change in SOD activity (p = 0.24) was found. The mitochondrial respiratory enzyme activities (complex I, II, III and IV) were assessed and found to be significantly reduced (p < 0.05) as compared to the control group (Fig. 4e, f, g and h). Therefore, CPSD resulted in significantly elevated oxidative stress, along with depleted mitochondrial complex activities. Serotonin levels in the brain were markedly reduced (p < 0.01) in CPSD animals as compared to the control group (Fig. 4i). The levels of TNF alpha and IL-6 were significantly increased (p < 0.01 and p < 0.05 respectively) in whole brain homogenates of CPSD mice as compared to the control group (Fig. 4k and l), thus suggesting neuroinflammation as a consequence of CPSD.Serum was also collected after 9 weeks of CPSD and serum levels of cortisol were not changed (p = 0.23) (Fig. 4j).
Effect of chronic partial sleep deprivation on oxidative stress, mitochondrial complex activities, serotonin, serum cortisol, and neuro-inflammatory markers in the brain (n = 5). a The activity of superoxide dismutase b The activity of catalase c Reduced glutathione levels d Malondialdehyde levels e Complex I activity f Complex II activity g Complex III activity h Complex IV activity I. Serotonin levels j Serum cortisol levels k Tumor necrosis factor-alpha in brain l Interleukin-6 levels in the brain. CPSD denotes chronic partial sleep-deprived animals. Values plotted are mean ± S.E.M. *p < 0.05, $p < 0.01 vs. respective control group
Discussion
The present study was designed to mimic the condition of night shift workers and assess its neurobehavioral consequences. Our results demonstrated a cluster of behaviors suggesting hyperactivity, such as hyperlocomotion in the open field test and actophotometer, along with no depression-like behavior in the sucrose preference and forced swim tests in CPSD animals. This group also showed stereotyped behaviors such as popping, excessive grooming, jumping, licking, etc. Moreover, CPSD animals showed an increase in the number of entries in an open and closed compartment at an elevated zero maze, which testifies hyperactivity. Our findings are consistent with a previous study, wherein animals were sleep deprived for 96 h in a group using MMP, showed more ambulations, number of entries and also spent more time in the open arm of EPM (Suchecki et al. 2002). Hyperlocomotion and stereotyped behavior are considered hallmarks for the occurrence of mania and psychosis in animal models (Young et al. 2011; Forrest et al. 2014). Likewise, sleep loss is stated to act as a trigger for mania in bipolar patients (Lewis et al. 2017) and induces positive symptoms in psychotics (Waters et al. 2018). In preclinical studies, mania and psychotic behaviors have been reported with ASD (Kanazawa et al. 2016; Frau et al. 2017). To the best of our knowledge, such behavior has not been reported with CPSD using MMP employing night shift conditions.
The circadian rhythm is linked anatomically and genetically to the brain serotonergic system. The observed decrease in serotonin levels in CPSD mice is consistent with a previous report of reduced hippocampal and frontal cortical serotonin levels during the 8 h of sleep deprivation in rats (Bjorvatn et al. 2002). Indeed, serotonin deficiency in the adult mouse brain has been associated with hyperactivity and circadian disruption (Whitney et al. 2016). In our study, CPSD also resulted in elevated levels of TNF-α and IL-6 in whole brain homogenates. Preclinical studies suggest that sleep deprivation results in elevated pro-inflammatory cytokines (Nadjar et al. 2017a).
Furthermore, elevated neuro-inflammatory cytokines have been found to deplete serotonin in the brain through hyperactivation of the kynurenine pathway (Watkins et al. 2014). Hyperactivation of the kynurenine pathway is well implicated in psychotic and bipolar patients (Erhardt et al. 2017). In line with these reports, the observed increase in brain TNF-α and IL-6 levels might account for reduced brain serotonin levels, which could underlie the observed hyperactivity through the kynurenine pathway over-activation. Furthermore, the MMP method used in the current study excludes immobility stress and social isolation stress (Suchecki and Tufik 2000). In line with this, we also found unaltered levels of cortisol in CPSD animals.
Mitochondrial dysfunction is also well evidenced in bipolar and psychotic patients, including reduced complex I activity, damaged mitochondrial proteins (Andreazza et al. 2010b; Da Silva et al. 2018), abnormal mitochondrial structure, and polymorphism in mitochondrial DNA (Cataldo et al. 2010). In the current study, decreased mitochondrial respiratory enzymes activity in CPSD mice also support this correlation. Moreover, impaired mitochondrial functioning enhances the generation of reactive oxygen species (ROS). The observed decrease in GSH and catalase levels in the whole brain depicted the decreased antioxidant defense mechanism and the increase in lipid peroxidation. Studies suggest that the ROS may mediate the interplay between mitochondrial dysfunction and neuroinflammation (Di Filippo et al. 2010; Witte et al. 2010; Rimessi et al. 2016; Garabadu et al. 2019). The excessive production of ROS in the form of superoxide, hydroxyl radicals, or hydrogen peroxide exceeds the capacity of antioxidant defense mechanism and damages neuronal proteins, DNA, and lipids, eventually culminating in declined synaptic signaling and brain plasticity, which could underlie the behavioral outcomes (Villafuerte et al. 2015; Salim 2017; Nadjar et al. 2017).
Moreover, accumulated evidence indicates the involvement of neuroinflammation and mitochondrial dysfunction in sleep deprivation-induced neuronal and behavioral outcomes (Streck et al. 2015; Chanana and Kumar 2016; Yin et al. 2017; Wadhwa et al. 2019). Our results are also according to previous reports on sleep deprivation models in which increased oxidative stress and elevated neuroinflammatory markers in the brain underlie anxiety-like behavior (Chanana and Kumar 2016) and mania (Kanazawa et al. 2016; Valvassori et al. 2017). However, there is an unlikely possibility that behavioral testing could have affected the observed biochemical measures. However, based on our protocol design and experimental approach, which is in concordance with other studies of sleep deprivation (Frau et al. 2017; Manchanda et al. 2018; Ma et al. 2019; Andrabi et al. 2020), it is more likely that the changes seen are due to CPSD. Moreover, the observed differences in the control and CPSD groups cannot be attributed to behavioral testing and handling, as both groups underwent behavioral testing using the same approach.
Along with the behavioral and biochemical findings, marked physiological alterations were observed in CPSD animals, including body weight and rectal temperature. The decreased body weight was consistent with the increased rectal temperature at weeks 6 and 9. Our findings are consistent with a previous report (Caron and Stephenson 2010). Further, the body weight pattern observed in our study has been reported in previous studies (Martins et al. 2006; Caron and Stephenson 2010; Everson and Szabo 2011; Barf et al. 2012). In a CSD study conducted by Everson and Szabo (2011), rats were sleep deprived for 10 days using a rotating platform method followed by 2 days of free sleep period and the protocol was repeated 6 times. The physiological effects of repeated sleep deprivation included decreased body weight and increased food intake. Their results pointed toward the increased energy expenditure and adaptation of the body. The body weight pattern in the above study was similar, as observed in the current study, a steep decline during the first few days, followed by stabilization of body weight (Everson and Szabo 2011). In another chronic sleep restriction study, where rats were sleep deprived for 18 h per day for 10 days, the trend in body weight decline was similar to our study (Caron and Stephenson 2010).
The abrupt decrease in body weight in the first two weeks could be due to increased energy expenditure. The increase in energy expenditure can occur due to several reasons. The energy expenditure can be increased due to prolonged wakefulness as extended wakefulness demands more energy than sleep (Jung et al. 2011). Secondly, it could also be the effect of chronic sleep deficit on energy balance (Caron and Stephenson 2010). Thirdly, increased energy expenditure could also be due to increased motor activity in the MMP due to more swimming in the initial two weeks. Since the food intake was not significantly altered in our study, the primary energy source is likely to be body mass rather than food intake.
Moreover, the failure to increase food intake with respect to increased energy expenditure could lead to a state of energy deficit in the animal during sleep deprivation (Martins et al. 2006; also see Barf et al. 2012). Therefore, we suspect that all these above mentioned factors or at least some of them could have contributed to the decline in body weight. In our study, the stabilization of body weight after the 2nd week points to adjustments in physiological processes related to energy expenditure, such as achieving a neutral energy state (Caron and Stephenson 2010) to adapt to the continued disruption of adequate sleep.
Interestingly, we also found that the CPSD did not impair memory consolidation, as evidenced by initial transfer latency and retention latency from EPM, object exploration time and percent recognition index (NOR), and mean escape latency from MWM. These results are in contrast with a previous study, which reported the long-term and short-term memory impairment with six weeks of CPSD in rats (Alzoubi et al. 2012). It is likely that the difference in protocol design, particularly the inclusion of the weekend as the recovery period and different tasks, is responsible for the results observed in our study. Alzoubi et al. (2012) induced sleep deprivation in rats by using MMP for 8 h/day for 6 weeks. In contrast, our study design included CPSD of mice using MMP for 8 h/day from Monday to Friday for 9 weeks, including weekends as the sleep recovery period. The sleep recovery period has been found to reverse a few ASD-induced alterations in catecholamines and hormones (Andersen et al. 2005).
Similarly, differential effects on behavioral parameters have been observed in a chronic sleep restriction study conducted in neonatal mice when assessed in pre-and post-recovery period (Saré et al. 2016). Sleep recovery of 2.5 h has been reported to reverse the profound effects of ASD of 5 h on hippocampal genes (mTOR pathway) expression involved in memory and cognition (Vecsey et al. 2012). Further, Alzoubi et al. (2012) looked at spatial memory using radial arm water maze, whereas we assessed spatial learning and memory using MWM and EPM, recognition memory using NOR.
Our data from the NOR memory test, a non-hippocampus dependent task, is in line with a previous study of ASD where NOR memory was reported to be unimpaired (Ishikawa et al. 2014). In the same study, impairment in object-place recognition memory, which is hippocampal-dependent, was reported. In contrast, we did not observe any impairment in our MWM task. Our findings from MWM could also be explained based on data from Hagewoud et al. (2010), where they suggested that sleep-deprived animals could adopt a more rigid, egocentric strategy dependent on striatum instead of a more flexible strategy which was suggested to be hippocampus-dependent to solve a Y-maze task (Hagewoud et al. 2010). Similarly, in our study, it is possible that sleep-deprived mice used a rigid strategy dependent on striatum (Woolley et al. 2013), which could account for unimpaired performance in the MWM task. It is also possible as discussed above that the inclusion of the sleep recovery period in our study could be responsible for unimpaired performance in the hippocampus-dependent task, MWM.
Electroencephalographic recordings are important for studying sleep. Using the MMP method for sleep deprivation for 96 h, Machado et al. (2004) reported that the model abolishes the REM sleep completely and reduces the SWS by 31 % (Machado et al. 2004). However, in another long term sleep deprivation study using a different protocol for sleep deprivation for 18 h per day for 21 days, Machado et al. (2006) reported that SWS increased on some recording days while REM was found to be increased on most recording days as compared to the baseline. The differences reported by the same group may be due to the time of recordings chosen, i.e., recording during the sleep deprivation (Machado et al. 2004) versus recording during the free sleep window in chronic sleep restriction protocol (Machado et al. 2006). In another study, recordings were done during 20 h of sleep deprivation along with 4 h of free sleep period, reported that REM sleep was completely abolished while non-REM sleep was significantly decreased in sleep-deprived animals during 20 h sleep deprivation in MMP; however, no change in REM and non-REM sleep was observed during the 4 h free sleep period (Gomez-Gonzalez et al. 2013). Based on these previous reports, we expect that our CPSD of 8 h/day for 9 weeks with a weekend sleep recovery period would have altered the sleep cycle, with REM being more affected than SWS. Further, as our study was for a longer period, with intermittent recovery than the studies mentioned above, we expect the total sleep deficit to be more profound in our protocol (Caron and Stephenson 2010) and hence, it is even more likely to affect sleep cycle.
The lack of electroencephalographic data is a limitation of our study. The EEG recordings would have allowed to determine the extent of sleep disturbance induced by the current study protocol and differences in REM and NREM sleep in the sleep deprivation and recovery phases of the protocol. Further EEG and unit recordings could also point to any subtle differences in the behavior of CPSD and control mice. It would be interesting to explore biochemical measures in different brain regions, including the hippocampus and associated cortices, as the whole brain homogenate provides nonspecific information. Nevertheless, the whole brain data did provide information about how the brain could be affected by a model of CPSD, which mimics night shift work condition with intermittent recovery.
In conclusion, the present study provides comprehensive behavioral and neurobiological evidence for pronounced hyperactivity in a mouse model of chronic partial sleep deprivation, which mimics the night shift work condition.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Alzoubi KH, Khabour OF, Rashid BA et al (2012) The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: The role of oxidative stress. Behav Brain Res 226:205–210. https://doi.org/10.1016/j.bbr.2011.09.017
Andersen ML, Martins PJF, D’Almeida V et al (2005) Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res 14:83–90. https://doi.org/10.1111/j.1365-2869.2004.00428.x
Andrabi M, Andrabi MM, Kunjunni R et al (2020) Lithium acts to modulate abnormalities at behavioral, cellular, and molecular levels in sleep deprivation-induced mania‐like behavior. Bipolar Disord 22:266–280. https://doi.org/10.1111/bdi.12838
Andreazza AC, Andersen ML, Alvarenga TA et al (2010a) Impairment of the mitochondrial electron transport chain due to sleep deprivation in mice. J Psychiatr Res 44:775–780. https://doi.org/10.1016/j.jpsychires.2010.01.015
Andreazza AC, Shao L, Wang JF, Young LT (2010b) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67:360–368. https://doi.org/10.1001/archgenpsychiatry.2010.22
Bansal Y, Singh R, Saroj P et al (2018) Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicol Appl Pharmacol 355:257–268. https://doi.org/10.1016/J.TAAP.2018.07.010
Barf RP, Van Dijk G, Scheurink AJW et al (2012) Metabolic consequences of chronic sleep restriction in rats: Changes in body weight regulation and energy expenditure. Physiol Behav 107:322–328. https://doi.org/10.1016/j.physbeh.2012.09.005
Belenky G, Wesensten NJ, Thorne DR et al (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res 12:1–12. https://doi.org/10.1046/j.1365-2869.2003.00337.x
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J Neurochem 73:1127–1137. https://doi.org/10.1046/j.1471-4159.1999.0731127.x
Berry B, McMahan R, Gallagher M (1997) Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a hippocampal-dependent task. Behav Neurosci 111:267–274. https://doi.org/10.1037/0735-7044.111.2.267
Bjorvatn B, Gronli J, Hamre F et al (2002) Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience 113:323–330. https://doi.org/10.1016/S0306-4522(02)00181-1
Brown JP, Martin D, Nagaria Z et al (2020) Mental Health Consequences of Shift Work: An Updated Review. Curr Psychiatry Rep 22:7. https://doi.org/10.1007/s11920-020-1131-z
Carey MG, Al-Zaiti SS, Dean GE et al (2011) Sleep problems, depression, substance use, social bonding, and quality of life in professional firefighters. J Occup Environ Med 53:928–933. https://doi.org/10.1097/JOM.0b013e318225898f
Caron AM, Stephenson R (2010) Energy expenditure is affected by rate of accumulation of sleep deficit in rats. Sleep 33:1226–1235. https://doi.org/10.1093/sleep/33.9.1226
Cataldo AM, McPhie DL, Lange NT et al (2010) Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol 177:575–585. https://doi.org/10.2353/ajpath.2010.081068
Chanana P, Kumar A (2016) GABA-BZD receptor modulating mechanism of Panax quinquefolius against 72-h sleep deprivation induced anxiety like behavior: Possible roles of oxidative stress, mitochondrial dysfunction and neuroinflammation. Front Neurosci 10:1–16. https://doi.org/10.3389/fnins.2016.00084
Conklin AI, Yao CA, Richardson CG (2018) Chronic sleep deprivation and gender-specific risk of depression in adolescents: A prospective population-based study. BMC Public Health 18:1–7. https://doi.org/10.1186/s12889-018-5656-6
Cordeira J, Kolluru SS, Rosenblatt H et al (2018) Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res 339:124–129. https://doi.org/10.1016/j.bbr.2017.11.033
Da Silva T, Wu A, Laksono I et al (2018) Mitochondrial function in individuals at clinical high risk for psychosis. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-24355-6
Dhir A, Naidu PS, Kulkarni SK (2010) Modulatory effect of nimesulide on apomorphine induced stereotypy cind MK-801 induced hyperlocomotion. Ann Neurosci 12:71–73. https://doi.org/10.5214/ans.0972.7531.2005.120402
Di Filippo M, Chiasserini D, Tozzi A et al (2010) Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimer’s Dis 20:369–379. https://doi.org/10.3233/JAD-2010-100543
Erhardt S, Schwieler L, Imbeault S, Engberg G (2017) The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 112:297–306. https://doi.org/10.1016/j.neuropharm.2016.05.020
Everson CA, Szabo A (2011) Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS One 6:e22987. https://doi.org/10.1371/journal.pone.0022987
Forrest AD, Coto CA, Siegel SJ (2014) Animal models of psychosis: current state and future directions. Curr Behav Neurosci Rep 1:100–116. https://doi.org/10.1007/s40473-014-0013-2
Frau R, Bini V, Soggiu A et al (2017) The neurosteroidogenic enzyme 5α-reductase mediates psychotic-like complications of sleep deprivation. Neuropsychopharmacology 42:2196–2205. https://doi.org/10.1038/npp.2017.13
Ftouni S, Sletten TL, Howard M et al (2013) Objective and subjective measures of sleepiness, and their associations with on-road driving events in shift workers. J Sleep Res. https://doi.org/10.1111/j.1365-2869.2012.01038.x
Garabadu D, Agrawal N, Sharma A, Sharma S (2019) Mitochondrial metabolism. Behav Pharmacol 30:641–651. https://doi.org/10.1097/FBP.0000000000000505
Gomez-Gonzalez B, Hurtado-Alvarado G, Esqueda-Leon E et al (2013) REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res 10:197–207. https://doi.org/10.2174/15672026113109990002
Gonzalez-Castañeda RE, Galvez-Contreras AY, Martínez-Quezada CJ et al (2016) Sex-related effects of sleep deprivation on depressive- and anxiety-like behaviors in mice. Exp Anim 65:97–107. https://doi.org/10.1538/expanim.15-0054
Graves LA (2003) Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 10:168–176. https://doi.org/10.1101/lm.48803
Hagewoud R, Havekes R, Tiba PA et al (2010) Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep 33:1465–1473. https://doi.org/10.1093/sleep/33.11.1465
Healy SD, Braham SR, Braithwaite VA (1999) Spatial working memory in rats: no differences between the sexes. Proc R Soc London Ser B Biol Sci 266:2303–2308. https://doi.org/10.1098/rspb.1999.0923
Ishikawa H, Yamada K, Pavlides C, Ichitani Y (2014) Sleep deprivation impairs spontaneous object-place but not novel-object recognition in rats. Neurosci Lett 580:114–118. https://doi.org/10.1016/j.neulet.2014.08.004
James SM, Honn KA, Gaddameedhi S, Van Dongen HPA (2017) Shift work: disrupted circadian rhythms and sleep—implications for health and well-being. Curr Sleep Med Rep 3:104–112. https://doi.org/10.1007/s40675-017-0071-6
Jollow D, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Jung CM, Melanson EL, Frydendall EJ et al (2011) Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589:235–244. https://doi.org/10.1113/jphysiol.2010.197517
Kanazawa LKS, Vecchia DD, Wendler EM et al (2016) Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free Radic Biol Med 99:79–86. https://doi.org/10.1016/j.freeradbiomed.2016.07.027
King TE (1967) [58] Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol 10:322–331. https://doi.org/10.1016/0076-6879(67)10061-X
King TE, Howard RL (1967) Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10:275–294
Koehl M, Battle SE, Turek FW (2003) Sleep in female mice: A strain comparison across the estrous cycle. Sleep 26:267–272. https://doi.org/10.1093/sleep/26.3.267
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195. https://doi.org/10.1016/0003-9861(78)90479-4
Leger M, Quiedeville A, Bouet V et al (2013) Object recognition test in mice. Nat Protoc 8:2531–2537. https://doi.org/10.1038/nprot.2013.155
Lewis KS, Gordon-Smith K, Forty L et al (2017) Sleep loss as a trigger of mood episodes in bipolar disorder: Individual differences based on diagnostic subtype and gender. Br J Psychiatry 211:169–174. https://doi.org/10.1192/bjp.bp.117.202259
Lindseth KA, Hicks RA, Leon HA (1980) Inhibition of the estrous cycles of rats by REM sleep deprivation. Bull Psychon Soc 16:380–380. https://doi.org/10.3758/BF03329571
Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593. https://doi.org/10.1046/j.1471-4159.1997.69020581.x
Lowry HO, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma W, Song J, Wang H et al (2019) Chronic paradoxical sleep deprivation-induced depressionlike behavior, energy metabolism and microbial changes in rats. Life Sci 225:88–97. https://doi.org/10.1016/j.lfs.2019.04.006
Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S (2004) Sleep deprivation induced by the modified multiple platform technique: Quantification of sleep loss and recovery. Brain Res 1004:45–51. https://doi.org/10.1016/j.brainres.2004.01.019
Machado RB, Suchecki D, Tufik S (2006) Comparison of the sleep pattern throughout a protocol of chronic sleep restriction induced by two methods of paradoxical sleep deprivation. Brain Res Bull 70:213–220. https://doi.org/10.1016/j.brainresbull.2006.04.001
Malmberg B, Kecklund G, Karlson B et al (2010) Sleep and recovery in physicians on night call: a longitudinal field study. BMC Health Serv Res 10:1–10. https://doi.org/10.1186/1472-6963-10-239
Manchanda S, Singh H, Kaur T, Kaur G (2018) Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem 449:63–72. https://doi.org/10.1007/s11010-018-3343-7
Martins PJF, D’Almeida V, Nobrega JN, Tufik S (2006) A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep 29:1233–1238. https://doi.org/10.1093/sleep/29.9.1233
Mhaidat NM, Alzoubi KH, Khabour OF et al (2015) Exploring the effect of vitamin C on sleep deprivation induced memory impairment. Brain Res Bull 113:41–47. https://doi.org/10.1016/j.brainresbull.2015.02.002
Monfort P, Gomez-Gimenez B, Llansola M, Felipo V (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem Neurosci 6:1420–1427. https://doi.org/10.1021/acschemneuro.5b00096
Nadjar A, Wigren H-KM, Tremblay M-E (2017) Roles of microglial phagocytosis and inflammatory mediators in the pathophysiology of sleep disorders. Front Cell Neurosci 11:1–11. https://doi.org/10.3389/fncel.2017.00250
Opperhuizen A, Kerkhof LWM, Van, Proper KI (2015) Rodent models to study the metabolic effects of shiftwork in humans. 6:1–20. https://doi.org/10.3389/fphar.2015.00050
Park SJ, Lee JY, Kim SJ et al (2015) Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci Rep 5:8502. https://doi.org/10.1038/srep08502
Patti CL, Zanin KA, Sanday L et al (2010) Effects of sleep deprivation on memory in mice: Role of state-dependent learning. Sleep 33:1669–1679. https://doi.org/10.1093/sleep/33.12.1669
Pires GN, Bezerra AG, Tufik S, Andersen ML (2016) Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med 24:109–118. https://doi.org/10.1016/j.sleep.2016.07.019
Rabbani M, Wright J, Butterworth AR et al (1994) Possible involvement of NMDA receptor-mediated transmission in barbiturate physical dependence. Br J Pharmacol 111:89–96. https://doi.org/10.1111/j.1476-5381.1994.tb14028.x
Rajaratnam SMW, Howard ME, Grunstein RR (2013) Sleep loss and circadian disruption in shift work: health burden and management. Med J Aust 199:S11–S15. https://doi.org/10.5694/mja13.10561
Reynolds AC, Banks S (2010) Total sleep deprivation, chronic sleep restriction and sleep disruption. In: Progress in Brain Research. pp 91–103
Rimessi A, Previati M, Nigro F et al (2016) Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int J Biochem Cell Biol 81:281–293. https://doi.org/10.1016/j.biocel.2016.06.015
Rodríguez CA, Aguilar R, Chamizo VD (2011) Landmark learning in a navigation task is not affected by the female rats’ estrus cycle. Psicológica Rev Metodol y Psicol Exp 32:279–299
Sachdeva AK, Dharavath RN, Chopra K (2018) Time-response studies on development of cognitive deficits in an experimental model of insulin resistance. Clin Nutr 1–10. https://doi.org/10.1016/j.clnu.2018.06.966
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205. https://doi.org/10.1124/jpet.116.237503
Santhi N, Lazar AS, McCabe PJ et al (2016) Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci 113:E2730–E2739. https://doi.org/10.1073/pnas.1521637113
Saré RM, Levine M, Hildreth C et al (2016) Chronic sleep restriction during development can lead to long-lasting behavioral effects. Physiol Behav 155:208–217. https://doi.org/10.1016/j.physbeh.2015.12.019
Singh K, Bishnoi M, Kulkarni SK (2007) Elevated Zero-maze: A paradigm to evaluate anti-anxiety effects of drugs. Methods Find Exp Clin Pharmacol 29:343. https://doi.org/10.1358/mf.2007.29.5.1117557
Singh T, Kaur T, Goel RK (2017) Adjuvant quercetin therapy for combined treatment of epilepsy and comorbid depression. Neurochem Int 104:27–33. https://doi.org/10.1016/j.neuint.2016.12.023
Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol 32:415–438. https://doi.org/10.1083/jcb.32.2.415
Stranges S, Tigbe W, Gómez-Olivé FX et al (2012) Sleep problems: an emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries Across Africa and Asia. Sleep 35:1173–1181. https://doi.org/10.5665/sleep.2012
Streck EL, Scaini G, Jeremias GC et al (2015) Effects of mood stabilizers on brain energy metabolism in mice submitted to an animal model of mania induced by paradoxical sleep deprivation. Neurochem Res 40:1144–1152. https://doi.org/10.1007/s11064-015-1575-4
Strekalova T, Steinbusch HWM (2010) Measuring behavior in mice with chronic stress depression paradigm. Prog Neuro-Psychopharmacol Biol Psychiatry 34:348–361. https://doi.org/10.1016/j.pnpbp.2009.12.014
Suchecki D, Tufik S (2000) Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav 68:309–316. https://doi.org/10.1016/S0031-9384(99)00181-X
Suchecki D, Tiba PA, Tufik S (2002) Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol 14:549–554. https://doi.org/10.1046/j.1365-2826.2002.00812.x
Valvassori SS, Resende WR, Dal-Pont G et al (2017) Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord 19:246–258. https://doi.org/10.1111/bdi.12503
Vecsey CG, Baillie GS, Jaganath D et al (2009) Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461:1122–1125. https://doi.org/10.1038/nature08488
Vecsey CG, Peixoto L, Choi JHK et al (2012) Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics 44:981–991. https://doi.org/10.1152/physiolgenomics.00084.2012
Villafuerte G, Miguel-Puga A, Murillo Rodríguez E et al (2015) Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid Med Cell Longev 2015:1–15. https://doi.org/10.1155/2015/234952
Wadhwa M, Prabhakar A, Anand JP et al (2019) Complement activation sustains neuroinflammation and deteriorates adult neurogenesis and spatial memory impairment in rat hippocampus following sleep deprivation. Brain Behav Immun 82:129–144. https://doi.org/10.1016/j.bbi.2019.08.004
Waters F, Chiu V, Atkinson A, Blom JD (2018) Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry 9:1–13. https://doi.org/10.3389/fpsyt.2018.00303
Watkins CC, Sawa A, Pomper MG (2014) Glia and immune cell signaling in bipolar disorder: Insights from neuropharmacology and molecular imaging to clinical application. Transl Psychiatry 4:1–10. https://doi.org/10.1038/tp.2013.119
Whitney MS, Shemery AM, Yaw AM et al (2016) Adult brain serotonin deficiency causes hyperactivity, circadian disruption, and elimination of siestas. J Neurosci 36:9828–9842. https://doi.org/10.1523/jneurosci.1469-16.2016
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676. https://doi.org/10.1042/bj0990667
Witte ME, Geurts JJG, de Vries HE et al (2010) Mitochondrial dysfunction: A potential link between neuroinflammation and neurodegeneration? Mitochondrion 10:411–418. https://doi.org/10.1016/j.mito.2010.05.014
Woolley DG, Laeremans A, Gantois I et al (2013) Homologous involvement of striatum and prefrontal cortex in rodent and human water maze learning. Proc Natl Acad Sci 110:3131–3136. https://doi.org/10.1073/pnas.1217832110
Yin M, Chen Y, Zheng H et al (2017) Assessment of mouse cognitive and anxiety-like behaviors and hippocampal inflammation following a repeated and intermittent paradoxical sleep deprivation procedure. Behav Brain Res 321:69–78. https://doi.org/10.1016/j.bbr.2016.12.034
Young JW, Henry BL, Geyer MA (2011) Predictive animal models of mania: Hits, misses and future directions. Br J Pharmacol 164:1263–1284. https://doi.org/10.1111/j.1476-5381.2011.01318.x
Zou X, Huang W, Lu F et al (2017) The effects of Jiao-Tai-Wan on sleep, inflammation and insulin resistance in obesity-resistant rats with chronic partial sleep deprivation. BMC Complement Altern Med. https://doi.org/10.1186/s12906-017-1648-9
Acknowledgements
The authors gratefully acknowledge University Grant Commission (UGC), New Delhi, for providing a UGC-BSR RFSMS fellowship to Shiyana Arora. The authors are immensely thankful to the University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh and National Agri-Food Biotechnology Institute, Mohali, for providing the research infrastructure.
Author information
Authors and Affiliations
Contributions
SA, MB, KKK and KC were involved in the design of the hypothesis. SA, RND, and YB executed experiments and were involved in data collection and data analysis. SA and RND prepared the manuscript. MB, KKK and KC were involved in manuscript revision. All the authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare there is no conflict of interest.
Ethical approval
The experimental protocol was approved by the Institutional Animal Ethics Committee of Panjab University (PU/IAEC/S/16/16) and conducted as per the guidelines of the Committee for Control and Supervision of Experiments on Animals, India.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chronic partial sleep deprivation (CPSD) of 8-h/day/5 days in a week continued for nine weeks, with the weekend as a sleep recovery period in a modified multiple platform model, mimics the night shift condition in mice.
• CPSD induces a cluster of behavioral phenotypes in mice similar to hyperactivity seen in mania and psychosis, including hyperlocomotion, aggression, and stereotyped behavior.
• CPSD elevates neuro-inflammatory markers, which might underlie the observed decrease in brain serotonin levels.
• CPSD also induces mitochondrial dysfunction and oxidative stress in the brain, which may underlie the observed neurobehavioral deficit.
Rights and permissions
About this article
Cite this article
Arora, S., Dharavath, R.N., Bansal, Y. et al. Neurobehavioral alterations in a mouse model of chronic partial sleep deprivation. Metab Brain Dis 36, 1315–1330 (2021). https://doi.org/10.1007/s11011-021-00693-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00693-9