Abstract
Tibet is an area in China with a high incidence of stroke, typically attributed to hypobaric hypoxia. The present study aimed to observe the neuronal injury of ischemic stroke after hypobaric hypoxia and explore the mechanism by which N-methyl-D-aspartate receptor (NMDAR) and its downstream pathways are involved. This study employed a hypobaric chamber to imitate high altitude at 4000 m. After hypoxia, the middle cerebral artery occlusion (MCAO) model was used to mimic ischemic stroke. Behavioral tests and measurements of infarct area were used to observe neuronal injuries. The expression of NMDAR, Ca2+/calmodulin-dependent protein kinase II (CaMKII) and phosphorylated CaMKII (Threonine 286) (P-CaMKII) was tested by western blot, and hematological tests were used to count the number of red blood cells (RBCs) and hemoglobin. Compared with the plain+MCAO group, the neurological deficit scores and infarct area of rats in the 4000 m + MCAO group were all decreased, and the protein expression of NMDAR, CaMKII and P-CaMKII was reduced. Compared with the plain group, the numbers of RBCs, hemoglobin and hematocrit were increased in the 4000 m group; compared with the 4000 m groups, the three indexes were increased in the 4000 m + MCAO groups. The neuronal injuries after hypoxia were not more serious than those in rats enduring ischemia and reperfusion in plain. The underlying mechanisms were related to the decreased expression of NMDAR and CaMKII; furthermore, the increased numbers of RBCs and hemoglobin may be crucial mechanisms for the incidence and development of ischemic stroke at high altitude.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second most common cause of death and the third most common cause of disability worldwide (Wu and Tymianski, 2018). Stroke morbidity in China shows regionality, and the high-prevalence provinces of stroke are (in sequence) Heilongjiang, Tibet, Jilin, Liaoning, Hebei, Xinjiang, Inner Mongolia, Beijing and Ningxia. Stroke morbidity in the national minority is 14.8%, and that in the Han nationality is 6.98%(Xu et al., 2013). The two main types of stroke are ischemic and hemorrhagic; among these, approximately 87% of strokes are cerebral ischemic (Luo et al., 2019; Wu and Tymianski, 2018), and in China, patients with ischemic stroke account for 69.6% (Wang, et al. 2017).
The pathogenesis of ischemic stroke is stenosis or occlusion of the main artery supply to the cerebral artery, and during disease development or the later treatment period, the occluded vasculature recanalizes, resulting in cerebral ischemia and reperfusion injuries (Piccardi et al., 2018; Wu and Tymianski, 2018). The mechanisms underlying cerebral ischemic stroke include calcium overload, neuronal excitotoxicity induced by the overactivation of excitatory amino acid receptors, free radical-mediated cytotoxicity, inflammatory reactions, destruction of the blood-brain barrier, and so on (Luo et al., 2019; Wu and Tymianski, 2018). Furthermore, excitotoxicity plays an important role in the pathological process of acute cerebral stroke (Wu and Tymianski, 2018; Wu et al., 2019).
N-methyl-D-aspartate receptor (NMDAR) are one of the main receptors of excitatory amino acids (Luo et al., 2019). Structurally, NMDAR are constituted by two NR1 subunits and two other subunits, including NR2A-NR2D, as well as the NR3A and NR3B subunits (Wu and Tymianski, 2018). Ca2+/calmodulin-dependent protein kinase II (CaMKII) is the downstream signaling molecule of NMDAR. A great increase in intracellular calcium, resulting from influx through NMDAR, was reported to lead to the continuous activation of CaMKII through the autophosphorylation of threonine 286, which plays an important role in regulating excitotoxicity (Wang and Peng, 2016; Wu et al., 2019).
Due to the high morbidity of stroke in Tibet, where the hypobaric hypoxia is the typical feature of natural environment, therefore, the present study aimed to observe cerebral ischemia and reperfusion injury after hypobaric hypoxia simulation and analyze the pathophysiologic mechanism underlying NMDAR and its downstream pathway to provide further insight into ischemic stroke at high altitude.
Materials and methods
Animals and treatments
60 adult male Sprague-Dawley rats weighing 210–220 g were procured from the Dossy Experimental Animal Corporation (Chengdu, China) and allowed to acclimate for 1 week. The ambient temperature was set to 25 °C, with a 12/12 light/dark cycle, during which the rats were allowed free access to food and water. All procedures of animal handling were approved by the Ethics Committee for Experimental Research at Xizang Minzu University, and conformed to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
To imitate hypobaric hypoxia, the rats were placed into a hypobaric chamber (Xi’an Fukang Air Purification Equipment and Engineering Co., Ltd., Xi’an, China) for 4 days, and the altitude was set at 4000 m (the barometric pressure was about 61.3 Kpa). The speed for ascent and descent was set at 135 m/min. To maintain continuous hypoxic conditions, specially made bottles were placed in the rat cages to allow rats to drink freely. The plain group used the altitude of Xianyang in Shaanxi (410 m) as a reference.
As for the middle cerebral artery occlusion (MCAO) model, at first, rats were anesthetized with 6% chloralhydrate. A midline incision was made in the neck to expose the right common carotid artery and its branches. A small incision was made in the external carotid artery, and a special suture (Beijing Cinontech Co., Ltd., Beijing, China) was inserted and gently advanced through the internal carotid artery until the tip occluded the origin of the middle cerebral artery (Longa et al., 1989). The operation was conducted on an operation table with heating device to maintain a constant body temperature of 37 ± 0.5 °C. After 30 min of occlusion, the suture was withdrawn for 24 h of reperfusion.
The animals were randomly divided into two groups: plain+ MCAO group and 4000 m + MCAO group. For the 4000 m + MCAO group, the MCAO model was made immediately at the end of hypobaric hypoxia. Except for the treatment of hypobaric hypoxia, all operations were same for the two groups. In order to deeply analyze the role of red blood cells (RBCs) and hemoglobin counts in this research, plain group and 4000 m group were supplemented.
Evaluation of neurological deficits, brain edema and the infarct area
The animals were evaluated for neurological deficits 24 h after reperfusion in a double-blinded manner and scored as follows: 0, no observable neurological deficit; 1, failed to extend the left forepaw fully; 2, circled to the left side; 3, fell to the left; and 4, did not walk spontaneously and showed a depressed level of consciousness(Longa et al., 1989).
After scoring, all test subjects were deeply anesthetized with 6% chloralhydrate and sacrificed by decapitation. The brain index was adopted to reflect brain edema, which was calculated by the weight ratio of the whole cerebrum to the whole body.
For evaluation of the cerebral infarct area, whole rat brains were equally sectioned into 6 coronal slices and then stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min in the dark and subsequently fixed in 4% paraformaldehyde overnight. The viable brain tissues were stained red, and infarcted tissues could not be stained. Each slice was photographed and analyzed with Image J software (NIH, MD, USA). The area of infarction was calculated and is expressed as the percentage of infarct area to the total hemispheric area for each slice, and the third brain slice of each rat was adopted for statistical analysis.
Red blood cells (RBCs) and hemoglobin counts
Before decapitation, blood was collected from the abdominal aorta, and RBCs and hemoglobin were counted with a Mindray BC-2800 Vet Hematology analyzer (Mahwah, NJ, USA).
Western blot analysis
After 30 min of occlusion and 24 h of reperfusion, the animals were decapitated, and the right hippocampus was rapidly dissected separately at the desired time. The hippocampal tissue samples were homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) with protease and phosphatase inhibitors (Roche, Basel, CH), and the total protein concentration was measured by the bicinchoninic acid assay. Equal amounts of protein (50 μg) were loaded onto 10% SDS-polyacrylamide gels. After separation by electrophoresis, proteins were transferred to PVDF membranes (Millipore, MA, USA), which were blocked with 5% nonfat milk in TBST at room temperature for 1 h and then incubated with the indicated primary antibody overnight at 4 °C. The primary antibodies were as follows: rabbit anti-NR1 monoclonal antibody (1:1000; Cell Signaling Technology, MA, USA), rabbit anti-NR2A polyclonal antibody (1:1000; Millipore), rabbit anti-NR2B polyclonal antibody (1:1000; Millipore), rabbit anti-NR3A polyclonal antibody (1:1000; Millipore), mouse anti-CaMKII monoclonal antibody (1:1000; Abcam, Cambridge, UK), rabbit anti-CaMKII alpha (phospho T286) polyclonal antibody (1:1000; Abcam), and mouse anti-β-actin polyclonal antibody (1:3000; Millipore). Membranes were washed 3 times with TBST for 5 min each and then incubated for 1 h at room temperature with the indicated horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit antibody (1:1000; Millipore) and anti-mouse antibody (1:3000; Millipore)). After washing with TBST, membranes were revealed by ECL-Plus detection kit (Beyotime Institute of Biotechnology) using the ChemiDoc™ XRS + System and analyzed using the accompanying proprietary program Image Lab™ Software (Bio-Rad Laboratories, Inc., CA, USA).
Statistical analysis
The data are presented as the mean ± SEM. Statistical analyses were performed with one-way ANOVA or a paired Student’s t test (for comparisons of two groups) using SPSS 18.0 statistical software. Differences with P < 0.05 were considered statistically significant.
Results
Death rate of the MCAO model and weight loss after MCAO
For the plain +MCAO group, 14 rats were used, and 2 rats died; the death rate was 14.29%. For the 4000 m + MCAO group, 18 rats were used, and 5 rats died; the death rate was 27.78%.
Compared with the plain + MCAO group (24.38 ± 1.08), the weight loss of rats in the 4000 m + MCAO group (19.00 ± 2.04, P < 0.05) was less.
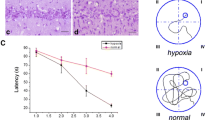
Neurological deficit score after MCAO
Compared with the plain + MCAO group (1.50 ± 0.19), the neurological deficit score of rats in the 4000 m + MCAO group (0.63 ± 0.18, P < 0.05) was decreased (Fig. 1a).
Changes in brain index after MCAO
Compared with the plain + MCAO group (0.60% ± 0.01%), the brain index of rats in the 4000 m + MCAO group (0.48% ± 0.01%, P < 0.01) was decreased ((Fig. 1b).
Variance in infarct area after MCAO
Compared with the plain + MCAO group (36.97% ± 3.85%), the area of cerebral infarction in the 4000 m + MCAO group (26.66% ± 3.19%, P < 0.05) was reduced ((Fig. 2).
Variance in parameters related to RBCs after hypoxia and MCAO
Compared with the plain group (7.97 ± 0.09; 146.5 ± 3.05; 41.24 ± 0.93), the RBCs, hemoglobin and hematocrit counts in the 4000 m group (9.49 ± 0.11, P < 0.01; 186.5 ± 1.18, P < 0.01; 52.78 ± 0.39, P < 0.01), plain+MCAO group (10.28 ± 0.70, P < 0.05; 194.88 ± 14.37, P < 0.05; 55.01 ± 3.35, P < 0.05), and 4000 m + MCAO group (12.16 ± 0.67, P < 0.01; 229.41 ± 12.35, P < 0.01; 65.79 ± 2.67, P < 0.01) were all significantly increased; compared with the 4000 m group, the above three indexes in the 4000 m + MCAO group were all increased (P < 0.05; P < 0.05; P < 0.05) ((Fig. 3).
Comparison of parameters related to red blood cell after hypoxia and MCAO. (a) red blood cell, (b) hemoglobin, (c) hematocrit. *P < 0.05, **P < 0.01, compared with the plain group; #P < 0.05, compared with the 4000 m group; \( \overline{\mathrm{x}} \) ± SEM, n = 8. MCAO: middle cerebral artery occlusion
Changes in NMDAR subunits in the hippocampus after MCAO
Compared with the expression of NR1 (1.03 ± 0.10), NR2A (0.98 ± 0.07), NR2B (0.96 ± 0.03), and NR3A (1.11 ± 0.10) in the plain+MCAO group, the expression of these proteins in the hippocampus of the 4000 m + MCAO group (0.49 ± 0.05, P < 0.01; 0.55 ± 0.05, P < 0.01; 0.40 ± 0.03, P < 0.01; 0.59 ± 0.04, P < 0.05) were all decreased ((Fig. 4).
Changes in the expression of NMDAR subunits. (a) representative blots showing the levels of NMDAR subunits, (b) semiquantitative analysis of the NR1 protein expression, (c) semiquantitative analysis of the NR2A protein expression, (d) semiquantitative analysis of the NR2B protein expression, (e) semiquantitative analysis of the NR3A protein expression. *P < 0.05, **P < 0.01, compared with the plain+MCAO group; \( \overline{\mathrm{x}} \) ± SEM, n = 6. MCAO: middle cerebral artery occlusion; NMDAR: N-methyl-D-aspartate receptor
Changes in CaMKII and P-CaMKII protein expression in the hippocampus after MCAO
Compared with the protein expression of CaMKII (0.90 ± 0.04) and P-CaMKII (0.97 ± 0.05) and the ratio of P-CaMKII to CaMKII (1.08 ± 0.05) in the plain+MCAO group, these values in the 4000 m + MCAO group (0.56 ± 0.02, P < 0.01; 0.51 ± 0.01, P < 0.01; 0.91 ± 0.03, P < 0.05) were all decreased ((Fig. 5).
Changes in the expression of CaMKII and P-CaMKII. (a) representative blots showing the levels of CaMKII and P-CaMKII, (b) semiquantitative analysis of the CaMKII protein expression, (c) semiquantitative analysis of the P-CaMKII protein expression, (d) the ratio of P-CaMKII/CaMKII. *P < 0.05, **P < 0.01, compared with the plain+MCAO group; \( \overline{\mathrm{x}} \) ± SEM, n = 6. MCAO: middle cerebral artery occlusion; CaMKII: Ca2+/calmodulin-dependent protein kinase II; P-CaMKII: phosphorylated CaMKII (Threonine 286)
Discussion
In our previous study, we found that MCAO damage was reflected not only in the brain but also in the whole body, as evidenced as weight loss in rats. In the present study, the weight loss of rats in the 4000 m + MCAO group was less than that in the plain +MCAO group. Then, we used the neurological deficit score, brain index and infarct area to observe differences in the degree of cerebral ischemia and reperfusion injury between plain and hypobaric hypoxia, two different circumstances. The more severe the injury is, the higher the neurological deficit scores. Brain edema, a serious complication of ischemic stroke, significantly affects mortality following ischemic brain injury (Wu et al., 2018). Hence, the brain index can be employed to reflect the degree of edema. The infarct area is the index used to represent damaged brain tissue directly. As shown in the results, the plain+MCAO group had higher neurological deficit scores than the 4000 m + MCAO group, and the brain index and infarct area in the plain +MCAO group were both larger than those in the 4000 m + MCAO group. The results were unexpected, and which induced us to explore further.
Regarding the death rate of the MCAO model in the present study, we performed an analysis and found that the death rate of rats in the plain+MCAO group was 14.29%, while that of rats in the 4000 m + MCAO group was 27.78%, which was higher than that of rats in the former group. These results show that the mortality of individuals suffering from ischemic stroke after hypobaric hypoxia is high, but damage to the cerebral system in survivors may not be too serious. Like some diseases, there may not be a positive correlation between the morbidity and the degree of injury. The mechanism underlying the disease may be complicated, that is why we carried out the study. Based on the results described above, we further speculate that hypoxic rats have some resistance to cerebral ischemia reperfusion injury, which may be related to the increase in antioxidant capacity in vivo. As we reported before, the ratio of superoxide dismutase to malondialdehyde increased with increasing altitude (Zhu et al., 2019).
NMDAR is an important receptor that causes excitotoxic injury. CaMKII is an important downstream signaling pathway molecule of NMDAR, and its phosphorylation state can also reflect the activation state of NMDAR and the degree of involvement of this signaling pathway in excitotoxic injury (Baucum et al., 2013; Wu et al., 2019). Selective inhibition of synaptic NMDAR can attenuate excitotoxicity induced by global NMDAR stimulation in primary hippocampal neurons (Lai et al., 2014). In an MCAO model with transient or permanent occlusion, NMDAR antagonists can also protect neurons from ischemic death (Wu and Tymianski, 2018). Some studies have reported that suppression of the expression of NR2B and P-CaMKII generates a protective effect against cerebral ischemia and reperfusion injury (Lai et al., 2014; Wu and Tymianski, 2018; Wu et al., 2019).
Compared with the plain+MCAO group, the protein expression of NR1, NR2A, NR2B, and NR3A, as well as CaMKII and P-CaMKII, was decreased in the 4000 m + MCAO group. Therefore, it can be inferred that compared with plain hypoxia, hypobaric hypoxia may protect rats with MCAO against ischemic and reperfusion injuries to some extent, which are related to the decreased expression of NMDAR subunits, CaMKII and its phosphorylated form.
Tibet is a high incidence area of stroke in China, the incidence of stroke was 466.9 per 100,000 per year (Xu et al., 2013). There were different opinions about the incidence of stroke in different subtypes. Hemorrhagic stroke may be common in Tibet due to high blood pressure, heavy drinking, a meat diet and genetic differences in the population (Fang et al., 2011). The relatively low incidence of ischemic stroke in Tibet does not exclude that some patients with ischemic stroke have mild symptoms and have not been admitted to a hospital for treatment and therefore not be included in the statistical analysis (Fang et al., 2011). Another study reported that ischemic stroke is the most common type of stroke in the population of state-owned enterprises and government institutions in Lhasa. Good medical services and healthy lifestyles may reduce the risk factors for hemorrhagic stroke in the research population (Fang et al., 2011; Zhao et al., 2010). At present, there are few epidemiological investigations on stroke at high altitude, and the existing studies have their own limitations. We hope that more information will be provided as references for future research.
In addition to the abovementioned behavioral and molecular detections related to cerebral functions, blood parameters were also our focus. Priti Azad et al. reported that between 1.2% and 33% of individuals living at high altitude suffer from chronic mountain sickness (CMS) (Azad et al., 2017). In high altitude areas, blood viscosity will increase with an increased hematocrit, leading to polycythemia. Although there is an increase in RBC compensation, there is still a lack of blood oxygen due to hypoxia. These two parameters are risk factors for cerebral stroke and myocardial infarction in patients with CMS (Azad et al., 2017). Jha SK et al. reported that the most common type of stroke at high altitude is ischemic stroke, and the important risk factor for its occurrence is an increase in blood viscosity (Jha et al., 2002). Another retrospective case control study found that the primary hemorrhagic neurovascular diseases patients in Tibetan with high blood viscosity due to high HGB levels easily formed tiny thromboses, and further caused cerebral infarction (Chen et al., 2017). This is basically consistent with our findings. For further exploration, four groups were employed here, and we found that hypoxia simulation at high altitude can induce an increase in the number of RBCs and hemoglobin in rats compared with that of rats in plain. In addition, regardless of the normoxic or hypoxic condition, the MCAO model exhibited a further increase in the number of RBCs and hemoglobin, and hence, the most significant increase in the numbers of RBCs and hemoglobin was observed in the MCAO group after hypoxia.
In conclusion, compared with plain circumstance, the mortality of individual rats suffering from ischemic stroke after hypobaric hypoxia at high altitude is higher; however, as long as the rats survive, damage to the cerebral system is slightly less. This finding can be explained by two reasons: the augmentation of antioxidant abilities with increased altitude and the reduced neuroexcitoxicity engendered by the decreased expression of NMDAR subunits and activation of the CaMKII pathways. Hypoxia can cause an increase in the numbers of RBCs and hemoglobin at high altitude, which would be further exacerbated by cerebral ischemia and reperfusion injury. The increase in blood viscosity is a risk factor for the incidence of ischemic stroke at high altitude and also a pathophysiological mechanism for neuronal injury during the development of ischemic stroke at high altitude.
Due to limitations in experimental instruments, the MCAO model can not be conducted under continuous hypoxic conditions. However, the conclusions reached in the present study represent a solid theoretical basis for further research on ischemic stroke at high altitude, and in future, we would explore the pathophysiological mechanism of the stroke at high altitude from other aspects, including dietary patterns, the variances in ethnic genetic, and so on. As we are in the primary stage of this experiment, there are some flaws in the grouping, but future experiments will include improvements in the experiment’s design like including the additional four groups added in the hematology analyzing.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Azad P, Stobdan T, Zhou D, Hartley I, Akbari A, Bafna V, Haddad GG (2017) High-altitude adaptation in humans: from genomics to integrative physiology. J Mol Med (Berl) 95:1269–1282
Baucum AN, Brown AM, Colbran RJ (2013) Differential association of postsynaptic signaling protein complexes in striatum and hippocampus. J Neurochem 124:490–501
Chen R, Xiao A, Ma L, Li H, Lin S, You C (2017) Elevated hemoglobin is associated with cerebral infarction in Tibetan patients with primary hemorrhagic neurovascular diseases. Clin Neurol Neurosurg 157:46–50
Fang J, Zhuo-Ga C, Zhao Y, Kong F, Si Y, Liu M, Zhou D (2011) Characteristics of stroke in Tibet autonomous region in China: a hospital-based study of acute stroke. Eur Neurol 66:151–158
Jha SK, Anand AC, Sharma V, Kumar N, Adya CM (2002) Stroke at high altitude: Indian experience. High Alt Med Biol 3:21–27
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Luo Y, Tang H, Li H, Zhao R, Huang Q, Liu J (2019) Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur J Med Chem 162:132–146
Piccardi B, Arba F, Nesi M, Palumbo V, Nencini P, Giusti B, Sereni A, Gadda D, Moretti M, Fainardi E, Mangiafico S, Pracucci G, Nannoni S, Galmozzi F, Fanelli A, Pezzati P, Vanni S, Grifoni S, Sarti C, Lamassa M, Poggesi A, Pescini F, Pantoni L, Gori AM, Inzitari D (2018) Reperfusion injury after ischemic stroke study (RISKS): single-Centre (Florence, Italy), prospective observational protocol study. BMJ Open 8:e21183
Wang H, Peng RY (2016) Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Mil Med Res 3:26
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL (2017) Prevalence, incidence, and mortality of stroke in China: results from a Nationwide population-based survey of 480 687 adults. Circulation 135:759–771
Wu QJ, Tymianski M (2018) Targeting NMDA receptors in stroke: new hope in neuroprotection. MOL BRAIN 11:15
Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X, Wu B, Liu M (2018) Early prediction of malignant brain edema after ischemic stroke. STROKE 49:2918–2927
Wu SP, Li D, Wang N, Hou JC, Zhao L (2019) YiQi Tongluo granule against cerebral ischemia/reperfusion injury in rats by freezing GluN2B and CaMK II through NMDAR/ERK1/2 signaling. Chem Pharm Bull (Tokyo) 67:244–252
Xu G, Ma M, Liu X, Hankey GJ (2013) Is there a stroke belt in China and why? STROKE 44:1775–1783
Zhao Y, Yao Z, D'Souza W, Zhu C, Chun H, Zhuoga C, Zhang Q, Hu X, Zhou D (2010) An epidemiological survey of stroke in Lhasa, Tibet, China. STROKE 41:2739–2743
Zhu M, Xu M, Zhang K, Li J, Ma H, Xia G, Li X, Zhang B, Shi H (2019) Effect of acute exposure to hypobaric hypoxia on learning and memory in adult Sprague-Dawley rats. Behav Brain Res 367:82–90
Acknowledgments
This work was supported by the Major Project of Science and Technology Department in Tibet Autonomous Region of China [grant number 2015XZ01G21]; and the Project of Testing Technique in Tibetan Medicine from Ministry of Education in China. Minxia Zhu was the recipient of the fundings.
Funding
This work was funded by the Major Project of Science and Technology Department in Tibet Autonomous Region of China [grant number 2015XZ01G21]; and the Project of Testing Technique in Tibetan Medicine from Ministry of Education in China.
Author information
Authors and Affiliations
Contributions
Minxia Zhu designed the project and approved the final manuscript for submission; Yaqi Lei and Kexin Zhang analyzed and interpreted the data; Yuwen Xia and Chenjing Li acquired data; Yaqi Lei wrote the original paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures of animal handling were approved by the Ethics Committee for Experimental Research at Xizang Minzu University, and conformed to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, M., Lei, Y., Zhang, K. et al. The pathophysiological mechanism of ischemic stroke after hypobaric hypoxia simulation at high altitude. Metab Brain Dis 36, 483–490 (2021). https://doi.org/10.1007/s11011-020-00653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00653-9