Abstract
D-glycerate 2 kinase (DGK) is an enzyme that mediates the conversion of D-glycerate, an intermediate metabolite of serine and fructose metabolism, to 2-phosphoglycerate. Deficiency of DGK leads to accumulation of D-glycerate in various tissues and its massive excretion in urine. D-glyceric aciduria (DGA) is an autosomal recessive metabolic disorder caused by mutations in the GLYCTK gene. The clinical spectrum of DGA is highly variable, ranging from severe progressive infantile encephalopathy to a practically asymptomatic condition. We describe a male patient from a consanguineous Arab family with infantile onset of DGA, characterized by profound psychomotor retardation, progressive microcephaly, intractable seizures, cortical blindness and deafness. Consecutive brain MR imaging showed an evolving brain atrophy, thinning of the corpus callosum and diffuse abnormal white matter signals. Whole exome sequencing identified the homozygous missense variant in the GLYCTK gene [c.455 T > C, NM_145262.3], which affected a highly conserved leucine residue located at a domain of yet unknown function of the enzyme [p.Leu152Pro, NP_660305]. In silico analysis of the variant supported its pathogenicity. A review of the 15 previously reported patients, together with the current one, confirms a clear association between DGA and severe neurological impairment. Yet, future studies of additional patients with DGA are required to better understand the clinical phenotype and pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycerate is a three-carbon carboxylic acid that is usually undetectable in urine organic acid analysis of healthy individuals. Two separate rare inherited metabolic disorders, caused by distinct genetic deficiencies, can result in significant excretion of urinary glycerate that differs in its configuration (Rashed et al. 2002). L-glyceric aciduria (OMIM #260000), also known as primary hyperoxaluria type 2, has a clear well defined clinical phenotype with renal manifestations including recurrent nephrolithiasis due to hyperoxaluria (Cramer et al. 1999). In contrast, the clinical spectrum of D-glyceric aciduria (DGA) (OMIM #220120) is more diverse and mainly involves the central nervous system (Dimer et al. 2015).
DGA is an autosomal recessive metabolic disorder caused by mutations in the GLYCTK gene (OMIM #610516), which encodes the enzyme D-glycerate 2 kinase (DGK) (EC 2.7.1.31) (Van Schaftingen 1989; Guo et al. 2006; Sass et al. 2010). DGK mediates the conversion of D-glycerate, an intermediate metabolite of serine and fructose metabolism, to 2-phosphoglycerate (Dimer et al. 2015). Central nervous system involvement in previously reported patients with DGA is highly variable, ranging from progressive encephalopathy, hypotonia and severe psychomotor retardation on one hand to a very mild neurologic dysfunction and even asymptomatic condition on the other hand (Sass et al. 2010).
We report a patient with DGA caused by a novel homozygous mutation in GLYCTK, who displayed severe progressive infantile encephalopathy with intractable seizures, profound psychomotor delay, severe hypotonia, cortical blindness and deafness. In addition, we review the previously reported cases of DGA, to further delineate the clinical spectrum of this metabolic disorder.
Case report
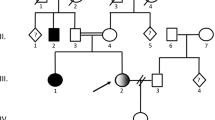
The patient is a nine year old boy, the first offspring of healthy first degree cousins of Israeli Arab origin. He was born following an unremarkable pregnancy by cesarean section due to maternal cause at 41 weeks of gestation, with a birth weight of 4280 g (97th percentile) and head circumference of 36.5 cm (90th percentile). At age 2.5 months he was initially admitted due to focal seizure during a febrile illness. Initial laboratory investigation results including complete blood count, blood chemistry and cerebrospinal fluid (CSF) analysis were normal. Brain computed tomography (CT) imaging was normal. The seizures were ceased by anti-convulsive therapy with phenobarbitone. At age 5 months he was readmitted due to a second episode of seizures. This time the seizures started focally with a later secondary generalization and required admission to the pediatric intensive care unit. Initial electroencephalography (EEG) revealed bilaterally asynchronous slow abnormal background activity with bilateral multifocal epileptic discharges. The first year of life was characterized by recurrent admissions due to refractory convulsions and recurrent episodes of aspiration pneumonia, which required insertion of a gastrostomy tube at age 7 months. Later, the seizures became variable and included clonic seizures of the left upper and lower limbs, which developed immediately into generalized tonic clonic seizures, as well as multifocal myoclonic spasms, and which were resistant to multiple anti-convulsive drugs including adrenocorticotropic hormone (ACTH) therapy, valproic acid, topiramate and levetiracetam. Repeated EEG studies displayed abnormally slow background activity and multifocal epileptic discharges (Supplementary Fig. 1).
The disease course was progressive, manifested mainly by failure to thrive, progressive microcephaly, generalized muscle atrophy, profound psychomotor retardation (with no eye contact, no communication skills and no motor milestones), cortical blindness, sensorineural hearing loss and severe hypotonia with evolving spasticity in all four limbs. At the age of 9.5 years, the patient succumbed, following a prolonged status epileptic episode triggered by febrile illness.
Brain MR imaging at age 5 months revealed significant enlargement of the cortical sulci and extremely wide open Sylvain fissures, consistent with an evolving brain atrophy (Fig. 1a, c). A follow up brain MR at age 7 years showed further enlargement of all CSF compartments. This implied progression of brain atrophy and thinning of the corpus callosum that was not present on the initial scan, and hyperintense T2 signals in the cerebellum and the occipital lobes, suggesting both white and gray matter lesions (Fig. 1b, d). Brain magnetic resonance spectroscopy (MRS) at age 7 years was unremarkable; specifically, no elevation of lactate peak was noted.
MRI images of the brain at age 5 months (a, c) and 7 years (b, d). At age 5 months, images show only mild volume loss, evident as mild enlargement of the Sylvian fissure bilaterally (c, asterisks) and mild ventricular enlargement. At age 7 years, volume loss is increased substantially, with thinning of the corpus callosum (b) and further enlargement of the third and lateral brain ventricles, and the Sylvian fissures (d, asterisks), as well as an increased T2 signal in the cerebellum and occipital lobes bilaterally (b, arrows), which involve grey and white matter
Extensive metabolic investigations were performed, which revealed normal results for serum creatine phosphokinase, liver transaminases, renal functions, acylcarnitine profile, serum transferrin isoelectric focusing, plasma lactate, amino acids, ammonia, very long chain fatty acids, urine alpha-aminoadipic semialdehyde, and CSF analysis of glucose, protein, amino acids, folate, pterins and neurotransmitters. Repeated urinary organic acid analyses revealed markedly increased excretion of glycerate.
Methods
Measurement of urinary D- and L-glyceric acid enantiomers
Measurement of the D- and L-glyceric enantiomers is based on the method of urinary measurement of D- and L-2-hydroxyglutarate enantiomers, by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride, as described previously (Struys et al. 2004) (more details are available in the supplementary data).
Biochemical assay of oxidative phosphorylation OXPHOS
Enzymatic activities of the five respiratory chain complexes, rotenone sensitive NADH CoQ reductase (Complex I), succinate cytochrome c reductase (complex II + III), succinate dehydrogenase (complex II) cytochrome c oxidase (COX complex IV) and Mg2+ATPase (complex V) were determined in isolated muscle and liver mitochondria from the patient, by spectrophotometric methods, as we have previously described (Saada et al. 2004). The activities were normalized to citrate synthase and compared to normal control means.
Whole exome sequencing (WES)
Exonic sequences were enriched in the DNA sample of the patient using SureSelect Human All Exon 50 Mb Kit V5 (Agilent Technologies, Santa Clara, California, USA(. Sequences were determined by HiSeq2500 (Illumina, San Diego, California, USA) and 100-bp were read paired-end. Reads alignment and variant calling were performed with DNAnexus software (Palo Alto, California, USA) using the default parameters with the human genome assembly hg19 (GRCh37) as a reference.
Results
Urinary organic acid analysis
Measurement of urinary D- and L-glyceric acid enantiomers in our patient revealed massive excretion of D-glyceric acid 4016 mmol/mol creatinine (control values <8 mmol/mol creatinine), as well as normal excretion of L-glyceric acid 11,4 mmol/mol creatinine (control values <63 mmol/mol creatinine) (Fig. 2a, b). Urinary glycerate excretion in both parents and 3 healthy siblings was in the normal range.
a-b D- and L-glyceric acid enantiomers in urinary samples of (a) a healthy control and (b) our patient. c-d Sanger sequencing of the T > C mutation shown by arrows in a homozygous state in (c) the patient and (d) in one of his heterozygous parents. e Amino acid sequence alignments showing high evolutionary conservation of the mutated residue in GLYCTK (arrow)
Biochemical assays of muscle and liver OXPHOS
Liver needle biopsy and muscle biopsy from the right quadriceps were obtained at the age of six months, while the patient was in the pediatric intensive care unit due to intractable seizures. At the time the biopsies were obtained, the patient received several anti-epileptic drugs including valproic acid. Light microscopy, immunohistochemical staining including COX, and electron microscopy were largely normal. Enzymatic activities of the five respiratory chain complex assays showed normal activities of complexes II, III and V, while complexes I and IV demonstrated mild to moderately reduced activities in both tissues but were more predominant in the liver (supplementary Table 1). In view of these results, we examined the effect of 1-5 mM D-glyceric acid on the activity of complexes I and IV in normal mitochondria in vitro, but detected no significant inhibition (supplementary Fig. 2).
Whole exome sequencing (WES)
Since the patient was the product of a consanguineous marriage, we performed WES under suspicion of a recessively inherited, rare, causal allele. WES yielded 47.2 million mapped reads with a mean coverage of X67. Following alignment and variant calling, we performed a series of filtering steps. These included removing variants that were called less than X8, were off-target, heterozygous, synonymous, had minor allele frequency (MAF) > 0.5% at Exome Aggregation Consortium (ExAC, Cambridge, Massachusetts, USA; http://exac.broadinstitute.org) or MAF > 1% at the Hadassah in-house database (∼1200 ethnic matched exome analyses). The remaining variants were filtered for conservation and pathogenicity; only four potential homozygous variants survived. Notably, none of them had any mitochondrial function or effect and only one, the homozygous missense GLYCTK variant [chr3:g.52,325,053 T > C (hg19), c.455 T > C, NM_145262.3], corresponded with the phenotype presented by our patient. This variant was not annotated in the ExAC database and was absent in the GnomAD, the dbSNP and the 1000 Genomes Project databases. The variant is predicted to change a highly evolutionary conserved leucine to a proline [p.Leu152Pro, NP_660305] (Fig. 2c, d). In silico web-based applications predicted this variant to be disease-causing by the MutationTaster software (Schwarz et al. 2014), “damaging” by SIFT (sorts intolerant from tolerant) and “probably damaging” by Polyphen 2. Sanger sequencing confirmed our patient to be homozygous, and both parents heterozygous, for this mutation, and it segregated perfectly in the family.
Discussion
Here we report a patient from a consanguineous marriage who presented with infantile onset progressive encephalopathy associated with DGA, confirmed both biochemically and genetically.
Since the first description in 1974 (Brandt et al. 1974), only 16 patients from 12 families were reported with DGA; of them, only six (including our patient) had a genetic confirmation (Kalim et al. 2017). The clinical, radiological and genetic characteristics of our patient and all patients reported until now are summarized in Table 1. Accordingly, DGA patients have a heterogeneous clinical presentation, with the majority (8/16) displaying a severe form dominated by progressive encephalopathy seizures and spastic quadriparesis (Brandt et al. 1974; Grandgeorge et al. 1980; Duran et al. 1987; Fontaine et al. 1989; Topcu et al. 2002; Insuga et al. 2010; Sass et al. 2010; Swanson et al. 2017). In contrast, some patients (5/16) present a milder neurological phenotype characterized by moderate developmental delay, autistic behavior and speech delay (Wadman et al. 1976; Bonham et al. 1990; Sass et al. 2010; Kalim et al. 2017), while a minority remain completely asymptomatic (3/16) (Bonham et al. 1990; Kalim et al. 2017). Interestingly, four siblings, documented almost three decades ago, had either no symptoms at all or mild neurological impairment (speech delay) (Bonham et al. 1990). This suggests a genotype phenotype correlation, as well as yet unidentified modifying genetic factors.
Refractory seizures and severe psychomotor retardation, manifesting during the first year of life, albeit non specific, are the core features of the severe form of DGA. Of note, seizure types are generally variable and include generalized seizures, focal seizures, infantile spasms, tonic-clonic spasms, clonic and myoclonic jerks and febrile induced seizures (Brandt et al. 1974; Grandgeorge et al. 1980; Duran et al. 1987; Fontaine et al. 1989; Topcu et al. 2002; Insuga et al. 2010; Sass et al. 2010; Swanson et al. 2017). Additional characteristics of severe DGA are hypotonia (7/8), microcephaly (4/8), spasticity (6/8) and failure to thrive (3/8) (Table 1).
Brain imaging has become an important tool in the diagnostic course of patients with epileptic encephalopathy. Only limited data of brain MR imaging is available for DGA patients. Accordingly, non specific cortical atrophy and white matter changes are the main features reported (Topcu et al. 2002; Insuga et al. 2010; Sass et al. 2010). Our report contributes to current knowledge, as it shows for the first time, by repeated MRI studies, progressive changes along the course of disease, and it further demonstrates the generalized white matter, and cerebellar and cortical involvement; this implies ongoing, destructive central nervous system pathology.
We described the fifth DGA mutation to be identified. Two previously reported GLYCTK mutations (p.F483Sfs*2 and p.Phe493Cys) cluster within the multi-organism fragment with the rich leucine (MOFRL) domain of the enzyme (Sass et al. 2010). This domain is distinctive for animal glycerate kinases. Although the precise function of MOFRL is not clear, mutations located in this domain are predicted to impair enzyme function and stability (Kehrer et al. 2007). Unsurprisingly, the two patients identified with mutations in the MOFRL domain presented with the severe form of DGA. The other two previously reported GLYCTK mutations are located in other areas of the protein and these patients were either asymptomatic or manifested a mild neurologic presentation (Sass et al. 2010; Kalim et al. 2017). The novel p.Leu152Pro mutation identified in our patient is located within the Domain of Unknown Function 4147 (DUF4147). This domain is frequently located at the N-terminus region of proteins carrying the MOFRL domain, and its precise function is currently unknown. Hence, we report here the only patient with the severe form of DGA with a mutation located in this domain. Accordingly, p.Leu152Pro should be considered a severe mutation associated with infantile onset and progressively deteriorating neurologic course and grave outcome.
The highly variable clinical spectrum of DGA, ranging from severe progressive infantile encephalopathy to a practically asymptomatic condition raises the question as to whether DGA is a true metabolic disorder or merely a biochemical phenotype (Kalim et al. 2017). In a historic patient of DGA, Swanson et al. recently identified the co-occurrence of non-ketotic hyperglycinemia caused by a homozygous pathogenic mutation in the AMT gene. This suggests that this patient’s severe neurological presentation may be related mainly to glycine encephalopathy (Swanson et al. 2017). In addition to the known clinical ambiguity, the mechanism of the neurological impairment remains unsolved. Our patient presented with a clinical phenotype that was initially assumed to be caused by a mitochondrial impairment. Therefore, muscle and liver biopsies were obtained (based on the apparent clinical involvement of these tissues) and were studied for the activity of the respiratory chain complexes. In view of the mild to moderate reduction in the activities of complexes I and IV in both muscle and liver, we hypothesized a secondary mitochondrial demise, caused by the massive tissue accumulation of D glycerate. Our results suggest that inhibition of OXPHOS by D glycerate is unlikely, implying an alternative mechanism for the disease. The decreased activities of complexes I and IV are probably secondary to valproic acid therapy (Finsterer 2017), and consequent to the generalized poor condition of the patient during his intensive care stay.
In conclusion, we describe a patient who presented with progressive epileptic encephalopathy presumably caused by DGA, due to a novel homozygous GLYCTK mutation. Our review of all the cases that have been reported with DGA, clearly highlight the severe neurological form as the most common presentation. The precise pathological mechanisms, as well as the causes for mild presentations (genetic, environmental), are yet to be determined. Additional studies of patients with DGA will further facilitate in resolving these matters.
References
Bonham JR, Stephenson TJ, Carpenter KH, Rattenbury JM, Cromby CH, Pollitt RJ, Hull D (1990) D(+)-glyceric aciduria: etiology and clinical consequences. Pediatr Res 28(1):38–41
Brandt NJ, Brandt S, Rasmussen K, Schnoheyder F (1974) Letter: Hyperglycericacidaemia with hyperglycinaemia: a new inborn error of metabolism. Br Med J 4(5940):344
Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP (1999) The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet 8(11):2063–2069
Dimer NW, Schuck PF, Streck EL, Ferreira GC (2015) D-glyceric aciduria. An Acad Bras Cienc 87(2 Suppl):1409–1414
Duran M, Beemer FA, Bruinvis L, Ketting D, Wadman SK (1987) D-glyceric acidemia: an inborn error associated with fructose metabolism. Pediatr Res 21(5):502–506
Finsterer J (2017) Toxicity of antiepileptic drugs to mitochondria. Handb Exp Pharmacol 240:473–488
Fontaine M, Porchet N, Largilliere C, Marrakchi S, Lhermitte M, Aubert JP, Degand P (1989) Biochemical contribution to diagnosis and study of a new case of D-glyceric acidemia/aciduria. Clin Chem 35(10):2148–2151
Grandgeorge D, Favier A, Bost M, Frappat P, Bon-Jet C, Garnel S (1980) L'acidemie D-glycerique: a propos d'une nouvelle obsevation anatomo-clinique. Arch Fr Pediatr (37):577–584
Guo JH, Hexige S, Chen L, Zhou GJ, Wang X, Jiang JM, Kong YH, Ji GQ, Wu CQ, Zhao SY, Yu L (2006) Isolation and characterization of the human D-glyceric acidemia related glycerate kinase gene GLYCTK1 and its alternatively splicing variant GLYCTK2. DNA Seq 17(1):1–7
Insuga VMS, Requena PT, Bermejo AM, Merino M, De La Puente AJA, Viana H, Murias S, Garcia MJ (2010) Aciduria D- glicerica. A proposito de un caso y revision de la bibiliografia. Acta Pediatr Esp (68):79–83
Kalim A, Fitzsimons P, Till C, Fernando M, Mayne P, Sass JO, Crushell E (2017) Further evidence that d-glycerate kinase (GK) deficiency is a benign disorder. Brain and Development 39(6):536–538
Kehrer D, Ahmed H, Brinkmann H, Siebers B (2007) Glycerate kinase of the hyperthermophilic archaeon Thermoproteus tenax: new insights into the phylogenetic distribution and physiological role of members of the three different glycerate kinase classes. BMC Genomics 8:301
Rashed MS, Aboul-Enein HY, AlAmoudi M, Jakob M, Al-Ahaideb LY, Abbad A, Shabib S, Al-Jishi E (2002) Chiral liquid chromatography tandem mass spectrometry in the determination of the configuration of glyceric acid in urine of patients with D-glyceric and L-glyceric acidurias. Biomed Chromatogr 16(3):191–198
Saada A, Bar-Meir M, Belaiche C, Miller C, Elpeleg O (2004) Evaluation of enzymatic assays and compounds affecting ATP production in mitochondrial respiratory chain complex I deficiency. Anal Biochem 335(1):66–72
Sass JO, Fischer K, Wang R, Christensen E, Scholl-Burgi S, Chang R, Kapelari K, Walter M (2010) D-glyceric aciduria is caused by genetic deficiency of D-glycerate kinase (GLYCTK). Hum Mutat 31(12):1280–1285
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11(4):361–362
Struys EA, Jansen EE, Verhoeven NM, Jakobs C (2004) Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin Chem 50(8):1391–1395
Swanson MA, Garcia SM, Spector E, Kronquist K, Creadon-Swindell G, Walter M, Christensen E, Van Hove JLK, Sass JO (2017) D-Glyceric aciduria does not cause nonketotic hyperglycinemia: a historic co-occurrence. Mol Genet Metab 121(2):80–82
Topcu M, Saatci I, Haliloglu G, Kesimer M, Coskun T (2002) D-glyceric aciduria in a six-month-old boy presenting with west syndrome and autistic behaviour. Neuropediatrics 33(1):47–50
Van Schaftingen E (1989) D-glycerate kinase deficiency as a cause of D-glyceric aciduria. FEBS Lett 243(2):127–131
Wadman SK, Duran M, Ketting D, Bruinvis L, De Bree PK, Kamerling JP, Gerwig GJ, Vliegenthart JF, Przyrembel H, Becker K, Bremer HJ (1976) D-Glyceric acidemia in a patient with chronic metabolic acidosis. Clin Chim Acta 71(3):477–484
Acknowledgments
We thank Cindy Cohen for professional language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Emek Medical Center ethics committee (study no. EMC-0067-09).
Informed consent
Informed consent for participation in the study and publication of the study case was obtained from all individual participants included in the study or their legally authorized representative (parents).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 334 kb)
Rights and permissions
About this article
Cite this article
Zehavi, Y., Mandel, H., Eran, A. et al. Severe infantile epileptic encephalopathy associated with D-glyceric aciduria: report of a novel case and review. Metab Brain Dis 34, 557–563 (2019). https://doi.org/10.1007/s11011-019-0384-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-0384-x