Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disturbance leading to memory deficit, cognitive decline, and behavioral disturbance. Deposition of Amyloid beta plaques, neurofibrillary tangle and mitochondrial impairment are common neuropathological signs in AD. In this study, the effect of standardized Cyperus rotundus(C. rotundus) extract in three different doses of 250, 500, and 750 mg/kg on memory, neurogenesis and mitochondrial mass in the beta amyloid rat model was assessed. For this purpose, 42 male Wistar rats were randomly divided into six groups (n = 7) to evaluate baseline training performance in Morris water maze test. Amyloid beta (Aβ) was injected in animal hippocampal CA1 bilaterally in four groups. After 21 days, a decrease was observed in spending time in target quadrant in the first probe trial in Aβ injected groups. Following that, 250, 500, and 750 mg/kg of C. rotundus extracts were administered to three out of four groups for a period of one month. BrdU (Bromodeoxyuridine) was intraperitoneally injected in all groups on the last 7 days of treatment. Then, 28 days after the last BrdU injection, the second probe trial was run, and rats were sacrificed. The neurogenesis and mitochondrial distribution were detected in hippocampus, by immunohistochemical staining. At last, it was observed that C. rotundus, almost recovered memory impairment, in addition to increasing in mitochondrial mass in CA1 and neurogenesis in dentate gyruse in the beta-amyloid rat model of Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AD is an age-related neurodegenerative disturbance (Šerý et al. 2013). The two key neuropathological alterations in AD consist of extracellular deposition of Aβ known as a senile plaque and intracellular neurofibrillary tangles (NFTs) in neocortex, hippocampus and other brain regions involved in cognitive functions (Moreira et al. 2010). NFT is formed from hyperphosphorlylated tau protein, while Aβ is produced by proteolysis of amyloid precursor protein (APP) with β and γ-secretase enzymes (Selkoe 2001). AD causes synaptic dysfunction and loss of neuronal cells in the hippocampus and entorhinal cortex leading to memory impairment and cognitive disability (Goedert and Spillantini 2006). Adult neurogenesis constitutively occurs in specific brain regions in the mammalian brain, including the hippocampal dentate gyrus and the subventricular zone (Bonfanti and Peretto 2011). Alterations in adult neurogenesis is another hallmark of different neurodegenerative diseases such as Parkinson’s disease (PD), Huntington’s disease (HD) and AD (Winner and Winkler 2015). Therefore, it shows that one of the main theraputic approaches to AD is the replacement of missed neuroun and an increase in neurogenesis.
Recent researches have demonstrated that Aβ high deposition is associated with great amount of oxidative products leading to oxidative stress and mitochondrial disturbance (Butterfield and Lauderback 2002). In the mitochondrial matrix, there are two main proteins of amyloid binding alcohol dehydrogenase (ABAD) and cyclophilin D (CypD) to which Aβ can be attached (Du et al. 2011). Deposition of Aβ in mitochondria leads to impaired activity of tricarboxylic acid cycle enzymes and a decrease in cytochrome c oxidase (COX), an increase in free radical generation, mutation in mtDNA, and a decrease in ATP production (Mohmmad Abdul et al. 2006). Consequently, reduction in the mitochondrial count in neuronal cell and synaptic terminals leads to functional disturbance of neuronal cells and death, thereby explaining the mechanism of neurodegeneration processes (Hirai et al. 2001). Recent findings in herbal medicines have indicated a source for new therapeutic approaches for dementia and AD (Mahboubi et al. 2016; Zhang et al. 2016). Experiments run in this area indicate that some of these herbs may have neuroprotective and neurogenesis effects on nerve tissues (Kim et al. 2007).
C. rotundus from Cyperaceae family, is a traditional medicinal herb widely grown in Iraq, Iran, and India. C. rotundus rhizome has antimalarial, analgesic, sedative, nootropic, and anti-inflammatory effects, and is effective in diarrhea, dysentery, indigestion and other gastrointestinal problems (Uddin et al. 2006). Several phytochemical studies into root extracts of this plant reported the presence of β-sitosterol, cyperene, cyperol, flavonoids, sesquiterpenoids, ascorbic acid and polyphenols (Sonwa and König 2001). Methanolic extract of C. rotundus rhizome was found to inhibit nitric oxide (NO) production in RAW 264.7 cells and was observed to exhibit anti-inflammatory effects (Seo et al. 2001). It was showed that the high level of flavonoid compounds (Yazdanparast and Ardestani 2007), particularly total oligomeric flavonoids (TOFs) of C. rotundus, was associated with different biological activities (Kilani-Jaziri et al. 2009). These flavonoids are commonly found in most of the plants belonging to the group of polyphenolic compounds (Manach et al. 2004). A number of studies have demonstrated that the consumption of flavonoid-rich plant extracts may cause improvement in cognitive performance (Spencer 2008). This major feature seems to be related with the role of flavonoids in interacting with intracellular neuronal and glial signaling pathways, affecting the peripheral and cerebral vascular system, and thereby reducing neuroinflammatory and neurodegenerative pathways. Flavonoids have a lipophilic nature, showing infusion in the brain by intracting with specific efflux transporters present in the blood-brain barrier, thereby enhancing cognitive performance and neuroprotective function (Youdim et al. 2004). A previous work reported that flavonoids increased antioxidant enzymes, leading to expression of proteins such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), transcription factor of cyclic AMP response element binding protein (CREB), and early growth response protein-1 (ERG-1, or Zif268) related to synaptic plasticity, thereby increasing protective effect on vulnerable neurons, improving the neuronal function, including neuronal regeneration, and causing neurogenesis (Dinges 2006; Mann et al. 2007).
It has been recently indicated that administration of total oligomeric flavonoids isolated from C. rotundus can significantly reduce neurological dysfunction in the middle cerebral artery occlusion (MCAO) of rats (Sunil et al. 2011).
Accordingly, its therapeutic potential on the nervous system has caused the authors here to design and assess the therapeutic effects of its rhizome extract on memory impairment, neurogenesis and mitochondrial mass in the Aβ rat model.
Material and method
Herbal extract preparation
Fresh rhizomes of C. rotundus (10 kg) were collected from Bahr Al-Najaf, Iraq on February 2016. Plant materials were identified and confirmed by Dr. Mustafa Ghanadian, Pharmacognosy Department, Faculty of Pharmacy, Isfahan University of Medical Sciences, Iran, according to the voucher specimen number 2262 deposited there. The plant was dried under shade and milled into powder using an electrical mill. After sieving, ethanol was added, mixed, left for two hours at room temperature. The mixture was poured into a percolator, and 6 Lethanol was added to cover mixure by approximately 5 cm. After three days, the extract was discharged with no break at a flow rate of 4 mL/min from bottom tap through cotton wool for a peroid of one week. The collected extract was evaporated through a Rotavapor (Büchi-Labortechnik Gmb, Konstanz, Germany) at 45 °C and 10 mbar. Then, the extract was suspended in a mixture of water (1000 mL) and chloroform (500 mL), shaked for 10 min, and then the chloroform and aqoeous parts were separated in a 2-l separating funnel. The Chloroform portion rich in oil, fat and nonpolar constiuents was separated. The defatted aqueous portion was filtered, and concentrated in a Rotavapor under the same conditions mentioned above and stored at 4 °C until use.
Extract standardization
C. rotundus was standaridized through the external stanardization method based on its Luteolin content. Luteolin was selected, since it is reported as a neuroprotectant against different Alzheimer’s pathogenesis. Therefore, it was selected for high-performance liquid chromatographic (HPLC) standardization of the extract as one of the components responsible for the observed effects. HPLC analysis was conducted on a 515 Waters HPLC pump, attached to a 2487 UV–Visible detector worked at 350 nm (Waters, Milford, MA, USA), and operated by the Millennium software to quantify and determine Luteolin. Before analysis, the defatted extract (187.9 mg) was hydrolysed by 2 N HCL (10 mL) at 90 °C for 1 h to release Luteolin from its conjugated sugars. Next, the hydrolyzed portions were extracted by diethyl ether at three times using a separating funnel. Organic solvent was air dried, and HPLC solvent A was added at 1 mL. Then, it was injected in a volume size of 5 μL into a Nova-Pak C18, 3.9 × 150 mm (Waters, Milford, MA, USA) by applying H3PO4 10 mM in CH3CN:H2O:H3PO4 (54.5: 445: 0.5) as solvent A and CH3CN:H2O:H3PO4 (345.5:150: 0.5) as solvent B with a stepwise gradient system of 1 mL/min flow rate in 14-min separation time. This process was begun with A: B (100:0) hold for 2 min, then 0–100% B for 12 min, with equilibration of 0%–100% A for 4 min. The flow rate was 1 mL/min at a temperature of 40 °C. Standards within the range of 2–20 μg/mL were also injected in the same way. A standard calibration curve was prepared through different concentrations of Luteolin (Sigma, USA) as standard material for quantitative analysis. The corelation between the concentration and the peak-area of standard was measured using the minimum square method (R2 value).
Animals
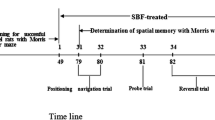
A total of 42 male Wistar rats (230–250 g) were obtained from Isfahan University of Medical Sciences and maintained in a 12-h light–dark cycle with food and water available ad libitum. The Ethics Committee for Animal Experiments (393,476, revised 1985) at Isfahan University of Medical Sciences approved the study, in accordance with the international guiding principles for the biomedical research involving animals. One week after adaptation to the environment, all animals were randomly divided into six groups (n = 7) for baseline training performance measurement in the MWM test, after which the animals were grouped as control, without any surgery and treatment, receiving ip injections of BrdU 50 mg/ kg twice a day for 7 days; sham, the operated rats received 5 μL normal saline into hippocampal CA1 bilaterally under operation, receiving 1 mL distilled water orally for 30 days after surgery and injections of BrdU during the last 7 days in addition to the distilled water consumption; AD, received 5 μg/μL Aβ1–42 into CA1 bilaterally, and 1 mL distilled water orally for 30 days after surgery and injections of BrdU during the last 7 days in addition to the distilled water consumption; treatments (250, 500,750 mg/kg), which received 5 μg/μL Aβ1–42 into CA1 bilaterally, and oral administration of the C. rotundus extract in three different doses of 250, 500, 750 mg/kg for 30 days after surgery and injections of BrdU during the last 7 days of the C. rotundus treatment. The Schematic experimental timeline during the course of study was repersented in (Fig. 1).
Morris water maze (MWM) test
Spatial learning and memory were evaluated through the MWM test modified for rats. MWM test was performed in a circular tank (diameter, 183 cm; height, 60 cm) filled with water (24 °C). This tank was divided into four quadrants of North West (NW), North East (NE), South West (SW), and South East (SE), which provided four alternative start positions as zone 1, zone 2, zone 3, and zone 4. A platform (12.5 cm in diameter and 38 cm high) was submerged 2 cm below the water surface in the SE quadrant (target quadrant) in the same position of every trial. A video camera was fixed at 1.4 m above the center of the water tank, and all swimming trials were recorded. The rats were trained for four times per day (with an inter trial interval of 5 min) for four days. At each trial, the rats were placed into the water in a different quadrant, allowed to find the platform in 60 s. All the rats were then allowed to stay on the platform for 30 s for training, before returning to the cage. In cases where rats failed to find the platform in the set time, they were guided again to the platform and allowed to stay there for 30 s. The rats’ behaviors were recorded by a computerized video tracking system measuring the time latency to find the platform. After completion of this phase, the rats were gently dried and returned to their home cages until the initiation of the retention phase (probe trials) on the test days. In probe trial sessions, the platform was removed, and the rats were allowed to swim freely for 60 s looking for the platform. In this test, the time to find the platform and the average time that the rats spent on the target quadrant for searching the platform were indexed to evaluate learning and memory.
Stereotaxic surgery
Rat amyloid β-protein Aβ1–42 (Sigma, St. Louis, MO, USA) was dissolved in sterile normal saline (1 μg/μL). The solution was incubated at 37 °C for one week before use. The rats were anesthetized with chloral hydrate (350 mg/kg). After anesthesia, the animal’s head was fixed in a stereotaxic instrument (Stoelting, Kiel, WI). The scalp was incised in the midline, and the related area was cleaned to exposed to bregma, lambda and sagittal sutures. Small holes were drilled into the skull above the injection sites. The Aβ was injected through microinjection at 5 μg/5 μL and delivered slowly at a rate of 1 μL /min in hippocampal CA1 region bilaterally (AP = −3.36 mm, ML = ±1.6 mm from the bregma and DV = 3.2 mm from top of the skull) according to Paxinos and Watson Atlas (George and Charles 2007). After injection, the needle was kept in a place for 5 min to allow complete diffusion. In the sham group, normal saline was injected into CA1. After surgery, the animals were placed in heated chambers, and allowed to recover for 5–7 days. Histological assessment was conducted to establish the correct injected area. Twenty-one days after surgery, the first probe trial of MWM test was conducted to find memory impairment in Aβ injected groups.
Congored staining
After the first probe trial, one rat from each group was transcardially perfused (subject to deep anesthesia with chloral hydrate, 350 mg/kg) with 100 mL saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate buffer saline (pH 7.4). After perfusion, the brain tissue was removed and post-fixed in the same fixative solution for one day at 4 °C. The hippocampus was separated, histological processing was conducted, and paraffin-embedded tissue sections were cut into 5 μm thick coronal serials sections and mounted on slides for hydration in water (2 min), staining in Congored solution (0.5% in 50% ethanol) for 45 min, rinsing in distilled water, rapid differentiation (8 s) in KOH solution, rinsing in tap water (2–3 s), and counterstaining with hematoxylin Harris. Through this protocol, Aβ plaques were stained in red, and the nuclei were stained in blue (Esfandiary et al. 2014).
C. rotundus treatment
After the first probe trial, C. rotundus was dissolved in distilled water and then it was administered through a gastric tube at 250, 500, and 750 mg/kg doses in treatment groups for 30 days. The sham and AD groups received distilled water in the same period.
Administrating Bromodeoxyuridine
The 5-bromo-20-deoxyuridine (BrdU, Sigma) was dissolved at 10 mg/mL in NaCl. All the animals received ip injections of 50 mg/ kg twice day during the last 7 days of gavages. To assess adult neurogenesis, on day 28 after the last BrdU injection, the rats’ behaviors were tested after which they were sacrificed (Esfandiary et al. 2014).
Immunofluorescence staining for neurogenesis
After the second probe trial, hippocampus was separated, processed and blocked for histological studies. Two series of coronal serial sections (4 μm thickness) with 480-μm intervals were obtained from the entire hippocampus. The first series were considered for MitoTracker Green FM staining and the second for BrdU and NeuN staining. After deparaffinization and rehydration of the sections, they were rinsed Tris-buffered saline (TBS). For DNA denaturation, the sections were incubated in 2XSSC formamide for 2 h at 60 °C. They were then rinsed with 2XSSC 10 min, incubated with H2O2 and methanol for 20 min, incubated in 2 N HCl for 30 min, rinsed for 10 min in 0.1 M boric acid, pH 8.5, and rinsed in TBS. Then, the sections were incubated in TBS 3% goat serum and 0.3% Triton X (TBS++) for 30 min. They were then incubated overnight at 4 °C temperature for double labeling in a mixture of primary antibodies, including mouse anti-NeuN (United Kingdom) catalog No. MAB377 [2:200], and rat anti-BrdU (USA) catalog No. OBT0030 [3:100]. On the next day, after rinsing with TBS, the sections were incubated in a mixture of secondary antibodies, including Alexa Fluor 568 goat anti-rat (catalog No. A-11077 (red; 2:150)) and goat anti mouse Alexa Fluor 488 (catalog No. A-11001 (green; 2:150)) in a humid and dark chamber at room temperature for 1 h and rinsed with TBS, and nuclear counterstaining was carried out with 4′,6′-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) (Sigma) for 3 min. All the antibodies were dissolved in TBS++. The sections were observed through a fluorescence microscope, and then they were photographed digitally (Zeiss, Axiophot, Germany) (Esfandiary et al. 2014).
MitoTracker green FM staining
To prepare a stock solution of MitoTracker, each lyophilized Mito Tracker vial (50 μg, Invitrogen) was dissolved in 74.4 μL high quality of anhydrous dimethylsulfoxide (DMSO). For staining, 1 μL of stock solution was dissolved in 3000 μL phosphate buffer saline (PBS). After deparaffinization and rehydration, the sections were rinsed with PBS then incubated in MitoTracker Green FM solution in a humid and dark chamber at room temperature for 30 min and then rinsed with PBS (de la Monte et al. 2000). For nuclear counterstaining, the sections were incubated with DAPI for 4 min, then they were observed through a fluorescence microscope and photographed digitally.
Statistical analysis
All the data were expressed as mean ± SEM and analyzed in the SPSS 23 software. The one way ANOVA post hoc LSD and Kruskal-Wallis tests were conducted to have a one way comparison. To observe frequencies in alternative times, the repeated measures and generalized linear models (GLM) tests were performed.
Result
Herbal extract standardization
Ethanolic extract of dried C. rotundus (2.5 kg) was prepared through the percolation method (180.3 g). After defatting and separation of apolar content,the aqoeus part of the ethanolic extract (130.4 g) was selected for this study through liquid liquid partitioning between chloroform and water. The correlation between concentration and peak area was determined through calibration curve of standard material (2, 4, 6, 8, 10 and 20 μg/mL). The regression equation of calibration curve was y = 17,096x + 8177.9, where x was the concentration of total luteoin in the defatted extract (μg/mL) with the correlation co-factor (R2) of 0.9943. Dried defatted extract was standarized to have 62.82 ± 3.81 μg luteolin per gram (Fig. 2).
Congored staining
Twenty one days after injection of Aβ, the plaques were detected through Congored staining in hippocampus, especially in CA1 (Fig. 3).
The impact of C. rotundus extraxt on memory
The results of statistical analyses, repeated measures and generalized linear models (GLM) indicated that there was no significant difference in the mean time latency to find hidden platform in all groups in 1–4 days spatial acquisition before Aβ injection (p = 0.81). Significant differences were observed in escape latency within days 1 and 4, in all the groups (p < 0.001), demonstrating that the learning performance in all the groups had improved significantly (Fig. 4).
The analysis of Kruskal-Wallis test in prob1 indicated a significant difference among the 6 groups (p = 0.03) as: control with AD and treatment groups individually (p < 0.05), and sham with AD and treatment groups individually(p < 0.05). There were no significant differences in the mean percentages of time spent on target quadrant between control and sham groups (p = 0.7). To assess the fluctuation of the second probe trial, the ANOVA test revealed no statistically significant difference among the six groups (p = 0.34). This occured when the treatment 500 mg/kg group revealed a none significant increase in (p < 0.05) in comparison with the AD group (Fig. 5).
The probe trials 1 and 2 in the experimental groups (prob 1 showing the average time rats spent on the target quadrant 21 days after Aβ injection, showed gray bars, * significant difference with the control group(p < 0.05), # significant difference with the sham group (p < 0.05)), (prob 2 showing the average time rats spent on the target quadrant after C. rotundus treatment, showed white bar, the treatment 500 mg/kg group revealed a none significant increase in (p < 0.05) in comparison with the AD group)
The impact of C. rotundus extract on adult neurogenesis
The differentiation of a new neuronal stem cell to neuron in the subgranolar zone (SGZ) of dentate gyrus through counting BrdU-positive cells 4 weeks after the last injection by double staining for BrdU and the neuronspecific marker NeuN was important (Fig. 6). To assess BrdU and BrdU/NeuN positive cells, Kruskal-Wallis test was conducted, indicating the existance of a statistically significant difference among the six groups in BrdU (p = 0.03). As to BrdU positive cells, this difference existed between the treatment 500 mg/kg group with both AD and treatment 250 mg/kg groups (p < 0.05) as well as between the control group with the AD group (p < 0.05). Furthermore, the Kruskal-Wallis test was performed, demonstrating the existance of a statistically significant difference among the six groups in BrdU/NeuN (p = 0.02). As to BrdU/NeuN, this difference existed between the treatment 500 mg/kg group with both AD and treatment 250 mg/kg groups (p < 0.05) as well as between the control with both AD and treatment 250 mg/kg groups (p < 0.05) (Fig. 6).
Double staining for BrdU (red), Neu N (green) and DAPI (blue) in dentate gyrus 28 days after the last BrdU injection(x = 200). Quantification of BrdU and BrdU/NeuN positive cells in dentat gyrus. * significant difference with treatment 500 mg/kg group (p < 0.05), # significant difference with control group (p < 0.05)
The impact of C. rotundus extract on mitochondria
In this staining, the mitochondria was shown in green dots, and the nuclei in blue. Quantitiy of mitochondrial mass was assess with image J (Analysis particles) software and Kruskal-Wallis test. This mitochondrial mass in control and treatment 500 mg/kg groups was more than that in other groups. There was a significant difference in (p < 0.1) between AD group with control and treatment 500 mg/kg groups. In the AD group, mitochondrial mass was less than that in other groups. Mitochondrial mass in treatment 250 and 750 mg/kg groups was not more than that in other groups (Fig. 7).
Disscusion
AD is the most current form of dementia characterized by memory impairment, behavioral deficit, progressive neurodegeneration, and mitochondrial damage in the brain (Reddy and Beal 2008). The most significant therapeutic agents proposed for AD consist of acetylcholinesterase inhibitors, which are palliative and temporary effective treatments, and cannot prevent the progressive process of this disease; these drugs are approved by the Federal Drug Administration (FDA) for AD treatment (Anekonda and Reddy 2005). The herbal medicines with anti-inflammatory, anti-oxidant, and anti-amyloid properties, and almost no side effects have been studied in recent decades for AD treatment (Kim et al. 2007). In this study, in the HPLC analysis, the concentration of flavonoids, especially Luteolin was more than other components in this extract. In previous studies, the neuroprotective effects of Luteolin on AD were considered to ehance acetylcholine, and its reversible effects on oxidative injuries in Aβ rats’ model were investigated (Yu et al. 2015). Here, the enhancing effects of the selected doses on memory impairment, neurogenesis and mitochondrial distribution in rat Alzheimer’s model induced by Aβ injection in CA1 were analyzed. Here, Aβ plaques accumulate in extracellular regions in synaptic spaces, 18–21 days after injection of this substance in CA1. According to some studies, microglia and astrocyte usually gather to clear these plaques. In this context, the findings here correspond to these studies (Kitazawa et al. 2004). Here, in the first probe trial, it was revealed that there existed a significant decrease in spending time on target quadrant, between AD, and control and sham groups. The findings above indicated interesting correspondence in the time needed to detect the plaque in CA1 and memory impairment appearance (21 days). In the secound probe trial, there was no significant increase in the treatment 500 mg/kg group. The 250 and 750 doses of this extract slightly increased spending time on target quadrant. Here, BrdU and Neu N positive cells were increased significantly after 28 days of BrdU injection in the treatment 500 mg/kg group. Double staining with BrdU and NeuN confirmed that the increase in BrdU- and NeuN-coexpressing cells might be basicaly owing to an increase in neurogenesis not in gliogenesis. The adult neurogenesis was associated with spatial learning and memory performance improvement (Kee et al. 2007; Clelland et al. 2009; Garthe et al. 2009). Flavonoids through interactions with some specific signalling pathways and increase in neurotrophins like BDNF lead to an increase in neurogenesis and neuronal plasticity (Lim et al. 2003). In the present study, it was suggested that C. rotundus extract might be through enhancing the expression of different neurotrophic factors such as BDNF and NGF, causing an incerase in neurogenesis. These functions lead to cognitive performance enhancement in neuro-cognitive disorders (Spencer et al. 2009). Mitochondrial mass in the immunohistochemical staining of CA1 was increased in the treatment 500 mg/kg group and similar to that in the control group. In the AD group, mitochondrial mass was sparsely scattered, since Aβ degenerated mitochondria, and consequently the neuronal cells. In the early stage of AD, mitochondria were degenerated, therefore, they cannot move toward the nerve terminals and produce the necessary ATP for communication with other neurons (Reddy and Beal 2005).
A recent study reveals that aging and neurodegenerative diseases, including AD, may be induced by impaired mitochondrial function resulting from oxygen free-radical damage and aggregation of mitochondrial (Mt) DNA mutations (Reddy 2009). Therefore, increased MtDNA damage results in reduced mitochondrial mass (MitoTracker Green labeling) and MtDNA content (de la Monte et al. 2000). In the previous study, it was showed that C. rotundus extract had antioxidant and free radical scavenging effects; therefore, in this study, it was supposed that C. rotundus extract through antioxidant effect caused to improve mitochondrial mass in AD. Mitochondrial mass in treatment 250 and 750 mg/kg groups was lower than that in the treatment 500 mg/kg group. High dose of C. rotundus might have toxic components inhibiting the curative effects of this extract. There exist very few studies on C. rotundus.
In the previous study, other effects of C. rotundus total extract on learning and memory and antioxidant plasma level in rat Alzheimer’s model induced by nucleus basalis of Meynert lesioned were measured (Rabiei et al. 2013). The results of this study reveal that C. rotundus ethanolic extract could prevent the behavioural disorders induced by NBM lesion in rats (Rabiei et al. 2013). This study was different from the type of model and investigated factors in our study. Therefore, in our study, the method of extraction, the type of model and the factors studied, according to the model, have sufficient innovation. In another study, neuroprotective properties of total flavenoids of C. rotundus (TOFs) in a model of cerebral ischemia and reperfusion were investigated (Sunil et al. 2011). In this experimantal study, TOFs in rats were found to be characterized by decreased glutamate, glutamine synthetase (GS) and increased Na+ K+ ATPase activity in a dose-dependent manner and significantly led to reducing the neurological deficits and reversing the anxiogenic behavior. However, it was found to significantly decrease MDA, and increase superoxide dismutase (SOD) and glutathione content in rats’ brains (Sunil et al. 2011). This investigation compared to our present study was different in experimental design regarding type of extract, which was only total flavenoid, AD model, and factores studied.
Mehdizadeh et al. showed the useful effects of C. rotundus extract (400 mg/kg, intraperitoneally) on memory impairment of Aβ rats model in Morris water-maze tasks (Mehdizadeh et al. 2017). These findings were partly conformed with our investigation in the present study. The effects of C. rotundus are assessed for its phenolic and flavonoid components due to antioxidant, neuroprotective, neurogenesis, enhancment of neuronal function, stimulation of neuronal regeneration and increase the number of mitochondria (Spencer 2010).
In colclusion, it was observed that C. rotundus, almost recovered spatial memory impairment, in addition to an increase in mitochondrial distribution in CA1 and an increase in nerogenesis in SGZ of dentate gyrus of the rats subject to dose of 500 mg/kg.
The limitation of our study was chronic toxicity observed in total extract in a pretest study, which led us to submit the total extract for solvent solvent partitioning and exclude cholorofom fraction rich in non-polar compounds responsible for toxicity from the total extract.
References
Anekonda TS, Reddy PH (2005) Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Res Rev 50:361–376
Bonfanti L, Peretto P (2011) Adult neurogenesis in mammals–a theme with many variations. Eur J Neurosci 34:930–950
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress1, 2. Free Radic. Biol Med 32:1050–1060
Clelland C, Choi M, Romberg C, Clemenson G, Fragniere A, Tyers P, Jessberger S, Saksida L, Barker R, Gage F (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213
de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR (2000) Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer's disease. Lab Investig 80:1323–1335
Dinges DF (2006) Cocoa flavanols, cerebral blood flow, cognition, and health: going forward. J Cardiovasc Pharmacol 47:S223–S225
Du H, Guo L, Zhang W, Rydzewska M, Yan S (2011) Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging 32:398–406
Esfandiary E, Karimipour M, Mardani M, Alaei H, Ghannadian M, Kazemi M, Mohammadnejad D, Hosseini N, Esmaeili A (2014) Novel effects of Rosa damascena extract on memory and neurogenesis in a rat model of Alzheimer's disease. J Neurosci Res 92:517–530
Garthe A, Behr J, Kempermann G (2009) Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4:e5464
George P, Charles W (2007) The rat brain in stereotaxic coordinates,6 ed Spiral-bound
Goedert M, Spillantini MG (2006) A century of Alzheimer's disease. science 314:777–781
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M (2001) Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21:3017–3023
Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362
Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Sghair MB, Bouhlel I, Bhouri W, Mariotte AM, Ghedira K (2009) Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact 181:85–94
Kim DS, Kim JY, Han YS (2007) Alzheimer's disease drug discovery from herbs: neuroprotectivity from beta-amyloid (1-42) insult. J Altern Complement Med 13:333–340
Kitazawa M, Yamasaki TR, Laferla FM (2004) Microglia as a potential bridge between the amyloid β-peptide and tau. Ann N Y Acad Sci 1035:85–103
Lim K-C, Lim ST, Federoff HJ (2003) Neurotrophin secretory pathways and synaptic plasticity. Neurobiol Aging 24:1135–1145
Mahboubi M, Taghizadeh M, Talaei SA, Firozeh SMT, Rashidi AA, Tamtaji OR (2016) Combined administration of Melissa officinalis and Boswellia serrata extracts in an animal model of memory. Iran J Psychiatry Behav Sci 10:e681
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Mann GE, Rowlands DJ, Li FY, de Winter P, Siow RC (2007) Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc Res 75:261–274
Mehdizadeh M, Dabaghian FH, Shojaee A, Molavi N, Taslimi Z, Shabani R, Asl SS (2017) Protective effects of cyperus rotundus extract on amyloid β-peptide (1-40)-induced memory impairment in male rats: a behavioral study. Basic & Clinical Neuroscience (BCN) 8:249
Mohmmad Abdul H, Sultana R, Keller JN, St. Clair D K, Markesbery WR, Butterfield DA (2006) Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid β-peptide (1–42), H2O2 and kainic acid: implications for Alzheimer's disease. J Neurochem 96:1322–1335
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta Biochim Biophys Acta 1802:2–10
Rabiei Z, Hojjati M, Rafieian-Kopaeia M, Alibabaei Z (2013) Effect of Cyperus rotundus tubers ethanolic extract on learning and memory in animal model of Alzheimer. Biomedicine & Aging Pathology 3:185–191
Reddy PH (2009) Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol 218:286–292
Reddy PH, Beal MF (2005) Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev 49:618–632
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14:45–53
Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Seo W-G, Pae H-O, Oh G-S, Chai K-Y, Kwon T-O, Yun Y-G, Kim N-Y, Chung H-T (2001) Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J Ethnopharmacol 76:59–64
Šerý O, Povová J, Míšek I, Pešák L, Janout V (2013) Molecular mechanisms of neuropathological changes in Alzheimer’s disease: a review. Folia Neuropathol 51:1–9
Sonwa MM, König WA (2001) Chemical study of the essential oil of Cyperus rotundus. Phytochemistry 58:799–810
Spencer JP (2008) Flavonoids: modulators of brain function? Br J Nutr 99:ES60–ES77
Spencer JP (2010) The impact of fruit flavonoids on memory and cognition. Br J Nutr 104:S40–S47
Spencer JP, Vauzour D, Rendeiro C (2009) Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys 492:1–9
Sunil A, Kesavanarayanan K, Kalaivani P, Sathiya S, Ranju V, Priya RJ, Pramila B, Paul FS, Venkhatesh J, Babu CS (2011) Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic–reperfusion injury in rats. Brain Res Bull 84:394–405
Uddin S, Mondal K, Shilpi J, Rahman M (2006) Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia 77:134–136
Winner B, Winkler J (2015) Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol 7:a021287
Yazdanparast R, Ardestani A (2007) In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J Med Food 10:667–674
Youdim KA, Shukitt-Hale B, Joseph JA (2004) Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med 37:1683–1693
Yu T-X, Zhang P, Guan Y, Wang M, Zhen M-Q (2015) Protective effects of luteolin against cognitive impairment induced by infusion of Aβ peptide in rats. Int J Clin Exp Pathol 8:6740
Zhang J, An S, Hu W, Teng M, Wang X, Qu Y, Liu Y, Yuan Y, Wang D (2016) The neuroprotective properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int J Mol Sci 17:1810
Acknowledgements
The authors would like to thank H. Vartevan and C. Larbie for proofreading, and N. Abrishami for conducting statistical analysis and they are grateful to Isfahan University of Medical Sciences, Isfahan, Iran, for funding the project no: 393476.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest between the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shakerin, Z., Esfandiari, E., Ghanadian, M. et al. Therapeutic effects of Cyperus rotundus rhizome extract on memory impairment, neurogenesis and mitochondria in beta-amyloid rat model of Alzheimer’s disease. Metab Brain Dis 35, 451–461 (2020). https://doi.org/10.1007/s11011-019-00493-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00493-2