Abstract
The present study investigated the therapeutic effects of probiotics on brain intoxication induced by clindamycin and propionic acid (PPA) in hamsters. Fifty golden Syrian hamsters were randomly divided into five experimental groups of ten animals each: (A) control group receiving phosphate buffered saline; (B) oral buffered PPA-treated group being administered with a neurotoxic dose of 250 mg/kg PPA during three days; (C) oral clindamycin-treated group receiving a single dose of 30 mg clindamycin/kg; and (D, E) the two therapeutic groups being administered the same doses of clindamycin and PPA followed by probiotics for three weeks at a daily dose of 0.2 g/kg. Biochemical parameters of energy metabolism and oxidative stress were examined in brain homogenates from all hamsters. The development of pathogenic bacteria was monitored on stool samples from all hamsters. Descriptive changes in fecal microbiota and overgrowth of Clostridium species in clindamycin and PPA treated hamsters were recorded. Interestingly, probiotics were shown effective to restore normal gut microbiota. Clindamycin and PPA treatments caused an elevation in lipid peroxidation and catalase activity, as oxidative stress markers, together with a reduction in GST activity and GSH level. Energy metabolism impairment was ascertained via the activation of creatine kinase and a decrease of lactate dehydrogenase. These findings suggest that bacteria overgrowth caused by PPA and clindamycin was efficient to illustrate signs of neuronal toxicity. The present study indicates that probiotic treatment can improve poor detoxification, oxidative stress, and altered gut microbiota as mechanisms implicated in the etiology of many neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence suggests that the gut microbiota plays a crucial role in the central nervous system (CNS) and any alterations in its composition can contribute later in life to the onset of brain disorders. Notably, these alterations might be vulnerable to environmental factors, such as stress and the use of antibiotics.
The gut-brain axis (GBA) is composed of bidirectional communication between the enteric nervous system (ENS) and the CNS and connects peripheral intestinal functions with cognitive and emotional centers of the brain. However, due to significant human health impact of the microbiota, the GBA has been widespread to the microbiota-gut-brain axis (Rhee et al. 2009; O'Mahony et al. 2011), which consists of a complex network of communications between the intestinal microbiota, the gut, and the brain, gastrointestinal tract (GI), modulating immune system, and the CNS functions (Rhee et al. 2009; Mayer 2011). Therefore, the bidirectional communication between the microbiota and the CNS plays an important role in the integration and monitoring of gut functions, as well as the connection of cognitive and emotional brain centers with peripheral intestinal mechanisms and functions such as intestinal permeability, immune activation, entero-endocrine signaling, and enteric reflex. Furthermore, the bidirectional communication between the microbiota and the CNS maintains homeostasis and can influence stress reactivity, pain perception, neurochemistry, and several GBA disorders (Collins et al. 2012; Cryan and Dinan 2012; Foster 2013).
Notably, several neuropathological, behavioral, and biochemical abnormalities similar to those reported in neurological patients have newly been observed following the intraventricular administration of propionic acid (PPA) in rats, probably by altering brain fatty acid metabolism (MacFabe et al. 2008). Physiologically, PPA gains access to the CNS after crossing the gut-blood and blood-brain barrier (BBB). The mechanisms by which PPA can affect CNS are multiple although they remain to be fully elucidated. The mitochondrial dysfunction by direct inhibition of oxidative phosphorylation (Brass and Beyerinck 1988), elevated levels of propionyl coenzyme A that might inhibit oxidation of short-chain fatty acids (SCFA), and sequestration of carnitine (Brass and Beyerinck 1987) are the most critical mechanisms described so far. Additionally, increased PPA may produce sensitivity to oxidative stress, which increases the harm caused by additional environmental toxic factors as heavy metals, hydrocarbons, or infectious agents (Wajner et al. 2004). Moreover, PPA involves promoting calcium release from intracellular stores (Nakao et al. 1992) that play a significant role in neuroplasticity (Bronstein et al. 1992) and can alter a number of neurotransmitter systems including dopamine, serotonin, glutamate and γ-Aminobutyric acid involved in the etiology of neurodevelopmental disorders (Sziray et al. 2007; El-Ansary et al. 2012, 2017).
As mentioned earlier, alterations in the gut microbiota could be vulnerable to environmental agents, such as stress, the use of antibiotics, infections, and poor diet, which could cause dysbiosis and metabolic neurodevelopment disorder. Clindamycin was utilized to provoke the overgrowth of pathogenic bacteria, especially the SCFAs producers. Buffie et al. (2012) have shown that a single dose of clindamycin can induce profound changes in mouse microbiota composition and, consequently, confer long-lasting susceptibility to Clostridium infection.
Interestingly, dietary probiotics may present a preventive or therapeutic strategy to improve the gut microflora profile and hence to resist neurodevelopmental disorder (Borre et al. 2014; El-Ansary et al. 2015). In fact, probiotics have been defined as microbial dietary adjuncts that positively affect the host physiology by ameliorating microbial and nutritional balance in the intestinal tract as well as modulating the systemic and mucosal immunity. Several studies have found that probiotics can maintain the integrity of the intestinal mucosal barrier and CNS by altering the gut microbiota composition (Cryan and Dinan, 2012; Farmer et al. 2014). Probiotics serve as selectively fermented elements, which cause specific modifications in the activity and/or composition of the gut microbiota that confers health benefits on the host (Gibson et al. 2004; Roberfroid 2007). In fact, probiotics can regulate the CNS through neural, endocrine, metabolic, and immunological mechanisms.
The interaction of gut microbiota with neural system opens new horizons for developing new therapeutic strategies based on the modulation of microbiota in early life to resist neurodevelopmental diseases, including autism spectrum disorder (ASD) (Fung et al. 2017).
Materials and methods
Animals
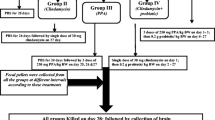
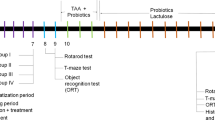
Fifty young golden Syrian hamsters, weighing about 60-70 g, were enrolled in the present study. The animals were randomly assigned into five experimental groups; each of them included ten hamsters. The hamsters in the control group were orally given phosphate buffered saline. The animals in the PPA-treated group were orally administered a neurotoxic dose of PPA (250 mg/kg body weight/day) for three days. The hamsters n the clindamycin-treated group were on day zero of the experiment administered a single orogastric dose of clindamycin (30 mg/kg body weight). The animals in the two therapeutic groups received the same doses of PPA or clindamycin followed by 0.2 g/kg body weight of probiotic (ProtexinR) for three weeks. ProtexinR (Somerset, UK) is a mixture of some healthy bacteria like Bifidobacterium infantis, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophiles with the concentration of 1 billion CFU per gram.
The hamsters were placed at 21 ± 1 °C with ad libitum access to water and standard chaw. Quantitative stool cultures were carried out both anaerobically and aerobically. All experiments, preapproved by the ethics committee of the College of Science at King Saud University, were performed in accordance with the national guidelines use and care of animals.
Preparation of brain tissue homogenates
By the end of the feeding trials, carbon dioxide anesthetized hamsters were decapitated. The brain tissues were taken from the hamsters in the five groups and dissected into small pieces, homogenized in bidistilled water (1:10, w/v), and stored at −80 °C until further use.
Biochemical analyses
Assay of creatine kinase activity
Creatine kinase (CK) activity was investigated according to Szasz et al. (1976) by following the rate of NADH formation, which is directly proportional to the sample CK activity, at 340 nm (Szasz et al. 1976).
Assay of lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity was investigated using the method described by Amador et al. (1963). Briefly, LDH catalyzes the conversion of lactate to pyruvate, and the forward reaction, conversion of pyruvate to lactate. The rate of NADH formation, which is directly proportional to the sample LDH activity is indicated by an elevation in the absorbance measured at 340 nm.
Measurement of glutathione and lipid peroxidation concentrations
The levels of glutathione (GSH) and lipid peroxidation in the brain samples were determined spectrophotometrically according to the protocols Beutler et al. (1963) or Ruiz-Larrea et al. (1994) respectively have described (Beutler et al. 1963; Ruiz-Larrea et al. 1994).
Assay of glutathione S-transferase and catalase activities
The activity of glutathione S-transferase (GST) was assessed at 25 °C by following the increase in A340 which parallels the conjugation of GSH to 1-chloro-2,4-dinitrobenzene (CDNB) (Vontas et al. 2000). Likewise, the activity of catalase was measured according to the method of Maehly and Chance (1954). I.e., the rate of the hydrogen peroxide (H2O2) dissociation/min by the catalase enzyme was followed measuring the change in absorbance at 240 nm.
Microbial analysis
Sample collection
In the present study, the fecal samples of the hamsters in the groups were collected in sterile tubes, and kept at −20 °C until further use. The microbial analysis involved the culturing of microorganisms on different media and at different incubation conditions for their preliminary identification and enumeration indicating as such the alteration of the gut microbiota in response to the treatment being tested.
Fecal sample preparation
Bacterial counts in the fecal samples from the hamsters were carried out according to Itoh et al. (1983). All fecal samples were diluted 1: 10 (w/v) in sterile phosphate buffer solution (0.1 M, pH 7.0; Oxoid), homogenized using a sonicator, and vortexed for 45 s to obtain an even suspension. After this, the fecal mixtures were centrifuged at 4500 rpm for three minutes at 4 °C. Ten-fold serial dilutions for all the fecal samples were performed. One milliliter of the fecal supernatant was added to 9 ml of PBS (dilution 1). The same process was repeated until dilution 4.
Of each of the prepared dilutions, 0.1 ml was loaded and spread on different media for the enumeration and identification of the microorganisms present in the intestinal gut. For the identification and quantification of Clostridium sp., Cefoxitin Cycloserine Fructose Agar (CCFA, Oxoid) plates were used; plates were incubated at 37 °C in an anaerobic jar supplied with 5% CO2 for three days. Aerobic microorganisms; however, were screened on Nutrient agar (NA), MacConkey agar (MAC), blood agar (BAP) and Sabouraud dextrose agar for yeast strains specifically. All the above-listed media plates were incubated aerobically at 37 °C for 18 - 24 h.
Bacterial enumeration and identification
Data from the culture methods were recorded according to the following scale as colony forming unit per gram of feces (CFU/g): 0 = no growth, ˂ 50 colony forming units (CFU)/g of feces; + = rare, 50 - 100 CFU/g of feces; ++ = few, 100 - 300 CFU/g of feces; +++ = moderate, ≥ 300 CFU/g of feces; ++++ = heavy. Plates with a bacterial count that ranged between 20 and 300 CFU/gram of feces were accepted and used to estimate the cultural count and bacterial identification.
Bacterial and yeast identification was performed macro and microscopically by cell morphology and gram staining technique. Single colonies obtained from the culture plates were selected for preliminary identification according to procedures Holdeman et al. (1977) previously have described. In particular, Clostridium sp. will appear identified as yellow fluorescent cells when grown on CCFA agar plates and as gram-positive rod when observed under the microscope following the gram staining technique.
Statistical analysis
The statistical package for the social sciences (SPSS, Chicago, IL, USA) was used to analyze the obtained data. The results were presented as mean and standard deviation (SD), and all statistical comparisons were performed using independent T-test. Correlations between all the investigated parameters were carried out using the Pearson’s correlation coefficient (r) whereas the receiver operating characteristics (ROC) curve together with the area under the ROC curve (AUC) were used as a fundamental tool for neurotoxicity evaluation in animal modeling. Actually, it is well known that ROC analysis can also be used in experimental work to measure the effectiveness of the studied variables as a marker of toxicity or therapeutic effect of treatment (El-Ansary et al. 2012; 2018). Only P value ≤0.005 was considered significant.
Results and discussion
The gut microbiota plays in mammals a critical role in modulating host physiology. These effects are mostly linked to nutrient intake and immunity. The gut microbial composition and metabolism is affected strongly by antibiotic abuse, thus affecting human health. The use of oral antibiotics to disturb the commensal bacteria is one of the most common strategies used for assessing the effect of the intestinal microbiota on CNS functioning (Finegold 2011). Table 1 shows the rates of bacteria growth in the clindamycin- and PPA-treated groups before and after probiotic treatments. In clindamycin-treated hamsters, there was found an overgrowth of several aerobic and anaerobic pathogenic bacteria with a marked increase in total clostridia species, which produce PPA as a metabolite. Also, the growth of clostridia species in clindamycin-treated hamsters was higher than in PPA-treated hamsters. This finding could be supported by the fact that clostridium can rebound from antibiotic treatment faster. This results in a huge proliferation of Clostridium with a massive secretion of its two toxins (A and B). These toxins, which work together, damage the intestinal epithelial cells and attract primarily neutrophils and leukocytes causing severe colitis (Sunenshine and McDonald 2006). However, Clostridium disappeared after treatment with probiotics in clindamycin- and PPA-treated hamsters. This indicates a role of probiotics for the release of antimicrobial factors that directly can target and limit Clostridium (Lee et al. 2013).
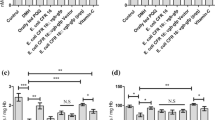
Oxidative stress can either occur as an increase in reactive oxygen species (ROS) production concomitant with a relative increase or decrease in antioxidant enzyme especially catalase and non-enzymatic antioxidants such as GSH (Chauhan and Chauhan 2006). GSH has important functions as antioxidant defense and detoxification of xenobiotics for a wide variety of environmental chemicals (James et al. 2004; Monks et al. 1999). In the present study, there was a marked diminution of GSH in hamsters treated with PPA and clindamycin compared to the control group (Table 2 and Fig. 1). Indeed, PPA caused a 45.95% decrease in GSH (P < 0.001) whereas clindamycin was found to be more efficient and induced a 53.76% decrease in GSH concentrations (P < 0.001). Therefore, clindamycin displayed a more significant reduction and seemed to be more toxic than PPA compared to the control group. These results may be explained with the background that hamsters with GSH deficiency present a model for realistic toxicological testing, similar to critically ill patients on drug or antibiotics treatment. The present findings collaborate with the previous results (MacFabe et al. 2008; El-Ansary et al. 2012), which demonstrated that high levels of PPA could induce oxidative stress in different brain regions through GSH depletion. Probiotics were found to be more efficient in restoring brain GSH levels in clindamycin-treated hamsters than in PPA-treated hamsters (with recorded values of 85.77 and 73.89%, respectively) (Table 2 and Fig. 1). The ameliorative effect of probiotics in the present study is supported by research on the probiotic Lactobacillus fermentum ME-3 (DSM14241), which is characterized by a complete glutathione system (synthesis, uptake, and redox turnover) (Kullisaar et al. 2010).
The accumulation of malondialdehyde (MD), a lipid peroxidation by-product considered a marker of cellular oxidation status, and highly elevated in many neurological disorders like for example Alzheimer’s and Parkinson’s diseases, ASD, and brain ischemia/reperfusion. There was a significant increase of lipid peroxidation in both PPA- and clindamycin-treated groups (P < 0.001) compared to the control group (Table 2 and Fig. 1). In contrast, PPA caused more lipid peroxidation in the hamster brains than did clindamycin, with obtained values of 194.34 and 172.32%, respectively. This finding may be related to the increased generation of ROS due to enormous oxidative damage in these animals. ROS, in turn, may oxidize several other key biomolecules, specifically membrane lipids (Frei, 1994). The elevated lipid peroxidation is attributed to the formation of free radicals that abstract hydrogen atoms from membrane phospholipids and lipoproteins, in particular, polyunsaturated fatty acids generating lipid peroxidation, of which MD is the primary product (Al-Gadani et al. 2009). The observed elevation in lipid peroxidation was in line with the work El-Ansary et al. (2013) carried out. Interestingly, an antioxidant effect of probiotics in the PPA-treated group significantly decreased the lipid peroxidation (Table 2 and Fig. 1). However, probiotics were found to be less efficient showing a reduced but still significant elevation (P < 0.024) of lipid peroxidation in the brains of clindamycin-treated hamsters compared to the control group. The suggested antioxidant effect of probiotics is in good agreement with a study by Shen et al. (2011), which demonstrated that supplementation with Bifidobacterium provided systemic protection against lipid peroxidation and decreased brain monoamine oxidase activity. Thereby treatment with probiotics may potentially increase the intersynaptic neurotransmitter levels.
Antioxidant enzymes, such as catalase, are a convenient indirect tool to assess the pro-oxidant/antioxidant status. Catalase is an important enzyme that catalyzes H2O2 dismutation into water and oxygen. Compared to the control group there was a significant elevation of catalase activity (P < 0.001) in the PPA- and clindamycin-treated groups (161.01 and 162.91%, respectively) (Table 2 and Fig. 1). This finding might be linked to the pathological effects of clindamycin and PPA as similarly elevated catalase levels were observed in H2O2-stressed Chinese hamster fibroblasts, U87MG glioblastoma cells, and A549 human lung adenocarcinoma cells (Bojes et al. 1998; Hunt et al. 1998). The increased catalase activity recorded in PPA-treated hamsters does not contradict the reduced catalase activity previously reported in PPA-treated hamsters (El-Ansary et al. 2013). The obtained result is expected as it is well established that, in vivo systems, if the oxidative stress is not very strong or very prolonged, the catalase activity raises, however, if its level is very high, or it is persistent, the protein damage becomes profound, and a decline in the catalase activity could take place (through oxidative stress-altered gene expression and/or direct oxidative damage of the catalase molecules) (El-Ansary et al. 2013). This supposition might be supported by the increase of lipid peroxidation, and the decrease of GSH (two potent biomarkers of oxidative stress) recorded in the current investigation. Probiotics were found to be capable of ameliorating brain catalase activity significantly in clindamycin-treated hamsters and, to a lesser extent, in the PPA-treated group. The effect of probiotics on catalase observed in the present study is consistent with a recent study of Amdekar and Singh (2016), in which the consumption of Lactobacillus acidophilus was found to decrease the level of catalase and lipid peroxidation in rats.
The recorded results also revealed a significant effect of clindamycin in altering the detoxification mechanisms in hamsters treated with 17.23% lower GST activity. GST is an important family of enzymes that detoxify pro-oxidative compounds by coupling them to GSH, which is the body’s primary antioxidant. On the other hand, PPA induced a nonsignificant reduction of GST activity (7.35%) compared to the control group (Table 1). Therefore, it can be observed that oxidative stress generated by bacteria overgrowth in the present study was not comparable to that provoked by the neurotoxic dose of PPA (El-Ansary et al. 2015). Furthermore, the GST activity in hamsters treated with clindamycin followed by probiotics was still significantly different (P < 0.025) compared to the results in the control group, but this activity was higher compared to the clindamycin-intoxicated group. Thus, the effectiveness of probiotics could be linked to its antioxidative properties. Therefore, probiotics may be safely used to improve poor detoxification, improve abnormal gut microbiota, and reduce oxidative stress that are well-known mechanisms involved in the etiology of many neurological disorders.
CK and LDH are two cytoplasmic enzymes that regulate energy metabolism in the cell. It is well known that CK is an enzyme that plays a critical role in ATP repletion, whereas LDH, a glycolytic enzyme might increase the influx of Kreb’s cycle through the conversion of pyruvate to acetyl CO A by pyruvate dehydrogenase complex. In the present study, it was found a significant increase of the CK activity in the four treated (129-274%, P < 0.001) groups relative to the control group (Table 2). The activity of LDH was highly reduced only in the clindamycin-treated hamsters (P < 0.034) (Table 2). The results previously reported by El-Ansary et al. (2012) is similar to those of the present study (El-Ansary et al. 2012) and may be explained by marked elevations in the activities of Ca2+/Mg2+ and Na+/K+ ATPases with a significant concomitant decrease in mitochondrial electron transport chain (ETC) complexes expression in various brain areas (Chauhan et al. 2011). Also, the increase of CK in the clindamycin-treated group can be related to the fact that pathogenic bacteria produce a high amount of SCFA, which in turn, activate CK enzyme (Chegwidden and Watts 1984). This assumption is consistent with a study by Chegwidden and Watts (1984), which demonstrated that acetate can activate CK in monkey skeletal muscle (Chegwidden and Watts 1984). Although the CK activity recorded in both probiotic-treated groups was markedly elevated compared to control, it remained much lower compared to the PPA- and clindamycin-intoxicated groups. This finding collaborates with a previous study by Vahdatpour et al. (2011), which demonstrated that Protexin® decreased the CK activity in serum of treated male birds. Notably, a dramatic decrease of LDH activity was observed in the clindamycin-treated group. In contrast, PPA caused a non-significant decrease (14%) of brain LDH activity relative to the control. Al-Dbass (2014) revealed a marked LDH activity increase in PPA-treated rats plasma compared to controls. This indicated a leakage of LDH from the brain into the plasma, which suggests a membrane and cellular damage.
The significant correlation observed (Table 3) may be explained by an association between oxidative stress (represented by lipid peroxidation), impaired antioxidant status (represented by GSH, catalase, and GST), as well as reduced energy metabolism (represented by CK and LDH) as etiological mechanisms related to brain neurotoxicity. The results of the ROC analysis in the present study demonstrate AUC together with the cut-off values of the eleven evaluated parameters (Fig. 2 and Table 4). The total AUC was determined as a measure of the performance of PPA or clindamycin treatment. The comparison of the AUC of the different markers in the five investigated groups revealed that lipid peroxidation, catalase, CK, and GSH can be used as excellent markers of both PPA and clindamycin neurotoxicity with AUC values equal to 1. Moreover, LDH and GST displayed good validity as measures of clindamycin neurotoxicity with AUC values of 0.875 and 0.819, respectively. The therapeutic potency of the tested probiotic, ProtexinR, can also be monitored through CK, GSH, catalase and lipid peroxidation as markers of ROS scavenging. This might also be supported by the marked specificity and sensitivity obtained for CK, GSH, lipid peroxidation, and catalase.
Conclusion
Overall, the findings presented in the present study indicate that overgrowth of gut pathogenic bacteria induced by PPA or clindamycin can be related to their neurotoxic effects through the gut-brain axis. Moreover, supplementation with healthy probiotic strains was effective in ameliorating the neurotoxic effects of both treatments.
Change history
31 August 2018
The original version of this article unfortunately contained a mistake. The family name of the fourth author listed in the title was incorrect, and the correct name is Nadine Moubayed, as noted in the addresses. Her name is now corrected in the author group of this article.
Abbreviations
- AUC :
-
area under the curve
- BAP :
-
Sheep Blood Agar plate
- BBB :
-
blood-brain barrier
- CDNB :
-
1-chloro-2,4-dinitrobenzene
- CFU :
-
Colony-forming unit
- CK :
-
creatine kinase
- CNS :
-
center nerves system
- ENS :
-
enteric nervous system
- ETC :
-
electron transport chain
- GI :
-
gastrointestinal tract
- GSH :
-
glutathione
- GST :
-
glutathione-s-transferase
- H 2 O 2 :
-
hydrogen peroxide
- HK :
-
hexokinase
- LDH :
-
lactate dehydrogenase
- MCA :
-
MacConkey Agar
- MD :
-
malondialdehyde
- PPA :
-
propionic acid
- ROC :
-
Receiver Operating Characteristics
- ROS :
-
reactive oxygen species
- SCFA :
-
Short-Chain Fatty Acids
- SDA :
-
Sabouroud Dextrose agar
References
Al-Dbass AM (2014) N-Acetylcysteine reduces the neurotoxic effects of propionic acid in rat pups. J King Saud Univ Sci 26:254–260
Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L (2009) Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem 42:1032–1040
Amador E, Dorfman LE, Wacker WE (1963) Serum lactic dehydrogenase activity: an analytical assessment of current assays. Clin Chem 12:391–399
Amdekar S, Singh V (2016) Lactobacillus acidophilus maintained oxidative stress from reproductive organs in collagen-induced arthritic rats. J Hum Reprod Sci 9:41–46
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bojes HK, Suresh PK, Millis EM, Spitz DR, Sim JE, Kehrer JP (1998) Bcl-2 and Bcl-xL in peroxide-resistant A549 and U87MG cells. Toxicol Sci 42:109–116
Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF (2014) The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 817:373–403
Brass EP, Beyerinck RA (1987) Interactions of propionate and carnitine metabolism in isolated rat hepatocytes. Metabolism 36:781–787
Brass EP, Beyerinck RA (1988) Effects of propionate and carnitine on the hepatic oxidation of short- and medium-chain-length fatty acids. Biochem J 250:819–825
Bronstein JM, Farber DB, Wasterlain CG (1992) Regulation of type-II calmodulin kinase: functional implications. Brain Res Brain Res Rev 18:135–147
Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG (2012) Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun 80:62–73
Chauhan V, Chauhan A (2006) Oxidative stress in Alzheimer's disease. Pathophysiology 13:195–208
Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, Chauhan V (2011) Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem 117:209–220
Chegwidden WR, Watts DC (1984) Anion activation of monkey muscle creatine kinase. Int J BioChemiPhysics 16:1171–1174
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
El-Ansary AK, Ben Bacha A, Kotb M (2012) Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation 9:74. https://doi.org/10.1186/1742-2094-9-74
El-Ansary AK, Al-Daihan S, Ben Bacha A, Shaker GH, Al-Ayadhi LY (2013) Comparative study on the protective effect of carnosine and carnitine against pro-inflammatory/pro-oxidant effects of clindamycin and propionic acid administrations to hamsters. Afr J Microbiol Res 7:103–114. https://doi.org/10.5897/AJMR12.1178
El-Ansary A, Bhat RS, Al-Daihan S, Al Dbass AM (2015) The neurotoxic effects of ampicillin-associated gut bacterial imbalances compared to those of orally administered propionic acid in the etiology of persistent autistic features in rat pups: effects of various dietary regimens. Gut Pathog 7:7. https://doi.org/10.1186/s13099-015-0054-4
El-Ansary A, Al-Salem HS, Asma A, Al-Dbass A (2017) Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis 16:96. https://doi.org/10.1186/s12944-017-0485-7
El-Ansary A, Bacha AB, Bjørklund G, Al-Orf N, Bhat RS, Moubayed N, Abed K (2018) Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis 33(4):1155–1164
Farmer AD, Randall HA, Aziz Q (2014) It's a gut feeling: how the gut microbiota affects the state of mind. J Physiol 592:2981–2988
Finegold SM (2011) Desulfovibrio species are potentially important in regressive autism. Med Hypotheses 77:270–274
Foster JA (2013) Gut feelings: bacteria and the brain. Cerebrum 2013:9
Frei B (1994) Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med 97:5S–13S discussion 22S-28S
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
Holdeman LV, Cato EP, Moore WEC (1977) Anaerobe laboratory manual. Anaerobe Laboratory, Virginia Polytechnic Institute and State University, Blacksburg
Hunt CR, Sim JE, Sullivan SJ, Featherstone T, Golden W, Von Kapp-Herr C, Hock RA, Gomez RA, Parsian AJ, Spitz DR (1998) Genomic instability and catalase gene amplification induced by chronic exposure to oxidative stress. Cancer Res 58:3986–3992
Itoh K, Mitsuoka T, Sudo K, Suzuki K (1983) Comparison of fecal flora of mice based upon different strains and different housing conditions. Z Versuchstierkd 25:135–146
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80:1611–1617
Kullisaar T, Songisepp E, Aunapuu M, Kilk K, Arend A, Mikelsaar M, Rehema A, Zilmer M (2010) Complete glutathione system in probiotic Lactobacillus fermentum ME-3. Prikl Biokhim Mikrobiol 46:527–531
Lee JS, Chung MJ, Seo JG (2013) In vitro evaluation of antimicrobial activity of lactic acid bacteria against clostridium difficile. Toxicol Res 29:99–106
MacFabe DF, Rodríguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Roy Taylor A, Boon F, Cain DP, Kavaliers M, Possmayer F, Ossenkopp KP (2008) A novel rodent model of autism: Intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotechnol 4:146–166
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Mayer EA (2011) Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12:453–466
Monks TJ, Ghersi-Egea JF, Philbert M, Cooper AJ, Lock EA (1999) Symposium overview: the role of glutathione in neuroprotection and neurotoxicity. Toxicol Sci 51:161–177
Nakao S, Fujii A, Niederman R (1992) Alteration of cytoplasmic Ca2+ in resting and stimulated human neutrophils by short-chain carboxylic acids at neutral pH. Infect Immun 60:5307–5311
O'Mahony SM, Hyland NP, Dinan TG, Cryan JF (2011) Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 214:71–88
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6:306–314
Roberfroid M (2007) Prebiotics: the concept revisited. J Nutr 137:830S–837S
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, De Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 59:383–388
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62:1097–1103
Sunenshine RH, McDonald LC (2006) Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med 73:187–197
Szasz G, Gruber W, Bernt E (1976) Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem 22:650–656
Sziray N, Leveleki C, Levay G, Markó B, Hársing LG Jr, Mikics E, Barsy B, Haller J (2007) Mechanisms underlying the long-term behavioral effects of traumatic experience in rats: the role of serotonin/noradrenaline balance and NMDA receptors. Brain Res Bull 71:376–385
Vahdatpour T, Nikpiran H, Babazadeh D, Vahdatpour S, Jafargholipour MA (2011) Effects of Protexin®, Fermacto® and combination of them on blood enzymes and performance of Japanese quails (Coturnix Japonica). Ann Biol Res 2:283–291
Vontas JG, Enayati AA, Small GJ, Hemingway J (2000) A simple biochemical assay for glutathione S-transferase activity and its possible field application for screening glutathione S-transferase-based insecticide resistance. Pestic Biochem Physiol 68:184–192
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Acknowledgments
This research project was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Ethical approval
This study was ethically approved by the Ethical Committee, College of Science, King Saud University.
Additional information
The original version of this article was revised: The family name of the fourth author listed in the title was incorrect, and the correct name is Nadine Moubayed, as noted in the addresses. Her name is now corrected in the author group of this article.
Rights and permissions
About this article
Cite this article
Al-Orf, N., El-Ansary, A., Bjørklund, G. et al. Therapeutic effects of probiotics on neurotoxicity induced by clindamycin and propionic acid in juvenile hamsters. Metab Brain Dis 33, 1811–1820 (2018). https://doi.org/10.1007/s11011-018-0284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0284-5