Abstract
Disruption of leptin signalling has been implicated as playing a role in the development of Alzheimer’s disease (AD). Leptin has previously been shown to be affected by amyloid-beta (Aβ)-related signalling; however, pathways that link leptin to the disease pathogenesis have not been determined. To characterize the association between increasing age-dependent Aβ levels with leptin signalling and the vulnerable brain regions in AD, we assessed the mRNA and protein expression profile of leptin and leptin receptor (Ob-Rb) at 9 and 18-month-age in APP/PS1 mice. Immunohistochemical labelling demonstrated that leptin and Ob-Rb proteins were localised to neocortical and hippocampal neurons in APP/PS1 and wildtype (WT) mice. Neuronal leptin and Ob-Rb immunolabelling was more prominent in the neocortex of both groups at 9 month of age, while, at 18 months, labelling was reduced in the hippocampus of APP/PS1 mice relative to WT. Immunoblotting analysis demonstrated decreased hippocampal leptin levels, concomitantly with an increased Ob-Rb levels, in APP/PS1 mice compared with WT controls at 18 month of age. While no leptin mRNA was found in either of the groups analysed, Ob-Rb mRNA was significantly decreased in the hippocampus of APP/PS1 mice at both ages analysed. In addition, a significant decreased protein kinase B (Akt) activity concomitantly with an upregulation of suppressor of cytokine signaling-3 (SOCS3) and protein-tyrosine phosphatase 1B (PTP1B) transcripts was present. Thus, these results collectively indicate alterations of leptin signalling in the hippocampus of APP/PS1 mice, providing novel insights about the pathways that could link aberrant leptin signaling to the pathological changes of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), the most common form of ageing-related dementia, is an irreversible neurodegenerative disorder that is characterized by the progressive loss of cognitive function associated with specific pathological changes in the brain (Hyman et al. 1989; Villemagne et al. 2013). With the increasing life span of the global population, AD is fast becoming a major health and socio-economic challenge for many countries, however, no current therapeutic disease-modifying intervention exists. Although the cellular basis for neurodegeneration in AD is not yet fully understood, numerous studies have shown that the underlying disease process involves multiple complex factors, including metabolic alterations (Chakrabarti et al. 2015; Pedros et al. 2014; Petrov et al. 2015).
With respect to potential metabolic effects on neural systems, leptin is a polypeptide hormone primarily secreted by adipocytes that exerts its main biological function in the brain (Stephens and Basinski 1995; Zhang et al. 1994). Leptin acts by binding to receptors that are structurally related to the cytokine receptor class I family. Alternative splicing generates distinct isoforms of the leptin receptor, including long (Ob-Rb) and short isoforms (Ob-Ra and Ob-Rc-f), however, Ob-Rb is thought to transmit the majority of leptin’s biological signals (Friedman and Halaas 1998). In addition to its classical role in the neuroendocrine regulation of food intake, the existence of leptin receptors in extra-hypothalamic brain regions such as the neocortex and hippocampus (Wilkinson et al. 2000) indicates that this hormone affects other biological processes. In the brain, the binding of leptin to the neuronal Ob-Rb receptor activates janus-tyrosine kinase 2 (JAK2), which in turn phosphorylates the insulin receptor substrate-1 and -2 (IRS 1/2) that results in the activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. JAK2 activation also leads to the phosphorylation of two tyrosine residues in the cytoplasmic tail of the Ob-Rb receptor, producing the activation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and the signal transducer and activator of transcription 3 (STAT3) signalling pathways.
Clinical and epidemiological studies support the concept that disrupted leptin metabolism and/or signalling contributes to the pathogenesis of AD (Bonda et al. 2014; Lieb et al. 2009; Power et al. 2001; Rosenbaum et al. 1996; Tezapsidis et al. 2009). It is possible that alterations in leptin signalling in the brain lead to altered leptin levels in AD. However, while new studies indicate a state of insulin resistance in AD (Pedros et al. 2014; Petrov et al. 2015; Watson and Craft 2003), there remains uncertainty about the role of leptin under these disease conditions. In this regard, circulating levels of leptin have been reported to be significantly lower in patients with AD as compared to controls (Power et al. 2001). Recently, Lieb et al. (Lieb et al. 2009) reported that high plasma concentrations of leptin correlate with a significantly lower risk of AD. In contrast, Rajagopalan et al. (Rosenbaum et al. 1996) showed that high leptin levels in plasma correlate with volume loss in several brain regions, regardless of the body mass index. Another recent study reported significant elevation in leptin levels in both the cerebrospinal fluid and hippocampus in AD (Bonda et al. 2014). Notably, upregulation of leptin has been suggested as a potential approach to therapeutic intervention in AD (Tezapsidis et al. 2009).
Moreover, experimental studies demonstrate that alterations in leptin signalling could significantly contribute to the development of ageing-related cognitive decline and impairment of synaptic plasticity (Farr et al. 2006; Harvey 2007; Moult et al. 2009). Indeed, it has been shown that leptin is apotential cognitive enhancer, as genetically obese rodents with dysfunctional Ob-Rb receptors display impairments in hippocampal synaptic plasticity. Direct administration of leptin into the hippocampus can facilitate hippocampal long-term potentiation (LTP) in vivo via enhancing NMDA receptor function (Harvey et al. 2006). Importantly, a growing body of studies have demonstrated leptin's beneficial effect on improving learning and cognitive function in AD (Farr et al. 2006; Sato et al. 2011; Searcy et al. 2012). In fact, the mechanism through which leptin modulates cognitive decline involves mechanism of action closely linked to the regulation of Aβ levels, which highlights its neuroprotective capacities. With respect to this, leptin has a strong in vitro (Fewlass et al. 2004; Greco et al. 2009b, 2008) and in vivo (Fewlass et al. 2004) anti-amyloidogenic effect, attributable to its transcriptional regulation capacity (Niedowicz et al. 2013) and inhibition of amyloid precursor protein (APP) processing (Perez-Gonzalez et al. 2014) through the amyloidogenic pathway. Accordingly, leptin reduces extracellular Aβ protein in vitro and this effect is dependent on activation of AMPK (Fewlass et al. 2004; Greco et al. 2009b, 2008). In addition, it has been reported that leptin can reduce total brain Aβ1–40 and Aβ1–42 in the Tg2576 mouse model of AD (Fewlass et al. 2004). Importantly, leptin reduces tau phosphorylation in neuronal cells through modulation of GSK-3β activity (Greco et al. 2009a), a protein kinase identified to be pathogenic in a range of neurodegenerative disorders (Koh et al. 2011). Therefore, these studies highlight leptin as an endocrine factor with a potentially important role in AD.
In the current study, biochemical, molecular biology and immunohistochemical techniques were used to examine the status of leptin signalling at 9 and 18 months of age in the APP/PS1 transgenic (Tg) line (Jankowsky et al. 2004). This Tg model of AD expresses mutant human presenilin 1 (PSEN1: deltaE9) and a chimeric mouse/human Aβ precursor protein harbouring the Swedish mutation (APP KM670/671NL). The APP/PS1 line demonstrates memory deficits as well asage-dependent Aβ plaque depositions in the brain (Arendash et al. 2001; Holcomb et al. 1998, 1999). Previous studies show that plaque deposition starts at between 4 and 6 months of age in these mice and steadily increasing with age (Jankowsky et al. 2004). Indeed, we detected very limited Aβ deposition around 6 months of age, whereas, from 9 months, plaque density and Aβ burden increased substantially up to 12-months of age (Fernandez-Martos 2017; Stuart et al. 2017). In this context, we examined leptin-related pathways through adulthood and aging to the background of increasing brain Aβ levels in these Tg mice. Our results indicated significant alterations in leptin and Ob-Rb levels in APPP/PS1 mice, corresponding with deregulated leptin-signalling at both 9 and 18 month of age, increasing with ageing. These findings suggest that AD may be associated with alterations in leptin signalling pathways that may result in a leptin resistant state, which could play a critical role to the irreversible cognitive decline and the characteristic pathological changes associated with this disease.
Methods
Animals
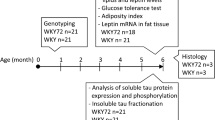
Cohorts of young (3 and 6 months age; n = 5 per group) and older (9 and 18 months; n = 10 per group) male APP/PS1 mice (Jankowsky et al. 2004) and age-matched WT littermates were used in this study. The maintenance and use of mice, and all experimental procedures, were approved by the Animal Ethics Committee of the University of Tasmania (Approval No A12780), in accordance with the Australian Guidelines for the Care and Use of Animals for Scientific Purposes. All analyses were conducted by personnel blinded to the genotype.
Tissue preparation
Animals were terminally anesthetized with sodium pentobarbitone (140 mg/kg) and transcardially perfused with 0.01 M phosphate buffered saline (PBS; pH 7.4) or 4% paraformaldehyde (PFA), in the middle of the light cicle (between 11 AM and 1 PM). Brains were immediately dissected, post-fixed overnight in the same fixative solution and then transferred to 18% then 30% sucrose solutions overnight (Liu et al. 2013). Serial coronal cryosections (40 μm thick) were cut on a cryostat (Leica CM 1850). For molecular biology experiments, the neocortex and hippocampus for each animal was split into two fractions and processed independently, for real time PCR or Western-blot analysis. Cortex and hippocampal samples were stored at −80 °C for later analysis.
RNA isolation and RT-qPCR
Total RNA was isolated from neocortex and hippocampus of APP/PS1 mice and WT mice tissue at 9 and 18-months of age (n = 5/age and genotype), respectively, using the RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized as described previously (Fernandez et al. 2009). Relative quantitation of leptin (assay ID: Mm00434759_m1) and Ob-Rb (assay ID: Mm00440181_m1) was performed using 10 ng of reverse transcribed total RNA (cDNA) in TaqMan One-Step real time PCR Master Mix (PE Applied Biosystem). β-Actin (assay ID: Mm00607939_s1) with VIC as real time reporter was used as a control to normalize gene expression. Relative quantitation of SOCS3 (Forward 5′- ATGGTCACCCACAGCAAGTTT -3′ and Reverse 5′- TCCAGTAGAATCCGCTCTCCT-3′; NM_007707.3) and PTP1B (Forward 5’-AGGAAGAGCTGTCCTCCACT-3′ and Reverse 5’-CACATTGACCAGGAAGGGCT-3′; NM_011201.3) was performed using 25 ng of reverse-transcribed total RNA, 20 pmol/ml of both sense and antisense primers and the SYBR Green PCR Master Mix (Applied Biosystems) in a final reaction volume of 10 μl (Fernandez-Martos et al. 2011). The reactions were run on a Light Cycler® 480 System instrument and software (Roche). Relative quantification for each gene was performed by the ∆∆Ct method (Livak and Schmittgen 2001). Primers were designed using NCBI/ Primer-BLAST software, and β-Actin (primer sequence obtained from (Gonzalez-Fernandez et al. 2014)) were used as an endogenous control.
Protein extraction and western blot analysis
Proteins from neocortex and hippocampus tissues of APP/PS1 mice and WT mice at 3, 6, 9 and 18-months of age (n = 5/age and genotype), were extracted with RIPA buffer (Sigma Aldrich) containing a cocktail of protease inhibitors (Roche). Denatured protein samples (20 μg) from each group were electrophoresed into Bolt® Bis-Tris Plus gels (Invitrogen), transferred to PVDF membranes (BioRad) and incubated with primary antibodies [rabbit anti-leptin (1:1000; Thermo Fisher), rabbit anti-Ob-Rb (1:500; Abcam), rabbit anti-Akt (1:1000; Cell signalling), rabbit anti-Akt (Ser473) (1:1000; Cell signalling), rabbit anti-STAT3(1:500; Santa Cruz), and rabbit anti-STAT3 (Tyr705) (1:1000; Cell signalling)] overnight. Subsequently, a corresponding anti-rabbit or anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (1:7000; Dako) was used, as described previously (Liu et al. 2015). GAPDH (1:5000, Millipore) was used as a loading control and band intensity was measured as the integrated intensity using ImageJ software (v1.4; NIH).
Histology
All immunohistochemical procedures were performed, as previously described (Fernandez-Martos et al. 2015), in one coronal 40 μm sections (bregma position −2.54 mm) of APP/PS1 mice and WT mice tissue at 9 and 18-months of age (n = 5/age and genotype). Antigen retrieval was carried out prior to immunohistochemistry using 10 mM citric acid, pH 6.0, in a pressure cooker for 14 mins. Endogenous peroxidase activity was inactivated in the sections by incubation with 2% H2O2 in a 70% methanol solution at RT for 10 min, and sections were then blocked for 1 h at RT in blocking buffer containing 10% horse serum, 0.3% bovine serum albumin and 0.3% Triton X-100 in TBS. The sections were then incubated overnight at 4 °C with the primary rabbit anti-leptin (1:250, Abcam) or rabbit anti-Ob-Rb (1:250; Abcam) antibodies in blocking buffer, plus 1 h at RT. Antibody binding was visualized by sequential incubations with a biotinylated anti-rabbit secondary antibody (1:500; VectorLabs) and HRP-linked streptavidin (1:500; Perkin Elmer), and with the “Nova Red Kit” (VectorLabs) according to the manufacturer’s instructions. The sections were finally dehydrated in graded ethanol, cleared with xylene and coverslipped with DPX (Panreac).Images were collected using the same acquisition parameters on an Axio lab A.1 microscope and AxioCam ICc5 camera (both Zeiss, Germany), with ×20 and ×40 objectives and Zen 2012 blue edition software (Zeiss, Germany). In all cases, the specificity of immunoreactivity was confirmed by processing sections lacking primary antibody (Suppl. fig. 1).
Statistical analysis
Values were reported as means ± standard error of the mean (SEM), and differences are considered significant at p < 0.05 (CI 95%). Differences between groups were evaluated using student’s t-test, and two-way anova using Dunett’s post-hoc test, to compare to WT 9 month of age group, and Sidak’s post-hoc test for multiple comparisons between groups. Statistical analysis was performed using GraphPad Prism software (version 6.0).
Results
Leptin and Ob-Rb levels in young APP/PS1 mice
We first examined the protein levels of leptin and Ob-Rb receptors at 3 and 6 months in APP/PS1 mice compared to age-matched WT mice, in order to determine both leptin and Ob-Rb expression profiles prior to significant cerebral Aβ deposition and before cognitive loss. Immunoblotting analysis demonstrated that leptin and Ob-Rb proteins were present in the neocortex of APP/PS1 mice and age-matched WT littermates at both these ages (Fig. 1a, b). While leptin levels were not significantly altered there was an age dependent reduction of Ob-Rb in the neocortex at 6 months of age in both APP/PS1 mice and WT. In the hippocampus, while leptin protein was not detectable at quatifiable levels in the APP/PS1 or WT mice at either ages (data not shown), there was also an age dependent reduction of Ob-Rb at 6 months of age in APP/PS1 and WT, with the level of the receptor being significantly enhanced in APP/PS1 relative to WT at this age (Fig. 1b).
Representative GAPDH-normalized immunoblot images and quantitation of leptin (a) and Ob-Rb proteins (b) in both neocortical and hippocampal extracts of APP/PS1 mice compared to WT controls at 3 and 6 month of age. Bar graphs represent the mean ± SEM. Statistical analyses were performed using two-way ANOVA where *p < 0.05 vs WT mice at 3 month of age; #p < 0.05 vs APP/PS1 mice at 3 month of age; **p < 0.05 vs WT mice at 6 month of age. The data are presented as a percentage (%) of the corresponding value in the WT control group at 3 month of age. Abbreviations: CTX, neocortex; HP, hippocampus; APP, APP/PS1 Tg mice
Leptin levels in the brain of aged APP/PS1 mice
We next determined leptin protein levels in the brain of APP/PS1 mice in the context of amyloid pathology (9 months of age) and amyloid pathology in ageing (18 months of age), in order to examine its expression relative to age-matched WT littermates. At 9 months of age, leptin was detected, while there was no difference in leptin levels between genotypes in the neocortex (Fig. 2a) but significantly (p = .048) higher levels in the hippocampus of APP/PS1 mice relative to WT controls (Fig. 2b). At 18 months, leptin levels were significantly (p = .003) lower in the hippocampus of APP/PS1 mice compared to age-matched WT (Fig. 2b). Leptin levels significantly (p = .02) decreased with ageing in the neocortex of WT mice (18 months relative to 9 months), while no differences where determined in the hippocampus of WT mice (Fig. 2b).
Representative GAPDH-normalized immunoblot images and quantitation of leptin protein in both neocortical (a) and hippocampal (b) extracts of APP/PS1 mice compared to WT controls at 9 and 18 month of age. Bar graphs represent the mean ± SEM. Statistical analyses were performed using two-way ANOVA where *p < 0.05 vs WT mice at 9 month of age; #p < 0.05 vs APP/PS1 mice at 9 month of age; **p < 0.05 vs WT mice at 18 month of age. The data are presented as a percentage (%) of the corresponding value in the WT control group at 9 month of age. Abbreviations: CTX, neocortex; HP, hippocampus; APP, APP/PS1 Tg mice
To identify the source of leptin in the brain of aged mice, RT-qPCR analysis was performed on leptin mRNA purified from neocortex and hippocampus of APP/PS1 mice and WT mice at 9 and 18 months of age. No leptin mRNA was detected in either of the groups analysed, while white adipose tissue, as a positive control, showed high levels of leptin transcript (data not shown).
Immunohistochemical staining further demonstrated the presence of leptin protein in neocortical (Fig. 3a-f) and hippocampal (Fig. 3g-l) neurons in APP/PS1 mice and WT mice, respectively, at 9 and 18 month of age. In the neocortex, at 9 months of age, leptin was localized to the pyramidal cell cytoplasm and neurites, as reflected by strong immunolabelling (Fig. 3a, b). At 18 month of age, qualitative analysis suggested lower neuronal leptin immunoreactivity in both genotypes, with the highest reduction in WT mice compared to APP/PS1 mice (Fig. 3g, h). Interestingly, while lepin immunoreactivity in WT animals showed a somal staining pattern within pyramidal neurons in the neocortex (Fig. 3g-g’), high immunolabelling was present in both cell bodies and neurites in APP/PS1 mice (Fig. 3h-h’). Furthermore, leptin showed a differential patterm of cellular immunolabelling in the hippocampal regions in APP/PS1 mice and WT mice, at 9 (Fig. 3c-f) and 18 month of age (Fig.3i-l), respectively. At 9 month of age, pyramidal cells in CA1 showed cytoplasmic immunostaining with dendrites labelled in the stratum radiatum of CA1 (Fig.3c, d), whereas, in areas CA2 and CA3, labelling of neurons was reduced (data not shown). In addition, granular cell bodies of the dentate gyrus and mossy cells in the hilus showed very strong immunolabelling for leptin (Fig. 3e, f). Nevertheless, at 18 months of age, leptin immunolabelling was lower in the hippocampus of APP/PS1 mice compared to age-matched WT mice (Fig. 3i-l). Taken together, the results of immunoblotting analysis and immunohistochemical staining suggest that leptin is present within neurons in the cortex and hippocampus, where it is localized to the cell soma and neurites and that there is a loss of leptin with ageing, which is most prominent in the hippocampus of APP/PS1 mice.
(a-l) Leptin immunoreactivity in WT mice and APP/PS1 mice at 9 (A-F) and 18 month of age (G-L). Photomicrographs of immunoperoxidae-labelled sections with leptin immunoreactivityin the neocortex (CTX) and regions of the hippocampus (CA1 and DG) from WT mice and APP/PS1 mice at 9 (A-E’ and B-F′) and 18 month of age (G-K′ and H-L’), respectively. Images were collected using the same acquisition parameters on an Axio lab A.1 microscope and AxioCam ICc5 camera (both Zeiss, Germany), with ×20 and ×40 objectives. Scale bars = 20 μm. Black square indicates the region of interest magnified with the ×40 objective. Arrows indicate soma and dendrites. Abbreviations: CTX, neocortex; CA1, CornuAmmonis area 1; DG, Dentate gyrus
Ob-Rb levels in the brain
We next determined whether altered leptin levels were associated with altered levels of its receptor Ob-Rb. RT-qPCR analysis demonstrated no significant differences in the expression levels of neocortical Ob-Rb mRNA between APP/PS1 and WT mice at 9 and 18 months of age (Fig. 4a). Interestingly, at both ages a significant (p = .002 and p = .003, respectively) downregulation of Ob-Rb transcript was detected in the hippocampus of APP/PS1 mice compared to age-matched WT controls. In addition, Ob-Rb mRNA levels were also significantly (p = .043) downregulated in APP/PS1 mice at 18-months compared to APP/PS1 mice at 9 month of age (Fig. 4b). These data suggest that Ob-Rb mRNA is downregulated with age and in the hippocampus of APP/PS1 mice.
Neocortical (a) and hippocampal (b) Ob-Rb mRNA expression was assessed by qRT-PCR in APP/PS1 mice as compared to WT controls at 9 and 18 month of age. (c-d) Representative GAPDH-normalized immunoblot images and quantitation of Ob-Rb protein in both neocortical (C) and hippocampal (D) extracts of APP/PS1 mice compared to WT controls at 9 and 18 month of age. Bar graphs represent the mean ± SEM. The white lines in the western blot analysis (F) represent the lanes that were run on the same gel but were non-contiguous. Statistical analysis were performed using two-way anova where *p < 0.05 vs WT mice at 9 month of age; #p < 0.05 vs APP/PS1 mice at 9 month of age; **p < 0.05 vs WT mice at 18 month of age. The data are presented as a percentage (%) of the corresponding value in the WT control group at 9 month of age. Abbreviations: CTX, neocortex; HP, hippocampus; APP, APP/PS1 Tg mice
Ob-Rb was also altered at the protein level in APP/PS1 mice. Immunoblotting analysis demonstrated significantly (p = .0006) lower neocortical Ob-Rb levels in APP/PS1 mice relative to WT controls at 9 month of age (Fig. 4c), while, in the hippocampus, Ob-Rb was signifcantly (p = .0003) increased in APP/PS1 mice relative to WT animals (Fig. 4d). Similarly, in 18 month mice, in the hippocampus levels of the receptor were significantly (p = .0002) higher in APP/PS1 mice relative to age-matched WT mice (Fig. 4d). Interestingly, there was also an age related effect of receptor expression in the neocortex with higher (p = .005 and p = .01, respectively) expression at 18 months in both WT and APP/PS1 mice relative to 9 months (Fig. 4c). This suggests that while at the mRNA level, Ob-Rb is downregulated with age, the protein expression levels are increased with age and in the hippocampus of APP/PS1 mice.
We performed immunohistochemical labelling to determine if the regional or cellular distribution of Ob-Rb was altered in APP/PS1 tissue and through ageing. Ob-Rb protein was detected in neocortical (Fig. 5a-f) and hippocampal (Fig. 5g-l) neurons in APP/PS1 mice and WT mice at both 9 and 18 month of ages. In the neocortex, Ob-Rb immunolabelling had a cytoplasmic expression in pyramidal neurons at 9 (Fig.5a, b) and 18 months of age (Fig.5g, h). In the hippocampus,at 9 month of age, CA1 and the granular cell bodies of the dentate gyrus and mossy cells in the hilus showed strong Ob-Rb immunolabelling (Fig. 5c-f), whereas, in areas CA2 and CA3, relatively low labelling was present in APP/PS1 and WT mice (data not shown). At 18 month of age, although the cellular labelling pattern remained the same, comparatively, there was a lower level of immunolabelling for Ob-Rb in WT mice as compared to APP/PS1 mice, especially in the dentate gyrus (Fig. 5k, l). These data suggest that the pattern of Ob-Rb expression in the neocortex and hippocampus reflect the localization of leptin to specific regions.
(a-l) Ob-Rb immunoreactivity in WT mice and APP/PS1 mice at 9 (A-F) and 18 month of age (G-L). Photomicrographs of immunoperoxidae-labelled sections with Ob-Rb immunoreactivity in the neocortex (CTX) and regions of the hippocampus (CA1 and DG) from WT mice and APP/PS1 mice at 9 (A-E’ and B-F′) and 18 month of age (G-K′ and H-L’), respectively. Images were collected using the same acquisition parameters on an Axio lab A.1 microscope and AxioCam ICc5 camera (both Zeiss, Germany), with ×20 and ×40 objectives. Scale bars = 20 μm. Black square indicates the region of interest magnified with the ×40 objective. Arrows in black indicate soma. Abbreviations: CTX, neocortex; CA1, CornuAmmonis area 1; DG, Dentate gyrus
Leptin signalling in the brain
Our immunoblotting analysis studies so far suggest that ageing is associated with lower levels of leptin and higher levels of Ob-Rb expression which is particularly prominent in the hippocampus of APP/PS1 mice. We next investigated the status of STAT3 (pTyr705-STAT3) and Akt (pSer473-Akt) pathways, which are downstream of the Ob-Rb receptor (Fig. 6), as well as, the expression levels of SOCS3 gene and PTP1B genes (Fig. 7), the main inhibitors of leptin signalling in the brain (Fig. 8).
Representative GAPDH-normalized immunoblot images and quantitation of pSTAT3 (pTyr705-STAT3) protein in both neocortical (a) and hippocampal (b) extracts of APP/PS1 mice compared to WT controls at 9 and 18 month of age. (c-d) Representative GAPDH-normalized immunoblot images and quantitation of pAkt (pSer473-Akt) protein in both neocortical (C) and hippocampal (D) extracts of APP/PS1 mice compared to WT controls at 9 and 18 month of age. Bar graphs represent the mean ± SEM. The white lines in the western blot analysis (F) represent the lanes that were run on the same gel but were non-contiguous. Statistical analysis were performed using two-way anova where *p < 0.05 vs WT mice at 9 month of age; #p < 0.05 vs APP/PS1 mice at 9 month of age; **p < 0.05 vs WT mice at 18 month of age. The data are presented as a percentage (%) of the corresponding value in the WT control group at 9 month of age. Abbreviations: CTX, neocortex; HP, hippocampus; STAT3, the signal transducer and activator of transcription 3; Protein Kinase B, Akt; APP, APP/PS1 Tg mice
Neocortical and hippocampal mRNA expression of SOCS3 (a-b) and PTP1B (c-d) was assessed by qRT-PCR in APP/PS1 mice compared to WT controls at 9 and 18 month of age. Bar graphs represent the mean ± SEM. Statistical analysis were performed using two-way anova where *p < 0.05 vs WT mice at 9 month of age; #p < 0.05 vs APP/PS1 mice at 9 month of age; **p < 0.05 vs WT mice at 18 month of age. The data are presented as a percentage (%) of the corresponding value in the WT control group at 9 month of age. Abbreviations: CTX, neocortex; HP, hippocampus; APP, APP/PS1 Tg mice; SOCS3, suppressor of cytokine signaling-3; PTP1B, protein-tyrosine phosphatase 1B
In the brain, the binding of leptin to the ObRb receptor activates janus-tyrosine kinase 2 (JAK2), which in turn phosphorylates the insulin receptor substrate-1 and -2 (IRS 1/2) that results in the activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. JAK2 activation also leads to the phosphorylation of two tyrosine residues in the cytoplasmic tail of the Ob-Rb receptor, producing the activation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and the signal transducer and activator of transcription 3 (STAT3) signaling pathways. SOCS-3 and PTP1B interfear with leptin receptor signal transduction
Immunoblotting analysis demonstrated an age dependent decrease (p = .017) in STAT3 activity in the neocortex, but not the hippocampus, of WT mice (Fig.6a). Conversely, at both ages, STAT3 significantly (p = .0002 and p = .0009, respectively) increase in the neocortex and hippocampus of APP/PS1 mice, and in both brain regions at 18 months relative to age-matched WT controls (p = .0001 and p = .001, respectively) (Fig. 6a, b). There was no difference in Akt activity between genotypes in either region (Fig.6c, d). Akt activity was significantly (p = .0009) decreased in the hippocampus, but not the neocortex, with age in APP/PS1 mice, and at 18 months relative to age-matched WT controls (p = .029). Interestingly, while there were no significant differences between genotypes at 9 months of age, ageing in both WT and APP/PS1 mice, was associated with higher Akt activity in the neocortex and lower activity in the hippocampus (Fig. 6c, d).
While no alterations in the SOCS3 transcript were present with aging in WT mice, at 18 months RT-qPCR analysis demonstrated that the SOCS3 transcript was significantly (p = .001 and p = .031, respectively) upregulated in the neocortex of APP/PS1 mice compared to WT controls and APP/PS1 mice at 9 month of age (Fig. 7a). Interestingly, in the hippocampus an age dependent increased (p = .047) SOCS3 transcript was determined in APP/PS1 mice (Fig.7b). However, PTP1B expression did not follow this pattern (Fig.7c, d). PTP1B mRNA was significantly (p = .0001 and p = .004, respectively) upregulated with ageing in the neocortex of WT controls and APP/PS1 mice, but significantly (p = .001) lower in APP/PS1 mice at 18 months than aged matched WT mice (Fig. 7c). In the hippocampus, however, there was a significant (p = .0001) upregulation only in the APP/PS1 mice with ageing (p = .0001), while PTP1B transcript was significantly (p = .0001 respectively) upregulated relative to WT mice at 9 and 18 month of age (Fig. 7d).
Discussion
A growing body of evidence supports a potential metabolic component in AD pathogenesis (Chakrabarti et al. 2015). Several studies have investigated insulin signalling in AD and suggest a state of insulin resistance (Pedros et al. 2014; Petrov et al. 2015; Watson and Craft 2003). While the importance of leptin in AD is suggested by several clinical and epidemiological studies (Bonda et al. 2014; Lieb et al. 2009; Power et al. 2001; Rosenbaum et al. 1996; Tezapsidis et al. 2009), the contributions of leptin to AD has not yet been fully characterized. Our study provides the first evidence of alterations in leptin and leptin signalling in the brain of the APP/PS1 Tg model of early AD pathology, providing novel insights about the pathways that could link alterations in leptin signalling with AD-like pathology.
According to our data, immunoblotting analysis showed that leptin and Ob-Rb proteins were present in the neocortex of young APP/PS1 mice and age-matched WT controls, while leptin protein was not detected at quatitable levels in the hippocampus of either groups analysed. In the APP/PS1 mice we did demonstrate a relatively enhanced hippocampal expression of Ob-Rb relative to WT controls, which could possibly contribute to leptin’s reported beneficial effect on improving learning and cognitive function in AD (Farr et al. 2006; Sato et al. 2011; Searcy et al. 2012). Relatedly, high levels of Ob-Rb immunoreactity have been reported in this region (Harvey et al. 2006). Interestingly, in older animals of the current study, leptin was detected in the hippocampus, and our data indicates that there are higher leptin levels in the hippocampus of APP/PS1 mice relative to WT controls at 9 months of age, followed by an ageing-related reduction in expression across both genotypes and regions, which was more prominent in the hippocampus of APP/PS1 mice. Immunolabelling for leptin in the granular cell bodies of the dentate gyrus and mossy cells in the hilus was qualitatively lower in APP/PS1 mice compared to age-matched WT controls. The reduction in leptin levels can not be explained by a downregulation of leptin mRNA in the brain as we did not detect leptin transcript expression in cortex or hippocampus, as has been reported previously (Bonda et al. 2014; Maioli et al. 2015). However, the localization of the protein to neurons, suggests that leptin is internalized by these cells (Fernandez-Galaz et al. 2010) and supports the concept that this hormone is synthesized in the periphery and actively transported across the BBB or blood–CSF barrier (Friedman and Halaas 1998). Hence, the alterations to leptin levels in the brain of the APP/PS1 mice may reflect altered blood–CSF concentrations, since leptin uptake by the target neuron or intracellular leptin-signalling resistance. In this regard, Holden et al. (Holden et al. 2009) reported that, in elderly individuals, higher serum leptin appears to protect against cognitive decline. Importantly, circulating levels of leptin were reported to be significantly lower in patients with AD as compared to controls (Power et al. 2001). In addition, it has been demonstrated that gliosis prevents circulating leptin from accessing neurons (Horvath et al. 2010) and interferes with neuronal leptin uptake in the brain (Jastroch et al. 2014). In this context, it will be interesting in future studies to determine the mechanism of leptin regulation in the brain of APP/PS1 mice through analysis of CSF samples and circulating leptin concentrations.
Notably, in the APP/PS1 model, robust Aβ deposition is detected in both neocortex and hippocampus up to 12 months of age (Garcia-Alloza et al. 2006; Jankowsky et al. 2004). A severe gliosis commences around 6 months, especially in the vicinity of plaques, and the number of Iba-1 and GFAP-positive cells progressively increases with age (Kamphuis et al. 2012). Importantly, studies show links between Aβ and leptin but cause and effect still unclear (i.e. which cause which and/ or is possible that both affect each other?) (Fewlass et al. 2004; Ishii 2016), suggesting that decreased levels of neuronal leptin in the hippocampus of APP/PS1 mice at 18 month of age may be associated with disease-Aβ associated pathology.
Along with the marked decrease of leptin protein in the hippocampus of aged APP/PS1 mice, our immunoblot results showed significantly increased Ob-Rb levels in APP/PS1 mice compared to WT controls at both ages analysed. In addition, our results confirm previous studies conducted in both human AD and Tg2576 mice (Bonda et al. 2014; Maioli et al. 2015), where we demonstrate a significant decrease in Ob-Rb mRNA levels in the hippocampus of APP/PS1 mice compared to WT controls, at both ages analysed. Thus, although our immunohiostochemical analysis primarily identified ObRb receptor labelled cells with a neuronal profile, it is possible that other cell types (i.e. astrocytes) contribute to increasing ObRb levels in the hippocampus of APP/PS1 mice. Indeed, Maioli et al. (Maioli et al. 2015) have found increased Ob-Rb levels in astrocytes in the hippocampus of old Tg2576 AD mice (2-year-old), while leptin protein was reduced in neurons when compared to age-matched WT controls. Interestingly, a significant upregulation of Ob-Rb levels was determined in cultured astrocytes treated with Aβ1–42 for 24 h (Maioli et al. 2015). Hence, these results may be indicative of age-dependent Aβ overproduction inducing alteration of Ob-Rb levels in the brain of APP/PS1 mice. However, the precise dynamics of Ob-Rb regulation in the brain are incompletely understood (Hikita et al. 2000; Mitchell et al. 2009), and the mechanism of Ob-Rb regulation in the APP/PS1 Tg line mice needs to be determined in future studies.
Alternatively, the alterations to leptin levels in the APP/PS1 mice may reflect intracellular leptin-signalling resistance. For that reason, we also investigated the association of leptin signalling pathways with increased Aβ pathology in aged APP/PS1 mice. The binding of leptin to the neuronal Ob-Rb receptor activates four major signal transduction pathways (Folch et al. 2015), JAK/STAT pathway, ERK pathway, PI3K/Akt/mTOR pathway and AMPK/SIRT1 pathway (Fig.8). However, we were particularly interested in STAT3 and Akt signalling downstream of Ob-Rb receptor, as the STAT3 pathway has been recently implicated in Aβ-mediated neurotoxicity in AD (Wan et al. 2010). Importantly, in aged rats, a decline in STAT3 activation is observed that is linked to a decrease in leptin responsiveness (Scarpace et al. 2000), which indicates that age-related changes in leptin receptor-dependent signalling cascades occur. In addition, new experimental studies conducted in mice have shown that high-fat diet consumption induced hippocampal leptin resistance produced by the desensitization of the Akt pathway downstream neuronal Ob-Rb receptors (Valladolid-Acebes et al. 2013). Importantly, leptin increases adult hippocampal neurogenesis in vivo and in vitro by a mechanism involving STAT3 and Akt signalling pathways (Garza et al. 2008). In the current study, an increased tyrosine phosphorylation of STAT3 was determined in the brains of aged APP/PS1 mice compared to WT controls, at both ages analysed. This is in agreement with previous work showing increased pSTAT3 levels in APP/PS1 mice (Wan et al. 2010). Notably, increased pSTAT3 has been determined in post-mortem studies of AD (Wan et al. 2010). However, a significant decrease in hippocampal Akt activity was found in APP/PS1 mice compared toWT mice at 18 month of age. Concomitantly, we determined a robust upregulation of SOCS3 and PTP1B transcripts, which could further contribute to desensitizing the Akt pathway by altering phosphorylation of Jak2, after ligand binding to an Ob-Rb homodimer, and may negatively regulate activaton of neuronal Ob-Rb in the hippocampus of AD mice. Indeed, it has been demonstrated that elevation of SOCS3 and PTP1B interferes with leptin receptor signal transduction in aged animals (Morrison et al. 2007; Peralta et al. 2002). Importantly, PTP1B regulates leptin signalling in vivo, by targeting Jak2 (Zabolotny et al. 2002). This was an interesting preliminary result of our study, because it may indicate the existence of a downregulation of signalling towards leptin resistance associated with age-related Aβ overproduction, which may support the link between age-related cognitive decline and impaired leptin responsiveness. Indeed, new preliminary data indicates that people with AD may benefit from leptin replacement therapy (Tezapsidis et al. 2009). In addition, a growing body of experimental studies have demonstrated leptin’s beneficial effect on improving learning and cognitive function in AD (Farr et al. 2006; Sato et al. 2011; Searcy et al. 2012). Leptin has also been shown to enhance performance in memory tasks in SAMP8 mice, which display Aβ-induced neuronal toxicity (Farr et al. 2006). Leptin treatments leads to improvements in novel object recognition in APP/PS1 (Perez-Gonzalez et al. 2014) and TgCRND8 mice (Greco et al. 2010). In addition, improvements in contextual and cued fear conditioning tests have also been reported following 8 weeks of leptin treatment in TgCRND8 mice (Greco et al. 2010). Leptin has been shown to have strong in vitro (Fewlass et al. 2004; Greco et al. 2009b, 2008) and in vivo (Fewlass et al. 2004) anti-amyloidogenic effect, attributable to its transcriptional regulation capacity (Niedowicz et al. 2013) and inhibition of amyloid precursor protein (APP) processing (Perez-Gonzalez et al. 2014) that contributes to the amyloidogenic pathway. Accordingly, leptin reduces extracellular Aβ protein in vitro and this effect is dependent on activation of AMP-activated protein kinase (AMPK) (Fewlass et al. 2004; Greco et al. 2009b, 2008). It has also been reported that leptin can reduce total brain Aβ1–40 and Aβ1–42 in Tg2576 mice (Fewlass et al. 2004) and leptin reduces tau phosphorylation in neuronal cells through modulation of GSK3β activity (Greco et al. 2009a), a protein kinase identified to be pathogenic in a range of neurodegenerative disorders (Koh et al. 2011). This suggests a feedback loop where high concentrations of Aβ or metabolic disease could cause a state of leptin resistance and further contribute to AD pathology.
In terms of study limitations, our research provides the first experimental evidences of disruption of leptin signalling in the brain of the APP/PS1. Further mechanistic studies on the consequences of leptin signalling’s alterations may require larger sample sizes at defined timepoints. In addition, immunohistochemical staining was conducted to evaluate protein localization within brain between genotypes on sections relative to Bregma. Further quantitation of immunohistochemistry studies, investigating both expression profiles, may be warranted.
In summary, the data reported here provide the first experimental evidence for the pathways that could link aberrant leptin signalling to evolving pathology in the hippocampus of aged APP/PS1 mice. Altered leptin signalling may play a critical role in the pathophysiological changes of AD. Therefore, although the primum movens cause of AD is not yet fully understood,determining the role of leptin and related signalling in the brain may provide a new avenue for therapeutic development for this condition.
References
Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM (2001) Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res 891:42–53
Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, Jicha G, Casadesus G, Smith MA, Zhu X, Lee HG (2014) Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem 128:162–172. https://doi.org/10.1111/jnc.12380
Chakrabarti S, Khemka VK, Banerjee A, Chatterjee G, Ganguly A, Biswas A (2015) Metabolic risk factors of sporadic Alzheimer's disease: implications in the pathology, pathogenesis and treatment. Aging Dis 6:282–299. https://doi.org/10.14336/AD.2014.002ad-6-4-282
Farr SA, Banks WA, Morley JE (2006) Effects of leptin on memory processing. Peptides 27:1420–1425. https://doi.org/10.1016/j.peptides.2005.10.006
Fernandez CM et al (2009) The expression of rat resistin isoforms is differentially regulated in visceral adipose tissues: effects of aging and food restriction. Metabolism 58:204–211
Fernandez-Galaz MC, Fernandez-Agullo T, Carrascosa JM, Ros M, Garcia-Segura LM (2010) Leptin accumulation in hypothalamic and dorsal raphe neurons is inversely correlated with brain serotonin content. Brain Res 1329:194–202. https://doi.org/10.1016/j.brainres.2010.02.085
Fernandez-Martos CM (2017) Combination treatment with leptin and pioglitazone in a mouse model of Alzheimer’s disease. Alzheimers Dement: Transl Res Clin Interv 3:92–106
Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ (2011) Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One 6:e27000. https://doi.org/10.1371/journal.pone.0027000PONE-D-11-06684
Fernandez-Martos CM, King AE, Atkinson RA, Woodhouse A, Vickers JC (2015) Neurofilament light gene deletion exacerbates amyloid, dystrophic neurite, and synaptic pathology in the APP/PS1 transgenic model of Alzheimer's disease. Neurobiol Aging 36:2757–2767. https://doi.org/10.1016/j.neurobiolaging.2015.07.003S0197-4580(15)00359-0
Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N (2004) Obesity-related leptin regulates. Alzheimer's Abeta FASEB J 18:1870–1878. https://doi.org/10.1096/fj.04-2572com
Folch J, Patraca I, Martínez N, Pedrós I, Petrov D, Ettcheto M, Abad S, Marin M, Beas-Zarate C, Camins A (2015) The role of leptin in the sporadic form of Alzheimer's disease. Interactions with the adipokines amylin, ghrelin and the pituitary hormone prolactin. Life Sci 140:19–28. https://doi.org/10.1016/j.lfs.2015.05.002S0024-3205(15)00258-1
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770. https://doi.org/10.1038/27376
Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24:516–524. https://doi.org/10.1016/j.nbd.2006.08.017
Garza JC, Guo M, Zhang W, Lu XY (2008) Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem 283:18238–18247. https://doi.org/10.1074/jbc.M800053200M800053200
Gonzalez-Fernandez C, Fernandez-Martos CM, Shields SD, Arenas E, Javier Rodriguez F (2014) Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma 31:565–581. https://doi.org/10.1089/neu.2013.3067
Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N (2008) Leptin reduces Alzheimer's disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun 376:536–541
Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N (2009a) Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett 455:191–194
Greco SJ, Sarkar S, Johnston JM, Tezapsidis N (2009b) Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun 380:98–104
Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G (2010) Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 19:1155–1167. https://doi.org/10.3233/JAD-2010-1308
Harvey J (2007) Leptin: a diverse regulator of neuronal function. J Neurochem 100:307–313
Harvey J, Solovyova N, Irving A (2006) Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res 45:369–378. https://doi.org/10.1016/j.plipres.2006.03.001
Hikita M, Bujo H, Hirayama S, Takahashi K, Morisaki N, Saito Y (2000) Differential regulation of leptin receptor expression by insulin and leptin in neuroblastoma cells. Biochem Biophys Res Commun 271:703–709. https://doi.org/10.1006/bbrc.2000.2692S0006-291X(00)92692-5
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100
Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D (1999) Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 29:177–185
Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K (2009) Serum leptin level and cognition in the elderly: findings from the health ABC study. Neurobiol Aging 30:1483–1489. https://doi.org/10.1016/j.neurobiolaging.2007.11.024S0197-4580(07)00454-X
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107:14875–14880. https://doi.org/10.1073/pnas.1004282107
Hyman BT, Damasio H, Damasio AR, Van Hoesen GW (1989) Alzheimer's disease. Annu Rev Public Health 10:115–140. https://doi.org/10.1146/annurev.pu.10.050189.000555
Ishii M (2016) The role of the adipocyte hormone leptin in Alzheimer's disease. Keio J Med 65:21. https://doi.org/10.2302/kjm.65-002-ABST
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170. https://doi.org/10.1093/hmg/ddh019
Jastroch M, Morin S, Tschop MH, Yi CX (2014) The hypothalamic neural-glial network and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab 28:661–671. https://doi.org/10.1016/j.beem.2014.02.002
Kamphuis W, Mamber C, Moeton M, Kooijman L, Sluijs JA, Jansen AHP, Verveer M, de Groot LR, Smith VD, Rangarajan S, Rodríguez JJ, Orre M, Hol EM (2012) GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One 7:e42823. https://doi.org/10.1371/journal.pone.0042823PONE-D-12-13276
Koh SH, Baek W, Kim SH (2011) Brief review of the role of glycogen synthase kinase-3beta in amyotrophic lateral sclerosis. Neurol Res Int 2011:205761. https://doi.org/10.1155/2011/205761
Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S (2009) Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302:2565–2572. https://doi.org/10.1001/jama.2009.1836302/23/2565
Liu Y, Staal JA, Canty AJ, Kirkcaldie MTK, King AE, Bibari O, Mitew ST, Dickson TC, Vickers JC (2013) Cytoskeletal changes during development and aging in the cortex of neurofilament light protein knockout mice. J Comp Neurol 521:1817–1827. https://doi.org/10.1002/cne.23261
Liu Y, Atkinson RA, Fernandez-Martos CM, Kirkcaldie MT, Cui H, Vickers JC, King AE (2015) Changes in TDP-43 expression in development, aging, and in the neurofilament light protein knockout mouse. Neurobiol Aging 36:1151–1159. https://doi.org/10.1016/j.neurobiolaging.2014.10.001S0197-4580(14)00637-X
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)). Method Methods 25:402–408
Maioli S, Lodeiro M, Merino-Serrais P, Falahati F, Khan W, Puerta E, Codita A, Rimondini R, Ramirez MJ, Simmons A, Gil-Bea F, Westman E, Cedazo-Minguez A, the Alzheimer's Disease Neuroimaging Initiative (2015) Alterations in brain leptin signalling in spite of unchanged CSF leptin levels in Alzheimer's disease. Aging Cell 14:122–129. https://doi.org/10.1111/acel.12281
Mitchell SE, Nogueiras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C, Williams LM (2009) Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol 587:3573–3585. https://doi.org/10.1113/jphysiol.2009.173328jphysiol.2009.173328
Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW (2007) Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148:433–440. https://doi.org/10.1210/en.2006-0672
Moult PR, Milojkovic B, Harvey J (2009) Leptin reverses long-term potentiation at hippocampal CA1 synapses. J Neurochem 108:685–696. https://doi.org/10.1111/j.1471-4159.2008.05810.xJNC5810
Niedowicz DM, Studzinski CM, Weidner AM, Platt TL, Kingry KN, Beckett TL, Bruce-Keller AJ, Keller JN, Murphy MP (2013) Leptin regulates amyloid beta production via the gamma-secretase complex. Biochim Biophys Acta 1832:439–444. https://doi.org/10.1016/j.bbadis.2012.12.009S0925-4439(12)00300-6
Pedros I et al (2014) Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer's disease. Biochim Biophys Acta 1842:1556–1566. https://doi.org/10.1016/j.bbadis.2014.05.025
Peralta S, Carrascosa JM, Gallardo N, Ros M, Arribas C (2002) Ageing increases SOCS-3 expression in rat hypothalamus: effects of food restriction. Biochem Biophys Res Commun 296:425–428
Perez-Gonzalez R et al (2014) Leptin gene therapy attenuates neuronal damages evoked by amyloid-beta and rescues memory deficits in APP/PS1 mice. Gene Ther 21:298–308. https://doi.org/10.1038/gt.2013.85gt201385
Petrov D et al (2015) High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta 1852:1687–1699. https://doi.org/10.1016/j.bbadis.2015.05.004S0925-4439(15)00147-7
Power DA, Noel J, Collins R, O'Neill D (2001) Circulating leptin levels and weight loss in Alzheimer's disease patients. Dement Geriatr Cogn Disord 12:167–170
Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL (1996) Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81:3424–3427. https://doi.org/10.1210/jcem.81.9.8784109
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2011) Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 32:1626–1633. https://doi.org/10.1016/j.neurobiolaging.2009.10.009S0197-4580(09)00339-X
Scarpace PJ, Matheny M, Shek EW (2000) Impaired leptin signal transduction with age-related obesity. Neuropharmacology 39:1872–1879
Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, Beckett TL, Murphy MP, Chen KC, Blalock EM, Landfield PW, Porter NM, Thibault O (2012) Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease. J Alzheimers Dis 30:943–961. https://doi.org/10.3233/JAD-2012-11166107T40865VP760877
Stephens TW, Basinski M (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377:530–532
Stuart KE, King AE, Fernandez-Martos CM, Dittmann J, Summers MJ, Vickers JC (2017) Mid-life environmental enrichment increases synaptic density in CA1 in a mouse model of Abeta-associated pathology and positively influences synaptic and cognitive health in healthy ageing. J Comp Neurol 525:1797–1810. https://doi.org/10.1002/cne.24156
Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, Wolozin B, Perry G, Zhu X, Greco SJ, Sarkar S (2009) Leptin: a novel therapeutic strategy for Alzheimer's disease. J Alzheimers Dis 16:731–740. https://doi.org/10.3233/JAD-2009-10213375752606620632
Valladolid-Acebes I, Fole A, Martin M, Morales L, Cano MV, Ruiz-Gayo M, Del Olmo N (2013) Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem 106:18–25. https://doi.org/10.1016/j.nlm.2013.06.012S1074-7427(13)00104-4
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL, Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group (2013) Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 12:357–367. https://doi.org/10.1016/S1474-4422(13)70044-9S1474-4422(13)70044-9
Wan J, Fu AKY, Ip FCF, Ng HK, Hugon J, Page G, Wang JH, Lai KO, Wu Z, Ip NY (2010) Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer's disease. J Neurosci 30:6873–6881. https://doi.org/10.1523/JNEUROSCI.0519-10.201030/20/6873
Watson GS, Craft S (2003) The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS Drugs 17:27–45
Wilkinson M, Morash B, Ur E (2000) The brain is a source of leptin. Front Horm Res 26:106–125
Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG (2002) PTP1B regulates leptin signal transduction in vivo. Dev Cell 2:489–495
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Acknowledgements
The authors would like to gratefully acknowledge Graeme McCormack for his excellent technical support. The authors declare that they have no conflict of interest. This work was supported by the funding from J.O. and J.R. Wicking Trust (Equity Trustees).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material

Suppl. Fig.1
IHC negative controls. (A-H) Representative images processed without the primary antibodies, leptin and Ob-Rb, as controls, in both WT (A-G) and APP/PS1 (B-H) sections at 9 month of age. No non-specific staining was observed in any of the immunohistochemical controls. Images were collected on an Axio lab A.1 microscope and AxioCam ICc5 camera (both Zeiss, Germany), with ×5, ×20 and ×40 objectives. Scale bars = 20 μm. Black square indicates the hippocampal region of interest magnified with the ×40 objective. Abbreviations: CTX, neocortex; HP, hippocampus. (JPEG 2521 kb)
Rights and permissions
About this article
Cite this article
King, A., Brain, A., Hanson, K. et al. Disruption of leptin signalling in a mouse model of Alzheimer’s disease. Metab Brain Dis 33, 1097–1110 (2018). https://doi.org/10.1007/s11011-018-0203-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0203-9