Abstract
The crucial role of xanthine oxidoreductase (XOR) gene and its active isoform, xanthine oxidase (XO), in purine metabolism and cellular oxidative status led us to investigative their fluctuations in food deprivation induced food hoarding in mice. After, 10 h food deprivation, mice that hoarded lesser than 5 g were considered as ‘low-hoarders’ while mice that hoarded higher than 20 g were considered as ‘high-hoarders’. Mice who hoarded between 5 to 20 g of food were excluded from study. An increase (1.133-fold) in encephalic XOR expression has been found in high-hoarders compared with low-hoarders without sex consideration. An increase (~ 50-fold) in encephalic XOR in female high-hoarders vs. female low-hoarders while a decrease (0.026-fold) in encephalic XOR in male high-hoarders vs. male low-hoarders demonstrated that food deprivation is associated with sex-dependent alteration in XOR expression. The encephalic and hepatic XO activities were not different in male high-hoarders vs. male low-hoarders while encephalic XO activity has been also increased significantly in female high-hoarders (~ 4 times) compared to female low-hoarders. The plasma and hepatic XO activities tended to be increased in female high-hoarders as compared to female low-hoarders, however the uric acid levels in plasma, liver and brain tissues were not altered in female high-hoarders as compared to female low-hoarders. In sum, this study generally proposed that different gene expression space is behind of hoarding behavior in a food-deprived mouse model. Specifically, this is the first study that examined the levels of encephalic XO activity and XOR expression in hoarding behavior, although additional studies are requested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most important adaptation strategies of animals to support their homeostasis involve decreasing energy costs through migration, weight gain, hibernation, fat storage and finally hoarding behavior (Daly et al. 1992; Erin 2002; Zhang and Wang 2011; Zhang et al. 2011).
Hoarding has been described as a human behavioral disorder although it is considered as an adaptive behavior evoked in other animals during times of capricious environmental conditions such as food scarcity. Several reports that examined the impact of food deprivation in animals acknowledged evidences of much greater burden of oxidative stress in brain and liver. For example, 36 h food-deprived rats showed amplified fat and protein oxidation along with reduced antioxidative glutathione amounts in liver tissues (Domenicali et al. 2001). Other study reported that increased free radical production and decreased antioxidative enzyme activities of catalase (CAT) and superoxide dismutase (SOD) in brain tissues of food-deprived rats (Santos et al. 2009). To date, there are no studies examining the consequences of food deprivation on brain oxidative status, however aforementioned studies support the likelihood that food deprivation may cause oxidative stress in brain and other tissues. As a housekeeping enzyme, xanthine oxidoreductase (XOR) isoforms perform many cellular protective and signaling functions associated with synthesis of uric acid (UA), reactive oxygen species (ROS), and reactive nitrogen species (RNS; Vorbach et al. 2006). The synthesis of both antioxidants (i.e. UA) and numerous prooxidants like hydrogen peroxide per a reaction makes XOR isoforms candidate regulators of the cellular oxidative status (Vorbach et al. 2003; Naseri et al. 2017; Asasi et al. 2016).
The XOR is a homodimer molybdoflavoenzyme which its subunits formed from iron-sulfur, flavin adenine dinucleotide and molyboprotein (Cheung et al. 2007). This enzyme has 2 interconvertible isoforms with identical XOR gene that are xanthine dehydrogenase (XD; EC 1.1.1.204) and xanthine oxidase (XO; EC 1.1.3.22). Both isoforms are generator of ROS, RNS (Vorbach et al. 2006) and UA as one of end product of purine catabolism (Vorbach et al. 2003). The XOR has been detected in brain cells, therefore, brain capillaries can be considered as a major source of oxygen free radicals produced throughout XO activity (Wakatsuki et al. 1999). Direct measurement of XOR is difficult because of its low activity in brain (Solaroglu et al. 2005) while activity of its XO isoform will increase in diabetic liver (Desco et al. 2002), brain ischemia (Solaroglu et al. 2005), gout, heat stress, hemorrhagic shock, viral infections (Dew et al. 2005), meningitis (Vorbach et al. 2003), xanthinuria-induced autism (Zannolli et al. 2003), and recurrent depression (Michel et al. 2010). Therefore, XO activity may alter in an array of behavioral paradigms. Based on these findings, we hypothesized that XO may contribute to food deprivation induced food hoarding in mice.
Materials and methods
Animal subjects
This study reviewed and approved by the Laboratory Animal Care Committee of Razi University (no.1392/1). Animals were maintained at 23 °C, under a 12:12 h light cycle with ad libitum access to water and food.
Behavioral screening
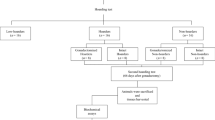
To evaluate hoarding behavior in BALB/c mice (3-month-old; 25–30 g), a special apparatus was designed and manufactured as reported previously (Deacon 2006). The hoarding apparatus was composed of chamber (13.0 × 32.0 × 6.5 cm3) with a hole (internal diameter 4 cm) in its front side, a chalky box and a water bottle. Hoarding tubes (length 45 cm, external diameter 4 cm) were connected to the holes of boxes equipped with a 10-cm plastic tube and joined to wire mesh tubes. The proximal end of designed hole was sealed with a plastic plug (see Fig. 1).
Each mouse was placed into chamber home box in the morning (8:00 a.m.) wherein water was available ad libitum during the experiment but access to hoarding tubes containing food pellets (100 g) was blocked via a plastic plug. At sundown (6:00 p.m.), the plugs were removed and the food-deprived mice were allowed access to hoarding tube. All hoarded food pellets distributed inside and outside of home box were weight at 8:00 to 8:30 a.m. next day. In this paradigm, mice that hoarded less than 5 g of food were considered “low-hoarders” while mice that hoarded more than 20 g of food were considered “high-hoarders”. Mice that hoarded between 5 to 20 g of food were considered as normal subjects and excluded from study.
After behavioral classification, thirty-two mice were retained and categorized into 4 groups (n = 8 for each), namely; female high-hoarders, male high-hoarders, female low-hoarders, and male low-hoarders. These mice experienced 14 h of food deprivation before tissue harvesting (vide infra).
Tissue harvesting
At the end of study, all fasted mice were weighed and deeply anesthetized intraperitoneally with anesthetic cocktail of ketamine (80 mg/kg; Alfasan, Netherland) and diazepam (0.5 mg/kg; Chemidarou Co. Tehran, Iran) and then mice were exsanguinated via cardiac puncture to prepare plasma. Brain and liver tissues also were weighted. Brain tissues were divided into 2 parts and submitted to enzymology and gene expression (vide infra).
Xanthine oxidase activity
All reagents were purchased from Sigma-Aldrich, UK unless otherwise stated. Lyophilized plasma, brain and liver tissues were homogenized in 5 to 10 volume of potassium phosphate buffer (pH 7.4) containing 5 mM ethylenediamine tetraacetic acid disodium salt (EDTA-Na2) and 1 mM phenylmethanesulfonyl fluoride using a WiseTis homogenizer (HG-15D, Korea). The homogenate was centrifuged at 12,000×g at 4 °C for 15 min. The supernatants were centrifuged at 12,000×g at 4 °C for 15 min once again and final supernatant was used to detect XO activity after Km calculation (data not shown).
Xanthine oxidase activity was spectrophotometrically assayed based on monitoring UA formation throughout reaction, xanthine + H2O + O2 ⇌ UA + H2O2, as described elsewhere (Hall et al. 1990; Sugawara et al. 1999). In brief, test reaction was started by adding 0.1 ml of the supernatant in 3.9 ml of a phosphate buffer solution (pH 8.0, 50 mM, containing 1 mM EDTA-Na2) with 1 ml of xanthine (500 μM, final concentration 100 μM) as the substrate. The mixture (total 5 ml) was incubated at 37 °C for 30 min and the reaction was stopped by the addition of 0.5 ml 0.58 M HCl. We considered a blank reaction containing all components of test reaction while stopped by the addition of 0.5 ml 0.58 M HCl at once. Then blank mixture (total 5.5 ml) was incubated at 37 °C for 30 min. The production of UA was determined by its UV absorbance (A) at 290 nm (A = A Test tube - A Blank tube). One unit of XO enzyme activity was calculated as the amount of enzyme required to convert 1 nmol of xanthine to UA per minute per mg protein at 37 °C using a standard curve of UA. Each assay was performed in triplicate and protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as standard.
Detection of uric acid
The UA levels of plasma and tissue homogenates were spectrophotometrically assayed based on phosphomolybdate reaction by a commercial kit (Pars Azmon, Tehran, Iran) using an autoanalyzer (Heitachi 896,Germany).
Real-time (RT)-qPCR assay
Total encephalic RNA was extracted using a commercial kit (Thermoscientific, England) as described by the manufacturer. Purity and RNA concentration were spectrophotometrically evaluated by optical density measurements at 260 and 280 nm. Total RNA (1 mg) was reverse transcribed using a complementary DNA (cDNA) synthesis kit (Bioneer, Germany) according to the manufacturer’s instructions and the prepared cDNA was stored at −20 °C till being used.
Primers of XOR as gene of interest and β-actin as house-keeping gene were purchased from Takapozist Co. Tehran, Iran (Table 1). The primer preparation was performed as described by the manufacturer. Diethylpyrocarbonate water was added into the primer tube in order to supply 100 pmol/μl after 30 min incubation at RT and mild pipetting; then a primer concentration of 10 pmol/μl was poured in RNase-free microtubes.
SYBR-Green Master Mix solution (Ampliqon, Den-mark) was prepared for real-time PCR reaction. For each sample, real-time quantitative reverse transcription (RT-qPCR) was performed in microtubes including 10 μl forward primer, 10 μl reverse primer, 20 μl SYBR-Green Master Mix, 5 μl cDNA with master cycler real-time PCR (Eppendorf, Germany) with cycling parameters: 95.0 °C for 2 min (initial temperature) and 40 cycles at 95.0 °C for 15 s, 56.5 °C for 15 s, and 72.0 °C for 15 s. The final stage was the application of a default temperature program for melting curve to make sure of absence of primer dimers.
For relative quantification (RQ) of gene expression, the mean CT values of the triplicates of the XOR and β-actin genes were calculated, followed by subtraction of the mean CT values of the β-actin gene to the mean CT values of the XOR gene (delta CT values). Then, the power of all delta CT values was calculated based on the formula power = 2-deltaCT (Livak and Schmittgen 2001).
Statistical analysis
Statistical analyses were performed using SPSS, version 16.0, statistical software (SPSS Inc., Chicago, IL, USA). Shapiro-Wilk test was utilized to determine whether data were normally distributed. Nonparametric Kruskal-Wallis analysis of variance (ANOVA) was employed when data are not normally distributed and post hoc pair-wise Mann-Whitney test was used where the overall Kruskal-Wallis revealed a significant effect. For normally distributed data, if ANOVA revealed a significant difference, post hoc Tukey’s test has been used to determine the difference between groups. The correlation between XO activity and food hoarding also checked with Pearson and Spearman tests depends on their normality. Data were expressed as the mean ± standard errors of the mean (S.E.M) and significance level was considered at p value <0.05.
Results
XO activity
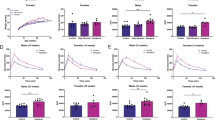
We found significant differences in the amount of hoarded food pellets (F3,28 = 51.180;, p = 0.000) among groups and an increase of hoarded food in high-hoarder versus low-hoarders in both male (p = 0.000) and female (p = 0.000) mice (Table 2).
Encephalic XO activity was different among groups (F3,26 = 4.890; p = 0.008). In this line, it significantly increased in female high-hoarder mice compared to female (p = 0.008) and male (p = 0.017) low-hoarder mice (Table 2). Hepatic XO activity showed significant difference among groups (F3,22 = 3.422; p = 0.035; Table 2). In this regard, results showed only significant difference between male high-hoarder and female low-hoarder (p = 0.045; Table 2).
Plasma XO activity (F3,23 = 0.193; p = 0.900) and plasma (F3,28 = 0.771; p = 0.520), hepatic (F3,28 = 1.422; p = 0.257), and encephalic (F3,28 = 2.625; p = 0.070) UA levels did not differ among groups (Table 2). The bodyweight (F3,28 = 1.794; p = 0.171), and relative weights of liver (F3,28 = 1.207; p = 0.325) and brain (F3,28 = 2.508; p = 0.079) were not different among groups (Table 2).
The correlation between amount of hoarded food and encephalic XO activity was positive (Spearman’s rho = 0.585; p = 0.001), while there was no difference between plasma, encephalic and hepatic XO activities (p > 0.05).
XOR gene expression
Total XOR mRNA and β-actin mRNA were measured by RT-qPCR in whole brain homogenate. Whole brain mRNA levels of housekeeping gene β-actin did not differ among studied groups and thus were used to normalize levels of XOR mRNA. According to the melting curve, there were no primer-dimers (data not shown). The amount of calculated XOR expressions in high-hoarder compared with low-hoarder without sex consideration were 1.133 fold changes. The XOR expressions in female and male high-hoarders showed 49.180 and 0.026 fold changes as compared to female and male low-hoarders, respectively.
Discussion
Food hoarding is characterized by frequently excessive food acquiring following a short period (10 h, in this study) of food deprivation. Many studies declared that animals do food hoarding that modulated by external (e.g. food deprivation) and internal factors (e.g., orexigenic neuropeptides like agouti-related protein; see seminal review Keen-Rhinehart et al. 2010). In a pioneered study (Karami et al. 2006), it has been reported that mice that were deprived of food, water, and bedding for upper than 6 h (between 9 and 24 h), when allowed to re-feed for up to 1 h, only 50% of the available food being consumed. Interestingly, feeding behavior appears retained for up to 6 h after deprivation. Thereafter, some mechanism inhibits the animals’ ability to fully feed, unlike continuously fed control animals (1 g of food/h). The biochemical basis of this process is not clearly understood (Karami et al. 2006). To the best of our knowledge, no study addressed how animals make decisions to hoard food in various amounts. For example, colonymate mice in the present study hoarded food in dissimilar amounts following 10 h food deprivation.
Keen-Rhinehart et al. (2010) proposed that ‘energy flux’ is a unifying factor controls food hoarding behavior. Based on ‘energy flux’ hypothesis, the signals that elicit foraging/hoarding are generated from central neuropeptides, endocrine or other circulating factors associated with mobilization of available metabolic fuels from energy reservoir compartment (e.g., liver) to energy utilizing tissues (e.g., brain). The mechanisms that differently alter XO activity after food deprivation (14 h before biochemical analysis) or differently ignite food hoarding behavior after food deprivation (10 h before refeeding) among a homogenous colony of mice are not investigated till now.
There are several evidences that hypothetically support putative role of XO in hoarding behavior. In this continuum, energy imbalance before or during hoarding behavior is one of probable key players. The purinergic system often relates to the level of adenine-based purines (ATP, ADP, AMP, and nucleoside adenosine; Murray 2003). The other side of this issue is involvement of XO in purine metabolism (Murray 2003). The pattern of encephalic XOR expression was sex-dependent in this study and showed ~ 50-fold increase in female high-hoarders compared to female low-hoarders. The encephalic XO activity was also increased in female high-hoarders compared to female low-hoarders. The encephalic UA level tended to increase in female high-hoarders compared to female low-hoarders. We can cautiously conclude that purine catabolism has been accelerated in brain tissues of female high-hoarders compared to female low-hoarders. In support of this notion, conditions characterized by augmented ATP degradation and/or diminished ATP synthesis (e.g., starvation, tissue hypoxia or strenuous exercise) burden the purine catabolic pathway accumulating degradation products (Becker 2001). Since XO activity will be increased in harsh (starvation/food deprivation) and pathological conditions (e.g., tissue ischemia or chronic sleep apnea; Parks et al. 1983; McCord 1985), we can cautiously conclude that high-hoarder mice may have a pathological condition as compared with low-hoarder mice.
Hoarding behavior may be a reflexive strategy against internal derangements like energy stagnation or hidden metabolic disorders (e.g., diabetes mellitus) or more possibly a neurochemical error. The hepatic XO activity and both hepatic and plasma UA level did not show any significant differences in female high-hoarders compared to female low-hoarders. Therefore, it seems that a partitioning of energy utilization is occurred between brain and other tissues like liver which works in higher intensity in female high-hoarders compared to female low-hoarders. The “selfish brain” theory supports this conclusion. This theory states that brain behaves greedily by adjusting energy fluxes in such a way that it allocates energy to itself before the requests of the other organs are gratified.
The hepatic XO activity in ovariectomized rats was higher than control rats (Khany et al. 2016). Accordingly, we can conclude that hormonal milieu may affect XO activity in various tissues and probably hormonal milieu of female high-hoarders are different as compared to female low-hoarders (for a review see Keen-Rhinehart et al. 2010). In this line, one study reported that the amount of hoarding in female mice is controlled with estrogen wherein hoarding is reduced during estrus and mating phases when estrogen is high (Bartness et al. 2011). Furthermore, XO activity has been reported to be inhibited by estradiol (Budhiraja et al. 2003). In this regard, more investigations including repetition of present study in estrous synchronized mice and a gonadal hormone assay in the presence of hoarding behavior are suggested to find an accurate explanation for this heterogeneity in hoarding behavior in colonymates. The encephalic XOR expression in male high-hoarders decreased by 0.026-fold as compared to male low-hoarders, while encephalic and hepatic XO activities were not different in male high-hoarders as compared to male low-hoarders. We cannot explain this heterogeneity in hoarding behavior in a homogeneous colony of male mice. It should be noted that there is no comparable study focused on XO activity in hoarder mice in the literature.
Food deprivation also alters mammalian antioxidative defense through ROS production, especially in the liver. In this line of findings, an increase in antioxidant enzyme and lipid peroxidation was found in food-deprived Atlantic cod (Furné et al. 2009) and reduced antioxidative enzyme (CAT and SOD) activities were identified in liver and erythrocytes following 36 h food deprivation in trouts. Finally, food deprivation has been reported to increase markers of oxidative stress in rat brains (Santos et al. 2009). Therefore, according to the higher brain XO activity in high-hoarders versus low-hoarders, it can be concluded that an oxidative stress and enhanced brain purine metabolism would be occurred in high-hoarders.
The increase (1.133-fold) in encephalic XOR expressions has been found in high-hoarder compared with low-hoarder without sex consideration. However, an increase (~ 50-fold) in XOR mRNA in brain tissue in female high-hoarders versus female low-hoarders and a decrease (0.026-fold) in XOR mRNA in brain tissue in male high-hoarders versus male low-hoarders demonstrate that fasting are associated with sex-specific changes in XOR expression. In this continuum, the encephalic XO activity has been also increased significantly in female high-hoarders (~ 4 times) compared to female low-hoarders. The plasma and hepatic XO activities tended to be increased in female high-hoarders as compared to female low-hoarders, however the UA levels in plasma, liver and brain were not altered in female high-hoarders as compared to female low-hoarders. These findings may explain that hoarding behavior of female mice is not modulated by the level of UA and it seems XO catalyzes reversible reaction that leads to UA reutilization and xanthine production for salvage purinogenesis (Asasi et al. 2016). In support of this explanation, Soñanez-Organis et al. (2012) showed that increased purine recycling coincident by upregulating hypoxanthine-guanine phosphoribosyl transferase (HGPRT) and XO mRNA and protein expression in a tissue-specific manner during fasting coincident with parallel increases in plasma HGPRT and XO activities and circulating hypoxanthine and xanthine content, suggesting an adaptive strategy to cope with prolonged fasting in post-weaned northern elephant seals. Accordingly, we postulate that encephalic XO catalyzes the reverse reaction during food restriction since mice encountered to a physiological energy shortage and based on ‘selfish theory’ that stated conservation of brain weight at this condition, brain tissues need molecular oxygen (De Oliveira et al. 2008) and hypoxanthine for synthesis of purine bases and nucleotides through salvage pathway (Stevens 1996).
In sum, this study provides insight into different gene expression space behind of hoarding behavior in a food-deprived mouse model. Specifically, this is the first study to examine levels of encephalic XO activity and XOR expression as candidate regulators in hoarding behavior, although additional studies are requested to thoroughly characterize molecular neuroeconomics of hoarding behavior.
References
Asasi K, Naseri D, Karimi I (2016) Ontogenetic study of enteric xanthine oxidase in the chick embryos: focus on the late embryogenesis. Comp Clin Pathol 25(3):625–629. https://doi.org/10.1007/s00580-016-2241-1
Bartness TJ, Keen-Rhinehart E, Daily MJ, Teubner BJ (2011) Neural and hormonal control of food hoarding. Am J Physiol Regul Integr Comp Physiol 301(3):R641–R655. https://doi.org/10.1152/ajpregu.00137.2011
Becker MA (2001) Hyperuricemia and gout. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic & Molecular Bases of inherited disease, vol 2. McGraw-Hill, New York, pp 2513–2535
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Budhiraja R, Kayyali US, Karamsetty M, Fogel M, Hill NS, Chalkley R, Finlay GA, Hassoun PM (2003) Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal 5(6):705–711. https://doi.org/10.1089/152308603770380007
Cheung KJ, Tzameli I, Pissios P, Rovira I, Gavriolova O, Ohutsubo T, Chen Z, Finkel T, Flier JS, Freidman JM (2007) Xanthine oxidoreductase is a regulator of adipogenesis and PPARI activity. Cell Metab 5(2):115–128. https://doi.org/10.1016/j.cmet.2007.01.005
Daly M, Jacobs LF, Wilson MI, Behrends PR (1992) Scatter hoarding by kangaroo rats (Dipodomys merriami) and pilferage from their caches. Behav Ecol 3(2):102–111. https://doi.org/10.1093/beheco/3.2.102
De Oliveira JE, Uni Z, Ferket PR (2008) Important metabolic pathways in poultry embryos prior to hatch. Worlds Poult Sci J 64(04):488–499. https://doi.org/10.1017/S0043933908000160
Deacon RM (2006) Assessing hoarding in mice. Nat Protoc 1(6):2828–2830. https://doi.org/10.1038/nprot.2006.171
Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J (2002) Xanthine oxidase is involved in free radical production in type 1 diabetes protection by allopurinol. Diabetes 51(4):1118–1124. https://doi.org/10.2337/diabetes.51.4.1118
Dew TP, Day AJ, Morgan MR (2005) Xanthine oxidase activity in vitro: effects of food extracts and components. J Agric Food Chem 53(16):6510–6515. https://doi.org/10.1021/jf050716j
Domenicali M, Caraceni P, Vendemiale G, Grattagliano I, Nardo B, Dall’agata M, Santoni B, Trevisani F, Cavallari A, Altomare E, Bernardi M (2001) Food deprivation exacerbates mitochondrial oxidative stress in rat liver exposed to ischemia-reperfusion injury. J Nutr 131(1):105–110
Erin S (2002) Illumination and food deprivation as determinants for hoarding in golden hamsters. Honors projects paper 35. http://digitalcommons.iwu.edu/psych_honproj/35
Furné M, Garcia-Gallego M, Hidalgo MC, Morales AE, Domezain A, Domezaine J, Sanz A (2009) Oxidative stress parameters during starvation and refeeding periods in Adriatic sturgeon (Acipenser naccarii) and rainbow trout (Oncorhynchus mykiss). Aquac Nutr 15(6):587–595. https://doi.org/10.1111/j.1365-2095.2008.00626.x
Hall IH, Scoville JP, Reynolds DJ, Simlot R, Duncan P (1990) Substituted cyclic imides as potential anti gout agents. Life Sci 46(26):1923–1927. https://doi.org/10.1016/0024-3205(90)90507-N
Karami KJ, Coppola J, Krishnamurthy K, Llanos DJ, Mukherjee A, Venkatachalam KV (2006) Effect of food deprivation and hormones of glucose homeostasis on the acetyl CoA carboxylase activity in mouse brain: a potential role of acc in the regulation of energy balance. Nutr Metab (Lond) 3(1):15. https://doi.org/10.1186/1743-7075-3-15
Keen-Rhinehart E, Dailey MJ, Bartness T (2010) Physiological mechanisms for food-hoarding motivation in animals. Philos Trans R Soc Lond Ser B Biol Sci 365(1542):961–975. https://doi.org/10.1098/rstb.2009.0225
Khany A, Karimi I, Alimoradian D, Zavareh S (2016) Distribution of xanthine oxidase in selected tissues of a mouse model of menopause. J Chem Health Risk 6(3):225–235
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
McCord J (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312(3):159–163. https://doi.org/10.1056/NEJM198501173120305
Michel TM, Camara S, Tatschner T, Frangou S, Sheldrick AJ, Reidere P, Grunblatt E (2010) Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry 11(2-2):314–320. https://doi.org/10.3109/15622970802123695
Murray RK (2003) Harper's illustrated biochemistry. McGraw-Hill, New York
Naseri D, Asasi K, Karimi I (2017) Similar developmental fluctuations of hepato-renal xanthine oxidoreductase gene expression and xanthine oxidase activity in layer and broiler chicken embryos. Br Poult Sci 58(2):144–150. https://doi.org/10.1080/00071668.2016.1268250
Parks D, Bulkley G, Granger D (1983) Role of oxygen-derived free radicals in digestive tract diseases. Surgery 94(3):415–422
Santos RX, Cardoso S, Silva S, Correia S, Carvalho C, Crisóstomo J, Rodrigues L, Amaral C, Louro T, Matafome P, Santos MS, Proença T, Duarte AI, Seiça R, Moreira PI (2009) Food deprivation promotes oxidative imbalance in rat brain. J Food Sci 74(1):H8–H14. https://doi.org/10.1111/j.1750-3841.2008.00982.x
Solaroglu I, Okutan O, Kaptanoglu E, Beskonakli E, Kilinc K (2005) Increased xanthine oxidase activity after traumatic brain injury in rats. J Clin Neurosci 12(3):273–275. https://doi.org/10.1016/j.jocn.2004.12.002
Soñanez-Organis JG, Vázquez-Medina JP, Zenteno-Savín T, Aguilar A, Crocker DE, Ortiz RM (2012) Prolonged fasting increases purine recycling in post-weaned northern elephant seals. J Exp Biol 215(9):1448–1455. https://doi.org/10.1242/jeb.067173
Stevens L (1996) Avian biochemistry and molecular biology. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511525773
Sugawara N, Ohta T, Lai YR, Sugawara C, Yuasa M, Nakamura M, Tamura M (1999) Iron depletion prevents adenine nucleotide decomposition and an increase of xanthine oxidase activity in the liver of the long Evans cinnamon (LEC) rat, an animal model of Wilson's disease. Life Sci 65(13):1423–1431. https://doi.org/10.1016/S0024-3205(99)00378-1
Vorbach C, Harrison R, Capecchi MR (2003) Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 24(9):512–517. https://doi.org/10.1016/S1471-4906(03)00237-0
Vorbach C, Capecchi MR, Penninger JM (2006) Evolution of the mammary gland from the innate immune system? BioEssays 28(6):606–616. https://doi.org/10.1002/bies.20423
Wakatsuki A, Okatani Y, Izumiya C, Ikenoue N (1999) Effect of ischemia-reperfusion on xanthine oxidase activity in fetal rat brain capillaries. Am J Obstet Gynecol 181(3):731–735. https://doi.org/10.1016/S0002-9378(99)70520-X
Zannolli R, Micheli V, Mazzei MA, Sacco P, Piomboni P, Bruni E, Miracco C, de Santi MM, Vagnoli PT, Volterrani L, Pellegrini L, Livi W, Lucani B, Gonnelli S, Burlina AB, Jacomelli G, Macucci F, Pucci L, Fimiani M, Swift JA, Zappella M, Morgese G (2003) Hereditary xanthinuria type II associated with mental delay, autism, cortical renal cysts, nephrocalcinosis, osteopenia, and hair and teeth defects. J Med Genet 40:e121. https://doi.org/10.1136/jmg.40.11.e121
Zhang H, Wang Y (2011) Differences in hoarding behavior between captive and wild sympatric rodent species. Curr Zool 57(6):725–730. https://doi.org/10.1093/czoolo/57.6.725
Zhang XY, Yang HD, Zhang Q, Wang Z, Wang DH (2011) Increased feeding and food hoarding following food deprivation are associated with activation of dopamine and orexin neurons in male Brandt’s voles. PLoS One 6(10):e26408. https://doi.org/10.1371/journal.pone.0026408
Acknowledgments
This study was supported by Razi University, Kermanshah, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Karimi, I., Motamedi, S. & Becker, L.A. An effort toward molecular neuroeconomics of food deprivation induced food hoarding in mice: focus on xanthine oxidoreductase gene expression and xanthine oxidase activity. Metab Brain Dis 33, 325–331 (2018). https://doi.org/10.1007/s11011-017-0166-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0166-2