Abstract
In this study, we present the clinical manifestations, brain magnetic resonance imaging (MRI) and concurrent polyneuropathies in two patients with non-alcoholic Wernicke’s encephalopathy (WE) after gastrojejunostomy (Billroth II) anastomosis procedures. These patients developed sub-acute onset of disorientation and disturbance of consciousness following several weeks of poor intake. Peripheral neuropathy of varying severity was noted before and after the onset of WE. Brain MRI of the patients showed cerebellar vermis and symmetric cortical abnormalities in addition to typical WE changes. Electrophysiological studies demonstrated axonal sensorimotor polyneuropathy. Prompt thiamine supplement therapy was initiated and both patients gradually recovered, however mild amnesia was still noted 6 months later. We reviewed non- alcoholic WE with atypical cortical abnormalities in English language literatures and identified 29 more cases. Eight out of 31 (25.8%) patients died during follow-up. Nine patients with gait disturbance or motor paresis had showed hyporeflexia in neurological examinations. In addition to classic triad, seizure was recorded in seven patients. Dietary deprivation is a risk factor for non-alcoholic WE among elderly patients receiving gastrointestinal surgery. The prognosis is good after thiamine supplement therapy. Recognizing the MRI features and predisposing factors in patients who have undergone gastrectomy can aid in the diagnosis and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide variety of neurological manifestations can occur after gastrectomy. Non-alcoholic Wernicke encephalopathy (WE) (Aasheim 2008; Haid et al. 1982), peripheral neuropathy (Thaisetthawatkul et al. 2004) and combined non-alcoholic WE and polyneuropathy have been reported after gastrectomy for morbid obesity (Cirignotta et al. 2000; Juhasz-Pocsine et al. 2007; Koffman et al. 2006), and highlight the importance of nutritional supplements after the procedure (Mechanick et al. 2013). In addition to nutritional deficiencies and significant weight loss, an inflammatory or immunological mechanism may play a role in WE and polyneuropathy (Landais 2014; Singh and Kumar 2007; Thaisetthawatkul et al. 2004). Non-alcoholic WE has been reported in patients without obesity or appreciable weight loss, and it has also been reported that thiamine deficiency (Rufa et al. 2011) and polyneuropathy (Koike et al. 2004; Koike et al. 2001) may occur after gastrectomy for indications other than morbid obesity. Herein, we report two patients, who developed non-alcoholic WE and peripheral neuropathy after gastrectomy, and whose clinical manifestations rapidly resolved after thiamine supplement therapy. We also compare our patients with those in previous reports of WE with atypical cortical abnormalities on brain magnetic resonance imaging (MRI), including the clinical manifestation and their prognosis.
Patients and methods
A clinical data analysis was carried out on two patients with WE and cortical involvement admitted to the neurology ward in a medical center in Taiwan. For literature reviewing, we used the PubMed data base to search the term of “Wernicke’s encephalopathy” and the words of “non-alcoholic”, “cortical” or “cortex”. We searched for case reports of non-alcoholic WE with cortical image abnormalities published in English languages with data reported at the individual level (Bonucchi et al. 2008; Chen et al. 2015; Cui et al. 2012; D'Aprile et al. 2000; Doss et al. 2003; Foster et al. 2005; Iannelli et al. 2010; Liu et al. 2006; Luigetti et al. 2009; Lyons et al. 2016; Mascalchi et al. 1999; Nolli et al. 2005; Ghosh et al. 2014; Pereira et al. 2011; Rufa et al. 2011; Sakurai et al. 2009; Santos Andrade et al. 2010; Sohoni 2014; Wicklund and Knopman 2013; Wu et al. 2016; Yamashita and Yamamoto 1995; Yoon et al. 2009; Zhong et al. 2005; Zuccoli et al. 2011; Zuccoli et al. 2009). One article discussing infant cases aged 2–10 months using soybean based diet was excluded (Kornreich et al. 2005). We focused on clinical manifestation of central and peripheral nervous systems, time window to intervention, clinical and brain MRI outcome. The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB: 102–0256B).
Case reports
Patient 1
A 66-year-old man was admitted because of progressive legs weakness and mild numbness with a glove and stocking distribution for 1 month for which he had become wheelchair bound. His medical history included hypertension and diabetes mellitus, both were well-controlled, hepatectomy for hepatocellular carcinoma (HCC) 10 years previously and Billroth II anastomosis for gastric signet ring cell carcinoma 14 months previously. He denied the use of alcohol or illicit substances, and his family claimed he was a picky eater. He had taken traditional Chinese medicine and eaten milled rice congee that resulted in a weight loss of 6 kg in 1 month.
After admission, he was afebrile but appeared to be lethargic and thin. His neurological examinations were abnormal, with 2 out of 5 on the Medical Research Council (MRC) muscle strength score of the lower extremities and 4 out of 5 in the upper extremities, diffuse tendon areflexia and diminished sensation to light touch over the fingertips on both hands and distal feet. His muscle strength progressively weakened, finally resulting in quadriplegia and hypercapnic respiratory failure. He was then intubated and plasmapheresis was begun for suspected Guillain-Barré syndrome. On the fourth day of admission, he developed a limitation in eyes movement, facial diplegia and slurred speech. The clinical picture was summary on Table 1.
Laboratory tests showed that electrolytes, complete blood count, renal function, liver function, protein electrophoresis, thyroid function, cortisol level, tumor markers and markers of autoimmune diseases (anti-gastric parietal cell autoantibodies, anti-acetylcholine receptor antibodies, antinuclear antibodies, rheumatic factor, and anti-neutrophil cytoplasmic antibodies) were normal. A serological test for syphilis was negative. His B-type natriuretic peptide level was 148 pg/mL (reference: <100 pg/mL), creatine phosphokinase (CPK) was 54 U/L (reference: <200 U/L), serum glycated hemoglobin was 6.5% (reference: <6.5%), vitamin B12 level was 821 pg/mL (reference: 211–946 pg/mL), and mild elevation of serum homocysteine was noted (12.2 μmol/L (reference: <12 μmol/L)). Urine heavy metal screen was unremarkable, and routine and biochemical examinations of cerebrospinal fluid were all normal.
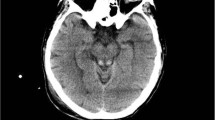
The chest plain film revealed cardiomegaly and the electrocardiogram showed sinus rhythm. The echocardiography disclosed 76% ejection fraction by M-mode and adequate left ventricle (LV) systolic function. Nerve conduction and electromyogram studies demonstrated motor-predominant axonal polyneuropathy with acute denervation five weeks after disease onset and became absence of sensory nerve action potential on both sural nerves ten weeks after onset (Table 2). Brain MRI revealed areas of symmetrically increased T2-weighted and fluid-attenuated inversion recovery signals without contrast enhancement on mammillary bodies, peri-aqueduct, medial thalami and peri-Rolandic cortex (Fig. 1a–d). Two months after the event, follow-up brain MRI showed a complete resolution of abnormal signal intensities in the cerebral cortex, and significant regression in the peri-aqueduct and periventricular thalamic regions (Fig. 1e–h).
Axial fluid-attenuated inversion recovery sequences of brain MRI in patients with non-alcoholic Wernicke’s encephalopathy. Symmetric hyperintensities in periaqueductal grey matter (white arrow), mammillary bodies (white arrowhead), medial thalami (black arrowhead), and bilateral peri-Rolandic cortices (curved arrow) and/or cerebellar vermis (black arrow) on initial images (a-b) of patient 1 and a significant resolution in periaqueductal grey matter, mammillary bodies and medial thalami (e-g) and complete resolution in the cerebral cortex (H) on follow-up images after 2 months of thiamine supplement in patient 1 and the initial images (i-l) of patient 2
Intravenous vitamin B complex supplements were given immediately and thereafter the intravenous supplements were kept for half month. Daily doses of vitamin B complex were thiamine 120 mg, riboflavin 6 mg, pyridoxine 6 mg, pantothenate 6 mg and nicotinamide 60 mg. The strength in his muscles recovered to 3 out of 5 in legs and 4 out of 5 in his upper limbs with free eyes movement on the 28th day of admission. He could walk with assistance and stand for more than 30 min 6 months later, although mild limb weakness and an ataxic gait remained. After 2 years of follow-up, his gait had become normal but amnesia remained.
Patient 2
A 70-year-old woman presented to our emergency department (ED) with episodic disturbance of consciousness and progressive mental deterioration for 7 days. Two weeks before this presentation she had been admitted for epigastric pain with severe vomiting, and a transient small bowel intussusception was diagnosed. Her nausea and retching improved and bowel function resumed after peripheral parenteral nutrition (a mixture of 20% fat emulsion and amino acid without vitamins supplements) for 1 week, however disorientation to time and place ensued after discharge. No headache or fever was noted. Her past medical history included a recurrent gastric ulcer treated with Billroth II anastomosis 42 years previously with ileus occurring several times after the operation, breast mass post mastectomy 10 years previously, chronic vertigo and well-controlled diabetes mellitus. The clinical manifestation was shown on Table 1. She denied the use of alcohol or illicit substances.
A physical examination of her heart, lungs and abdomen on admission to our hospital was normal. A neurological examination revealed obtunded consciousness and fixation in the movement of her extra-ocular muscles. Her facial expression was symmetric, and her motor response was movable bilaterally to sternal pressure, with a strength graded as at least 4 out of 5 on the MRC muscle strength score. Deep tendon reflexes were diffusely mute and Babinski signs were absent bilaterally.
Biochemical laboratory examinations showed that blood electrolytes, liver function, blood count, creatinine, glucose, calcium, magnesium, copper (89.1 μg/dL, reference: 80–153 μg/dL), albumin, ammonia, CPK (174 U/L), vitamin B12 level (2818 pg/mL and folate (12.07 ng/mL, reference: 4.6–18.7 ng/mL) were within normal limits. Mild elevations in glycated hemoglobin (6.8%, reference: <6.5%) and homocysteine (13.4 μmol/l, reference: <12 μmol/l) were noted. Antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-streptolysin O, anti-Ro antibodies, anti-La antibodies and Venereal Disease Research Laboratory serum test were undetectable. A test for anti-gastric parietal cell autoantibodies was positive (1:80, reference: negative). Elevated levels of anti-thyroid peroxidase antibodies (87.5 IU/ml, reference: <5.6 IU/ml) and normal anti-thyroglobulin antibodies (39.41 IU/ml, reference: <115 IU/ml) were thought to be secondary to hypothyroidism (free T4, 1.05 ng/dL; T3, 43.5 ng/dL; TSH, 0.097 uIU/mL). A urine toxicological screen for heavy metals was unremarkable. Routine and biochemical tests of her cerebrospinal fluid were sterile.
A short-term hypotension episode happened in the second admission day. Immediate echocardiography showed 54% ejection fraction by M-mode, fair left ventricular wall movement and mild mitral valve regurgitation and the electrocardiogram showed sinus rhythm with a rate of 96 bpm and non-specific ST-T changes. Mild cardiomegaly was found in chest plain film and otherwise normal. Her lactate level was 15.4 mg/dL (reference: 4.5–19.8 mg/dL). Hypotension improved 1 day after hydration. Her troponin I level was 1.812 ng/mL (cutoff: <0.5 ng/mL, 10% coefficient of variation: 0.06 ng/mL) initially and returned to 0.662 ng/mL within a 6-h interval.
Nerve conduction studies on the 10th day of admission revealed mildly reduced amplitudes of sensory nerve action potentials on both sural nerves (Table 2) and there were neither spontaneous activities nor myopathic change in the electromyogram study. Brain MRI scans performed in the ED showed high signal intensities involving bilateral peri-Rolandic cortices, mammillary bodies, periaqueductal gray matter, medial thalamus and cerebellar vermis on fluid-attenuated inversion recovery imaging (Fig. 1i–l) and T2 weighted imaging, these lesions were not enhanced by contrast medium. Daily 120 mg intravenous thiamine supplement therapy was initiated since ED admissino and lasted for 1 month. After that treatment she became alert, however mild limitation in eyes movement and amnesia remained. Dementia was the only sequela 9 months later.
Discussion
Wernicke’s encephalopathy is an acute neurological disorder caused by thiamine deficiency. It was first described as superior acute hemorrhagic poliencephalitis according to the autopsy findings in three patients (two alcoholic males and one vomiting female) and was believed to be associated with thiamine deficiency (Sechi et al. 2016; Zuccoli and Pipitone 2009). At autopsy, WE has been observed in 0.4 to 2.8% of the general population in Western countries (Sechi and Serra 2007) and up to 23% WE patients are non-alcoholic individuals (Lindboe and Loberg 1989). WE is a clinical diagnosis characterized by the triad of mental status changes, ocular signs, and cerebellar dysfunction. However, only 16 to 38% of cases fulfill these diagnostic criteria (Harper et al. 1986; Zuccoli et al. 2009). Factors affecting nutrition other than alcoholism include malignancy, gastrointestinal surgery, prolonged intravenous feeding, protracted vomiting, starvation and hyperemesis gravidarum (Galvin et al. 2010). In non-alcoholic WE following bariatric surgery, patients typically have a weight loss greater than 7 kg per month with the symptoms and signs of WE occurring 2 to 8 months post-operatively (from 2 weeks to 20 years), and most patients are young women with vomiting before onset of disease (Aasheim 2008; Cirignotta et al. 2000; Sechi and Serra 2007; Singh and Kumar 2007). In our patients, surgery causing food stuff to circumvent the duodenum and jejuno-ileal tract, where absorption of thiamine mainly occurs, certainly played a role in the development of WE. Ordinary gastrectomy is not widely thought to be a possible cause of thiamine deficiency, especially when a patient’s clinical condition is favorable without complications for a long period after surgery. The underlying causes, inducers and clinical presentations of our patients and other non-alcoholic WE cases from literature review are listed in Table 3. There were 16 cases with underlying causes of abdominal or bariatric surgery and 14 cases undergoing parenteral supplement before the WE. At least twenty-one out of 31 patients developed neurological manifestations following poor appetite and/or gastrointestinal symptoms within a few weeks. We believe that non-serious predisposing factors in addition to abdominal surgery that occurred before the clinical events may have played an important role in nutritional deficiency. Because of that, our patient 2 developed WE in a latency of 42 years. In addition, high carbohydrate diets, like in our patient 1, can deplete thiamine storage (Sauberlich et al. 1979), and aging also seems increase the need for thiamine (Wilkinson et al. 2000).
Brain MRI has a sensitivity of 53% and a specificity of 93% for the diagnosis of WE (Antunez et al. 1998). In WE, the brain MRI findings are characterized by typical symmetrical signal changes in medial thalami, mammillary bodies, and periaqueductal areas and less commonly alterations in atypical areas like cerebellar vermis and cerebral cortex. All these findings can be used to support the diagnosis of either non-alcoholic or alcoholic WE (Galvin et al. 2010; Zuccoli and Pipitone 2009). Typical radiologic manifestations allow the diagnosis of non-alcoholic WE for our two patients. The cortical involvement was previously believed to be indicative of irreversible lesions with poor prognosis (Fei et al. 2008; Pereira et al. 2011; Wicklund and Knopman 2013; Zhong et al. 2005). Table 4 showed the time from disease onset to thiamine supplement, clinical and brain MRI outcome. Our patient 1 had a significant resolution in the brain MRI abnormalities after the thiamine supplement, mild dementia was only a sequel after follow-up for at least 24 months. Combined out patients with patients from literature review, a follow-up MRI after thiamine treatment showed regression or absence of abnormal cortical signals in 10 out of eleven patients. Although our report has several limitations, including retrospective designs and loss of those non-reported or miss-diagnosed cases in our review. In our opinion, a reversible cytotoxic edema of cortex may explain good improvement of cortical lesions, documented at MRI, following timely thiamine treatment in our patients with WE (Sakurai et al. 2009; Wu et al. 2016). In the prognosis, eight patients died following the WE event and most of other patients had partial or complete recovery in a follow-up period.
A variable severity of peripheral neuropathy has been reported in patients with chronic mild thiamine deficiency (Sechi et al. 2016; Takahashi and Nakamura 1976), and thiamine has been shown to be an important cause of polyneuropathy in patients after ordinary gastrectomy (Koike et al. 2004; Koike et al. 2001). The characteristics of thiamine deficiency-related neuropathy are thought to include a length-dependent dying-back axonal neuropathy, manifesting as symmetric polyneuropathy with greater involvement of the lower than upper limbs. Patient 1 was initially misdiagnosed as having an axonal variant of Guillain-Barré syndrome and received one course of plasmapheresis. He was subsequently diagnosed with WE based on clinical features and brain MRI findings. Acute axonal polyneuropathy has also been rarely reported in thiamine deficiency (Ishibashi et al. 2003; Koike et al. 2008). The nerve conduction study of patient 2 revealed mild axonal polyneuropathy, although the contemporary occurrence of early diabetic sensorimotor polyneuropathy could not be excluded. Although the serum thiamine levels were not tested, the clinical conditions of both patients dramatically improved after thiamine supplement therapy. In Table 3, ten patients had reduced deep tendon reflex at neurological examination, and those patients were thought to have both central and peripheral nervous system involvement. Only one patient died after diagnosing WE. Therefore, we believe that multiple factors are involved in determining prognosis, such as the coexisting deficiencies of other micronutrients, particularly vitamins B6, B12, folate and magnesium (Sechi et al. 2016).
Cardiovascular disturbances are also present in thiamine deficiency and the clinical manifestations are varied. Usually patients develop vascular resistance, high output heart failure, pulmonary and peripheral edema (Watson et al. 2011). Another fulminant type is called shoshin beriberi with predominant injury to the heart and the presentations include low cardiac output, hypotension and lactic acidosis (Phua et al. 1990). Our female patient had developed hypotension and elevating cardiac enzyme, but no lactic acidosis or heart failure. Although the symptoms onset was after thiamine initiation, thiamine deficiency might partly account for her presentations. Proximal weakness, myalgia and gastrointestinal symptoms might also be attributed to thiamine deficiency (Donnino 2004; Koike et al. 2006). Thiamine functions in glucose metabolism, Krebs cycle and pentose phosphate pathway (Sechi and Serra 2007), therefore thiamine deficiency results in adenosine triphosphate depletion and pyruvate accumulation, which may cause heart failure and muscle injury (Bakker and Leunissen 1995). Myopathy has been reported frequently in alcoholism and rarely in nonalcoholic thiamine deficiency, and our patients have no clinical and electromyographic evidence of myopathy. Our female patient had been hospitalized for severe gastrointestinal symptoms, but biochemical data showed no lactic acidosis.
Diseases related to thiamine deficiency are rare in developed countries, and routine thiamine supplement therapy and monitoring of thiamine levels are recommended in patients after bariatric surgery (Galvin et al. 2010). Attention should also be paid to elderly patients with multiple risk factors for nutritional deficiency, especially following gastrectomy. The early recognition of Wernicke’s encephalopathy is crucial, considering the poor prognosis without appropriate treatment. It should also be kept in mind that undergoing gastrojejunostomy is a risk factor for non-alcoholic Wernicke encephalopathy in elderly patients, particularly in those with poor intake status.
Change history
19 October 2017
A correction to this article has been published.
References
Aasheim ET (2008) Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg 248:714–720. doi:10.1097/SLA.0b013e3181884308
Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A (1998) Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol 171:1131–1137. doi:10.2214/ajr.171.4.9763009
Bakker SJ, Leunissen KM (1995) Hypothesis on cellular ATP depletion and adenosine release as causes of heart failure and vasodilatation in cardiovascular beriberi. Med Hypotheses 45:265–267
Bonucchi J, Hassan I, Policeni B, Kaboli P (2008) Thyrotoxicosis associated Wernicke's encephalopathy. J Gen Intern Med 23:106–109. doi:10.1007/s11606-007-0438-3
Chen MH, Lee JT, Peng GS, Sung YF (2015) Non-alcoholic Wernicke's encephalopathy as a cause of profound shock after abdominal surgery. QJM 108:661–663. doi:10.1093/qjmed/hct044
Cirignotta F, Manconi M, Mondini S, Buzzi G, Ambrosetto P (2000) Wernicke-korsakoff encephalopathy and polyneuropathy after gastroplasty for morbid obesity: report of a case. Arch Neurol 57:1356–1359
Cui HW, Zhang BA, Peng T, Liu Y, Liu YR (2012) Wernicke's encephalopathy in a patient with acute pancreatitis: unusual cortical involvement and marvelous prognosis. Neurol Sci 33:615–618. doi:10.1007/s10072-011-0771-5
D'Aprile P, Tarantino A, Santoro N, Carella A (2000) Wernicke's encephalopathy induced by total parenteral nutrition in patient with acute leukaemia: unusual involvement of caudate nuclei and cerebral cortex on MRI. Neuroradiology 42:781–783
Donnino M (2004) Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med 141:898–899
Doss A, Mahad D, Romanowski CA (2003) Wernicke encephalopathy: unusual findings in nonalcoholic patients. J Comput Assist Tomogr 27:235–240
Fei GQ et al (2008) Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol 29:164–169. doi:10.3174/ajnr.A0827
Foster D, Falah M, Kadom N, Mandler R (2005) Wernicke encephalopathy after bariatric surgery: losing more than just weight. Neurology 65:1987. doi:10.1212/01.wnl.0000188822.86529.f0
Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA (2010) EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol 17:1408–1418. doi:10.1111/j.1468-1331.2010.03153.x
Ghosh GC, Chatterjee K, Sharma B (2014) Non Alcoholic Wernicke’s Encephalopathy with Cortical Involvement in a Patient of Active Peptic Ulcer Disease. J Clin Case Rep 4:379. doi:10.4172/2165-7920.1000379
Haid RW, Gutmann L, Crosby TW (1982) Wernicke-Korsakoff encephalopathy after gastric plication. JAMA 247:2566–2567
Harper CG, Giles M, Finlay-Jones R (1986) Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 49:341–345
Iannelli A, Addeo P, Novellas S, Gugenheim J (2010) Wernicke's encephalopathy after laparoscopic Roux-en-Y gastric bypass: a misdiagnosed complication. Obes Surg 20:1594–1596. doi:10.1007/s11695-010-0116-0
Ishibashi S et al (2003) Reversible acute axonal polyneuropathy associated with Wernicke-Korsakoff syndrome: impaired physiological nerve conduction due to thiamine deficiency? J Neurol Neurosurg Psychiatry 74:674–676
Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI (2007) Neurologic complications of gastric bypass surgery for morbid obesity. Neurology 68:1843–1850. doi:10.1212/01.wnl.0000262768.40174.33
Koffman BM, Greenfield LJ, Ali II, Pirzada NA (2006) Neurologic complications after surgery for obesity. Muscle Nerve 33:166–176. doi:10.1002/mus.20394
Koike H, Iijima M, Mori K, Hattori N, Ito H, Hirayama M, Sobue G (2004) Postgastrectomy polyneuropathy with thiamine deficiency is identical to beriberi neuropathy. Nutrition 20:961–966. doi:10.1016/j.nut.2004.08.002
Koike H et al (2008) Rapidly developing weakness mimicking Guillain-Barre syndrome in beriberi neuropathy: two case reports. Nutrition 24:776–780. doi:10.1016/j.nut.2008.02.022
Koike H et al (2001) Postgastrectomy polyneuropathy with thiamine deficiency. J Neurol Neurosurg Psychiatry 71:357–362
Koike H, Watanabe H, Inukai A, Iijima M, Mori K, Hattori N, Sobue G (2006) Myopathy in thiamine deficiency: analysis of a case. J Neurol Sci 249:175–179. doi:10.1016/j.jns.2006.06.016
Kornreich L et al (2005) Thiamine deficiency in infants: MR findings in the brain. AJNR Am J Neuroradiol 26:1668–1674
Landais AF (2014) Rare neurologic complication of bariatric surgery: acute motor axonal neuropathy (AMAN), a severe motor axonal form of the Guillain Barre syndrome. Surg Obes Relat Dis. doi:10.1016/j.soard.2014.02.019
Lindboe CF, Loberg EM (1989) Wernicke's encephalopathy in non-alcoholics. An autopsy study J Neurol Sci 90:125–129
Liu YT, Fuh JL, Lirng JF, Li AF, Ho DM, Wang SJ (2006) Correlation of magnetic resonance images with neuropathology in acute Wernicke's encephalopathy. Clin Neurol Neurosurg 108:682–687. doi:10.1016/j.clineuro.2005.05.010
Luigetti M, De Paulis S, Spinelli P, Sabatelli M, Tonali P, Colosimo C, Cianfoni A (2009) Teaching NeuroImages: the full-blown neuroimaging of Wernicke encephalopathy. Neurology 72:e115. doi:10.1212/WNL.0b013e3181a82647
Lyons DA, Linscott LL, Krueger DA (2016) Non-alcoholic Wernicke Encephalopathy. Pediatr Neurol 56:94–95. doi:10.1016/j.pediatrneurol.2015.12.007
Mascalchi M et al (1999) Do acute lesions of Wernicke's encephalopathy show contrast enhancement? Report of three cases and review of the literature. Neuroradiology 41:249–254
Mechanick JI et al (2013) Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 21(Suppl 1):S1–27. doi:10.1002/oby.20461
Nolli M, Barbieri A, Pinna C, Pasetto A, Nicosia F (2005) Wernicke's encephalopathy in a malnourished surgical patient: clinical features and magnetic resonance imaging. Acta Anaesthesiol Scand 49:1566–1570. doi:10.1111/j.1399-6576.2005.00879.x
Pereira DB, Pereira ML, Gasparetto EL (2011) Nonalcoholic Wernicke encephalopathy with extensive cortical involvement: cortical laminar necrosis and hemorrhage demonstrated with susceptibility-weighted MR phase images. AJNR Am J Neuroradiol 32:E37–E38. doi:10.3174/ajnr.A2359
Phua KH, Goh LG, Koh K, Ong CN, Tan TC, Wong ML, Lee HP (1990) Thiamine deficiency and sudden deaths: lessons from the past. Lancet 335:1471–1472
Rufa A et al (2011) Wernicke encephalopathy after gastrointestinal surgery for cancer: causes of diagnostic failure or delay. Int J Neurosci 121:201–208. doi:10.3109/00207454.2010.544430
Sakurai K, Sasaki S, Hara M, Yamawaki T, Shibamoto Y (2009) Wernicke's encephalopathy with cortical abnormalities: clinicoradiological features: report of 3 new cases and review of the literature. Eur Neurol 62:274–280. doi:10.1159/000235596
Santos Andrade C, Tavares Lucato L, da Graca Morais Martin M, Joaquina Marques-Dias M, Antonio Pezzi Portela L, Scarabotolo Gattas G, da Costa LC (2010) Non-alcoholic Wernicke's encephalopathy: broadening the clinicoradiological spectrum. Br J Radiol 83:437–446. doi:10.1259/bjr/27226205
Sauberlich HE, Herman YF, Stevens CO, Herman RH (1979) Thiamin requirement of the adult human. Am J Clin Nutr 32:2237–2248
Sechi G, Sechi E, Fois C, Kumar N (2016) Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr Rev 74:281–300. doi:10.1093/nutrit/nuv107
Sechi G, Serra A (2007) Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 6:442–455. doi:10.1016/s1474-4422(07)70104-7
Singh S, Kumar A (2007) Wernicke encephalopathy after obesity surgery: a systematic review. Neurology 68:807–811. doi:10.1212/01.wnl.0000256812.29648.86
Sohoni C (2014) Usefulness of Magnetic Resonance Imaging in the Diagnosis of Non-Alcoholic Wernicke's Encephalopathy. Ann Med Health Sci Res 4:S163–S164. doi:10.4103/2141-9248.138049
Takahashi K, Nakamura H (1976) Axonal degeneration in beriberi neuropathy. Arch Neurol 33:836–841
Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ (2004) A controlled study of peripheral neuropathy after bariatric surgery. Neurology 63:1462–1470
Watson JT et al (2011) Outbreak of beriberi among African Union troops in Mogadishu, Somalia. PLoS One 6:e28345. doi:10.1371/journal.pone.0028345
Wicklund MR, Knopman DS (2013) Brain MRI findings in Wernicke encephalopathy. Neurol Clin Pract 3:363–364. doi:10.1212/CPJ.0b013e3182a1ba00
Wilkinson TJ, Hanger HC, George PM, Sainsbury R (2000) Is thiamine deficiency in elderly people related to age or co-morbidity? Age Ageing 29:111–116
Wu L, Jin D, Sun X, Liang L, Huang D, Dong Z, Yu S (2016) Cortical damage in Wernicke's encephalopathy with good prognosis: a report of two cases and literature review. Metab Brain Dis. doi:10.1007/s11011-016-9920-0
Yamashita M, Yamamoto T (1995) Wernicke encephalopathy with symmetric pericentral involvement: MR findings. J Comput Assist Tomogr 19:306–308
Yoon JH, Yong SW, Yong SW, Lee PH (2009) Dystonic hand tremor in a patient with Wernicke encephalopathy. Parkinsonism Relat Disord 15:479–481. doi:10.1016/j.parkreldis.2008.10.007
Zhong C, Jin L, Fei G (2005) MR Imaging of nonalcoholic Wernicke encephalopathy: a follow-up study. AJNR Am J Neuroradiol 26:2301–2305
Zuccoli G, Cravo I, Bailey A, Venturi A, Nardone R (2011) Basal Ganglia involvement in Wernicke encephalopathy: report of 2 cases. AJNR Am J Neuroradiol 32:E129–E131. doi:10.3174/ajnr.A2185
Zuccoli G, Pipitone N (2009) Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol 192:501–508. doi:10.2214/ajr.07.3959
Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, Pipitone N (2009) MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol 30:171–176. doi:10.3174/ajnr.A1280
Acknowledgements
This study is partially supported by a grant from Chang Gung Memorial Hospital (CMRPG3F0511).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure Statement
None declared.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s11011-017-0124-z.
Rights and permissions
About this article
Cite this article
Tsao, WC., Ro, LS., Chen, CM. et al. Non-alcoholic Wernicke’s encephalopathy with cortical involvement and polyneuropathy following gastrectomy. Metab Brain Dis 32, 1649–1657 (2017). https://doi.org/10.1007/s11011-017-0055-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0055-8