Abstracts
The impaired insulin signaling has been recognized as a common pathogenetic mechanism between diabetes and Alzheimer’s disease (AD). In the progression of AD, brain is characterized by defective insulin receptor substrate-1 (IRS-1) and increased oxidative stress. Thymol, a monoterpene phenol isolated from medicinal herbs, has exhibited robust neuroprotective effects. The present study was designed to investigate the protective effect of thymol on HFD-induced cognitive deficits, and explore the possible mechanisms. C57BL/6 J mice were fed for 12 weeks with either HFD or normal diet. The mice fed with HFD were dosed with metformin (200 mg/kg) or thymol (20, 40 mg/kg) daily. It was observed that thymol treatment significantly reversed the gain of body weight and peripheral insulin resistance induced by HFD. Meanwhile, thymol improved the cognitive impairments in the Morris Water Maze (MWM) test and decreased HFD-induced Aβ deposition and tau hyperphosphorylation in the hippocampus, which may be correlated with the inhibition of hippocampal oxidative stress and inflammation. In addition, thymol down-regulated the level of P-Ser307 IRS-1, and hence enhancing the expression of P-Ser473 AKT and P-Ser9 GSK3β. We further found that the protective effects of thymol on cognitive impairments were associated with the up-regulation of nuclear respiratory factor (Nrf2)/heme oxygenase-1(HO-1) pathway. In conclusion, thymol exhibited beneficial effects on HFD-induced cognitive deficits through improving hippocampal insulin resistance, and activating Nrf2/HO-1 signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s diseases (AD) and Type 2 diabetes (T2DM) are increasingly prevalent in twenty-first century, which bring a huge burden to the health care system. Progressive deterioration of cognition and independent living are the characteristics of AD, which is the common form of dementia (Chen et al. 2012; Sosa-Ortiz et al. 2012). Although molecular mechanisms of amyloid-β (Aβ) deposition and tau hyperphosphorylation have been studied extensively, the causes and effective treatments of Alzheimer’s disease are yet to be known. Characterized by insulin resistance, chronic inflammation and metabolic dysfunction, T2DM has shown the same characteristics with dementia. (Mittal and Katare 2016). Hence, in the past decades, many studies have concentrated on the correlation between T2DM and AD. It has been reported that a high-fat diet (HFD) consumption in animal can cause neuronal insulin resistance and subsequently diminished cognitive functions (Pipatpiboon et al. 2012; Tucsek et al. 2014). By improving insulin signaling pathway can ameliorate tau hyperphosphorylation, reverse hippocampal synaptic plasticity impairments and prevent neuroplasticity deficits elicited through intrahippocampal administration of Aβ (Biessels and Reagan 2015). Consequently, the brain insulin signaling is an important mechanism of T2DM and AD.
Insulin, secreted by the pancreas and transported across the blood-brain barrier via a saturable transport system (Banks 2004), plays an important role in human brain function such as the control of whole-body metabolisms (Heni et al. 2015). Insulin exerts its biological effects by binding to transmembrane insulin receptors (IRs) in target tissues. Yet IRs are wildly expressed in CNSs, including olfactory bulb, hypothalamus, cerebral cortex, thalamus and hippocampus (Grillo et al. 2015). Insulin binding to IRs induces receptor autophosphorylation, then recruits and phosphorylates insulin receptor substrates (IRS) as well as activates downstream cascades such as phosphatidylinositol 3-kinase(PI3K), protein kinase B(AKT) and glycogen synthase kinase 3β(GSK3β) (Kido et al. 2000; Steculorum et al. 2014; Hao et al. 2014). Furthermore, PI3K/AKT pathway affected dendritic spine and synapse formation and synaptic plasticity (Yin et al. 2015). GSK3β activity regulated amyloid-β (Aβ) deposition and tau protein phosphorylation (Phiel et al. 2003; Chen et al. 2016), which were major pathological markers of AD.
Insulin resistance (IR) refers to cells failing to respond to the normal actions of the hormone insulin in insulin-sensitive tissues such as the adipose, liver, skeletal muscle (Kang et al. 2016) and brain (Heni et al. 2015), which is one of the major risk factors driving the development of AD. However, the underlying mechanisms of this process have not been fully elucidated. Studies suggested that reactive oxygen species (ROS) or oxidative stress may interfere with insulin signaling (Moreira et al. 2007), contributing to insulin resistance by directly phosphorylating IRS-1 and activating inflammatory signaling (Wang et al. 2012). Nrf2 is a crucial mediator maintaining homeostasis of redox status in endogenous antioxidant systems. Once activated, Nrf2 translocated from cytoplasm to the nucleus and bond to antioxidant-responsive element (ARE), and then, initiated the transcription of anti-oxidant enzyme gene, including glutamate-cysteine ligase, NADPH quinone oxidoreductase 1, superoxide dismutase (SOD) and HO-1 (Fujita et al. 2012), which was able to improve insulin sensitivity and endoplasmic reticulum (ER) stress (Nicolai et al. 2009; Cai et al. 2016). Recently, a study showed that deletion of Nrf2 enhanced autophagic dysfunction in AD mice (Joshi et al. 2015). Likewise, Nrf2 reduced levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52 (Jo et al. 2014). The activation of Nrf2/HO-1 pathway attenuated Aβ-induced neurotoxicity in cell lines (Li et al. 2016; Kwon et al. 2015; Wang et al. 2016) and animal models (Zou et al. 2013). Previous studies also demonstrated that the consumption of a HFD increased hippocampal oxidative stress and damaged cognitive functions, which were correlated with decreased Nrf2 signaling (Morrison et al. 2010). Above all, these suggested that Nrf2/HO-1 pathway may be correlated with brain insulin resistance and cognitive dysfunctions.
Thymol (2-isopropyl-5-methylphenol), a monoterpene phenol, is isolated from oils of thyme and medicinal herbs, such as Thymus vulgaris, Thymbra spicata, Thymus ciliates, Trachyspermum ammi, Monarda fistulosa and Nigella sativa seeds (Saravanan and Pari 2016). It has been reported that thymol exhibited a variety of biological activities, including anti-diabetic (Saravanan and Pari 2015), anti-bacterial (Miladi et al. 2016), anti-inflammatory (Liang et al. 2014), and antioxidant effects (Saei-Dehkordi et al. 2012). Furthermore, our previous study revealed that thymol displayed antidepressant-like effects in mice model of depression (Deng et al. 2015). Reports showed that thymol enhanced cognitive activities on model of dementia (Azizi et al. 2012), but the exact mechanism has not been fully studied. Moreover, thymol treatment has been reported to increase antioxidant status in rat brain (Youdim and Deans 2000) and display neuroprotective effects involving with its potential action on GABA-mediated inhibition of synaptic transmission. (Marín et al. 2011). Considering the inspiring beneficial effects of thymol on the brain, the purpose of this study was to investigate the protective effects and the possible mechanisms of thymol on cognitive dysfunction in mice induced by HFD.
Materials and methods
Reagents
Thymol (purity > 98 %) was obtained from Xian Kailai Biotechnology Co., Ltd., (Shanxi, P.R. China). Metformin (Zhonglian Pharmaceutical Co., Ltd., Shenzhen, P.R. China). Glucose test kit (Nanjing Jiancheng biological technology Co., Ltd., Nanjing, P.R. China). Insulin, IL-1β and TNF-α ELISA kits (Dizhao biological technology Co., Ltd., Nanjing, P.R. China). BCA Protein Assay Kit (Beyotime Institute of Biotechnology Co., Ltd., Shanghai,China). SOD and MDA assay kits (Nanjing Jiancheng biological technology Co.,Ltd., Nanjing,P.R. China). Rabbit anti-Nrf2, anti-HO-1, anti-P-Tau (Ser396) and anti-Tau were purchased from Abcam (Cambridge, MA, USA). Rabbit anti-P-IRS-1 (Ser307), anti-IRS-1, anti-AKT, anti-P-AKT (Ser473), anti-GSK3β, anti-P-GSK3β (Ser9) were obtained from Cell Signaling Technology (Beverly, MA, USA), while β-actin antibody from Bioworld technology, Inc. (USA).
Animal
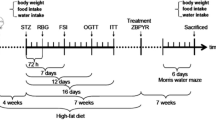
Male C57BL/6 J mice aged six weeks were purchased from Animal Breeder of Mount Qing long in Nanjing, China. All animals were group housed under standard housing conditions (temperature: 25 ± 2 °C; with a 12/12 h light/dark cycle). After one week of adaptive feeding, the mice were randomly divided into five groups (n = 12): normal group, HFD group, HFD + thymol(20, 40 mg/kg) groups, HFD + metformin(200 mg/kg) group and fed for 12 weeks with either High fat diet(HFD) or normal diet. Mice were administrated by gavage daily. Thymol and metformin were dissolved in 0.3 % carboxymethyl cellulose sodium. Mice were weighed once weekly and were sacrificed after 12 weeks. All experimental procedures were performed according to the provision and General Recommendation of Chinese Experimental Animals Administration Legislation and were approved by the science and Technology Department of Jiangsu Province.
Measurement of insulin resistance
Blood samples were collected from canthus after food deprivation for 12 h, and then the samples were centrifuged at 3000r for 15 min to obtain serum. Subsequently, the level of blood glucose and insulin were measured using a glucose detection kit and insulin ELISA kits respectively. Then, the Homeostasis Model Assessment (HOMA) was calculated by the follow formula (HOMA = fasting insulin (mIU/L / fasting glucose (mmol / L) / 22.5).
Morris water maze test
Morris water maze test were used to assess spatial learning and memory of mice. As described in reference (Chen et al. 2016), diameter of 100 cm water maze was divided into four quadrants. A platform (diameter 10 cm) was placed in a quadrant and hidden 1 cm below the surface of the water. During the training test, each group of mice were put into the maze, and the escape latency was recorded. If mice found the platform within 90 s, they were allowed to stay on the platform for 15 s, if failed, they were guided to the platform and stayed for 15 s. All mice were trained twice a day for 4 days. On the fifth day, the platform was removed. The time spent in the target quadrant was recorded.
Congo red staining
As described in reference (Liu et al. 2013), the brain sections were stained with hematoxylin for 5–10 min. The coloration of the sections was developed by Congo red in methanol for 3 min. Finally, the sections were cleared in xylene before mounting with neutral gum under a cover slip and taken a photograph under light microscopy by Leica Application suite V3.1.0 software.
Measurement of superoxide dismutase (SOD), malondialdehyde (MDA) in brain hippocampus
Mice hippocampus was separated out and homogenized in cold saline (w:v 1:9). The supernatants were collected by centrifuging at 3000 r/min for 10 min. The supernatant protein concentration was detected using BCA kit. Superoxide dismutase (SOD), malondialdehyde (MDA) were detected by SOD or MDA assay kits.
Measurement of IL-1β and TNF-α production
The levels of IL-1β and TNF-α in hippocampus were determined by ELISA assays. All the protocol were according to the manufacturer’s instruction. The data were shown as picogram per milliliter (pg/ml).
Western blot analysis
Mice hippocampus were homogenized in lysis buffer. The supernatants were collected by centrifuging at 12,000 r/min for 30 min. Protein concentrations were quantified by a BCA Kit. Equal amounts of proteins were separated on 10 % SDS-PAGE and transferred to PVDF membrane. Subsequently, the membrane was placed in 5 % skim milk for 2 h at room temperature, and incubated overnight with primary antibodies. The next day the membrane was washed three times, 10 min each. Then at room temperature with HRP-conjugated secondary antibodies were incubated for 2 h. The reactive bands were visualized using chemiluminescence (ECL) kits (Millipore, Billerica, MA, USA) and Molecular Imager Gel Doc XR System (Bio Rad). Quantifications were employed Image pro plus (IPP).
Statistics
The GraphPad Prism 5.0 software was used for data analyzed. All data were presented as the mean ± S.E.M. Differences between the two groups was analyzed by a one-way analysis of variance (ANOVA) with Tukey’s test. Statistical significance was considered as P-values <0.05.
Results
Effects of thymol on body weight and peripheral insulin resistance of HFD-fed mice
To examine the effect of thymol on body weight and insulin resistance induced by HFD, the body weight of the mice were recorded once a week, the plasma glucose (data no shown) and insulin were evaluated and then the HOMA was calculated as discussed above. At the beginning of the experiment, there was no difference in body weight of mice in each group. Figure 1a. After 4 weeks, we found that thymol and metformin treatment could significantly reduce the weight of mice and in the continuing 12 weeks. As shown in Fig. 1b c), the plasma insulin levels were remarkably elevated in model group. Thymol and metformin treatment could significantly decrease the insulin levels versus the model group. In addition, the HOMA index represents the degree of insulin resistance. Model group, compared with the normal group, had a significantly increase in HOMA index. Thymol or metformin treatment showed lower HOMA index. Thus, these results indicated that thymol or metformin treatment could efficiently improve the peripheral insulin resistance induced by HFD.
Thymol decreased the gain of body weight and insulin resistance in HFD-fed mice. High fat diet fed C57BL/6 J mice were dosed with 20, 40 mg/kg thymol or 200 mg/kg metformin for 12 weeks. (a) Body weight were recorded weekly (n = 10). (b) Plasma insulin concentrations were detected with commercial kits. (c) The HOMA index of all the groups (n≧5). Data were shown as means ± SEM, *p < 0.05 vs. Model. #p < 0.05 vs. Normal
Effects of thymol on HFD-induced learning and memory deficits
To explore cognitive function in mice fed with HFD, we used Morris water maze test. As shown in Fig. 2a b. After 12 weeks of HFD, the escape latency of the model group was remarkably increased during the training time versus that in the normal group. Time spent in the target zone was significantly decreased in the model group versus that in the normal group. Chronic administration of thymol and metformin ameliorated the alterations involved in escape latency and time spent in the target zone for the HFD-mice. Above all, these results indicated that thymol and metformin could improve the learning and memory performance and prevent the cognitive deficits during HFD feeding.
Data related to learning and memory performance were recorded by a video-tracking program. (a) Escape latency to find the hidden platform in the test during the four consecutive days training. (b) The time of the mice spent in the quadrant where the platform was once placed within 90 s (n = 8). Data were shown as means ± SEM, *p < 0.05 vs. Model. #p < 0.05 vs. Normal
Congo red staining
To explore the effect of thymol on HFD-induced Aβ expression, the congo red staining was carried out in hippocampus CA1 region. The data revealed that the Aβ deposition in the hippocampus was aggravated in model group compared with the normal group Fig. 3a, b. Remarkably, The Aβ deposition level was significantly reversed by the treatment with thymol and metformin. The results indicated that thymol and metformin could ameliorate the memory impairment due to Aβ deposition in HFD fed in mice.
Effects of thymol on the expression of P-tau
The phosphorylation of tau protein is mediated by proline-directed protein kinases (PDKs) and the abnormal tau phosphorylation at the Ser396 residue contributes to the reduction of microtubule binding affinity (Bramblett et al. 1993). After fed HFD for 12 weeks, P-Tau was significantly increased in mice hippocampus for the model group. Figure 3c, d; Treatments with thymol and metformin significantly reduced the levels of p-tau, indicating the neuroprotective effects of thymol on AD.
Effects of thymol on the oxidative stress and inflammation in the hippocampus of HFD-fed mice
The neuroinflammation and oxidative stress are closely related to cognitive impairment. So the effects of thymol on the levels of oxidative stress and inflammatory cytokines (IL-1β, TNF-a) in brain hippocampus were measured using assay and ELISA kits. As shown in Fig. 4a b. The SOD activities were significantly decreased in model group. Conversely, the MDA levels were dramatically increased. The treatment groups could increase SOD activities and reduce MDA levels in hippocampus. Elisa kit results showed that, compared with the normal group, the IL-1β and TNF-α levels were significantly elevated in model group. Figure 4c d HFD-induced inflammatory cytokines levels were significantly decreased after thymol and metformin treatments.
Effects of thymol on insulin signaling pathway
To examine whether thymol will affect HFD-triggered insulin resistance in the brain, we examined the expression of insulin signaling pathway related proteins. As described in Fig. 5, the levels of P-IRS Ser307 were significantly increased by HFD. Additionally, Ser473 of phosphorylation at protein kinase B (AKT) and glycogen synthase kinase 3β (GSK3β) Ser9 were obviously reduced by HFD. However, the thymol and metformin treatment significantly inhibited the increase levels of P-IRS Ser307 and the decreased expressions of P-AKT Ser473 and P-GSK3β Ser9. These result indicated that thymol and metformin treatment could significantly restore insulin signaling impairment.
Effects of thymol on brain insulin signaling, the levels of P-IRS-1, P-AKT and P-GSK in hippocampus were determined by western blot. (a) The relative levels of IRS-1, P-IRS-1, P-AKT, and P-GSK-3β were detected by western blotting. (b)(c)(d) The quantitative analysis of IRS-1, P-IRS-1 P-AKT, and P-GSK-3β respectively. Data were shown as means ± SEM (n = 3), *p < 0.05 vs. Model. #p < 0.05 vs. Normal
Effects of thymol on Nrf2/HO-1signaling pathway
To further evaluate the underlying mechanisms of thymol on cognitive deficits, we explored the expression of HO-1 and Nrf2 in hippocampus. As shown in Fig. 6. HFD significantly decreased the levels of HO-1 protein in the model group compared with the normal group. Either thymol or metformin treatment was able to restore the HFD-induced decrease in levels of HO-1. As a redox sensor mediating the antioxidant response, Nrf2 was significantly decreased after HFD in mice. Oral administrated thymol was able to greatly enhance the expression of Nrf2 protein levels. These results indicated that thymol treatment effectively activated Nrf2/HO-1 signaling pathway.
Discussion
The present study was designed to evaluate the effects of thymol on HFD-induced cognitive deficits, and explore the possible mechanisms underlying the therapeutic effects. Our results showed that long-term HFD consumption induced cognitive deficits, Aβ deposition, tau protein phosphorylation, oxidative stress and inflammation in the hippocampus. These alterations were significantly reversed by thymol treatments. Thymol pretreatment also improved brain insulin signaling pathway and increased expression of Nrf2 and HO-1 in the hippocampus. Thus, our present study, for the first time, investigated the protective effects of thymol on the cognitive deficits induced by HFD via improving brain insulin signaling and activating Nrf2/HO-1 signaling.
It is recognized that impairments of insulin signaling increases the risk of AD and T2DM. HFD in mice not only causes peripheral insulin resistance, but also impairs neuronal insulin signaling, thereby damaging cognitive functions (Greenwood and Winocur 2005; Arnold et al. 2014). Previous studies indicated that HFD induced hyperphosphorylation of Ser307 IRS-1 and inhibited downstream AKT/GSK3β cascade. As a result, GSK3β activation aggravated tau phosphorylation and Aβ deposition in AD. In turn, abnormal tau phosphorylation and Aβ deposition triggered brain insulin resistance, oxidative stress, mitochondrial dysfunction and ES stress leading to neuronal death (Najem et al. 2016; Costa et al. 2013; Chiang et al. 2016; Lesné et al. 2006; Hoozemans et al. 2012), which have been recognized as an important mechanism for the initiation and progression of cognitive deficits and AD. Consistent with previous studies, we demonstrated that long-term consumption of a HFD significantly eliminated learning and memory ability and induced Aβ deposition and tau hyperphosphorylation. These results further demonstrated the important role of insulin resistance in the development of AD. However, administration of thymol significantly improved cognitive impairments, Aβ deposition and tau phosphorylation induced by HFD. These indicated the important role of thymol against brain insulin resistance in AD.
A robust redox homeostasis is required for insulin to exert its physiological actions. Conversely, oxidative stress or ROS overproduction damage insulin signaling and cognitive ability (D’Apolito et al. 2010; Matsuzawa-Nagata et al. 2008). In the present study, chronic consumption of a HFD largely increased the level of MDA and decreased the level of SOD, which were reversed by thymol treatment. Increasing evidence has shown that SOD is a reflection of cellular free radicals scavenging, while MDA refers to the condition of lipid peroxidation, which are two primary pathophysiological factors in evaluating free radical metabolisms (Zheng et al. 2008). Moreover, inflammatory cytokines can be repressed by thymol. Elevated brain proinflammatory cytokines under insulin resistance and oxidative stress will maintain a proinflammatory environment until further activate proinflammatory pathways (NF-ƙB and JNKs) and impair insulin signaling pathways (Verdile et al. 2015). Furthermore, thymol could decrease P-IRS Ser307 expression, subsequently enhance AKT/GSK signaling. These results further support the protective effect of thymol on insulin signaling via inhibiting oxidative stress and inflammation.
Nrf2, as a key molecule of the endogenous defense system against oxidative stress, regulates a battery of antioxidant enzymes. The antioxidant stress protein HO-1 is widely recognized as cytoprotective molecule against oxidative insults (Lim et al. 2007). On one hand, with the activation of Nrf2/HO-1 pathway, oxidative stress is inhibited and subsequently insulin signaling dysfunction, tau phosphorylation and Aβ deposition were ameliorated in the hippocampus. On the other hand, Nrf2 is negatively regulated by the activity of GSK-3β which can be inhibited via GSK-3β phosphorylation at Ser 9 caused by AKT (Hong and An 2015). Therefore, improving the insulin signaling and increasing the phosphorylation of AKT lead to GSK-3β (Ser 9) phosphorylation and Nrf2 activation. In the present study, Nrf2 level was significantly decreased in the hippocampus of model group, reflecting an attenuated antioxidant and homeostatic capacity. Administration of thymol increased the HO-1 expression and Nrf2 translocation, which suggested that its neuroprotective effects were possible through activating the Nrf2/HO-1 pathway. Moreover, activated Nrf2 signaling could inhibit declines in cognitive performance contributing to the improvement of brain insulin resistance (IR), as well as through more direct mechanisms.
Additionally, metformin, an anti-diabetic agent, has been shown to reverse the cognitive deficits induced by HFD via attenuating peripheral insulin resistance, decreasing plasma and brain oxidative stress, and restoring brain mitochondrial function (Pintana et al. 2012). But there are also reports that long-term administration of metformin may increase the risk of cognitive impairments in diabetic patients (Moore et al. 2013). In our study, administration of metformin significantly improved cognitive deficits, insulin resistance, oxidative stress and neuroinflammation. However, compared with the model group, metformin treatment was no significant difference in the expression of Nrf2. Additionally, Allard’s research indicated that prolonged metformin treatment (six month) leads to reduced Nrf2 expression without cognitive impairment in older C57BL/6 J mice (Allard et al. 2016). It is an interesting result that needs further study.
Hence, our results suggested that administration of thymol and metformin improved brain insulin resistance, as well as significantly reduced Aβ deposition and tau phosphorylation in hippocampus. Furthermore, treatment of thymol attenuated oxidative stress and inflammation and markedly increased the expression of Nrf2 and HO-1.
Conclusion
In conclusion, thymol possesses the ability of improving cognitive dysfunction caused by HFD and the mechanisms are partly through ameliorating brain insulin resistance and enhancing Nrf2/HO-1 signaling. But the specific mechanisms needs to be further explored. This suggests an important role of thymol in response to insulin resistance, and provides an insight into the potential therapeutic implications of thymol for AD and T2DM.
References
Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R (2016) Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6 J mice. Behav Brain Res 301:1–9
Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF (2014) High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67:79–87
Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N (2012) Cognitive- enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol 23(3):241–249
Banks WA (2004) The source of cerebral insulin. Eur J Pharmacol 490:5–12
Biessels GJ, Reagan LP (2015) Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16:660–671
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099
Cai M, Wang H, Li JJ, Zhang YL, Xin L, Li F, Lou SJ (2016) The signaling mechanisms of hippocampal endoplasmic reticulum stress affecting neuronal plasticity-related protein levels in high fat diet-induced obese rats and the regulation of aerobic exercise. Brain Behav Immun 57:347–359
Chen L, Magliano DJ, Zimmet PZ (2012) The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol 8:228–236
Chen J, Deng X, Liu N, Li M, Liu B, Fu Q, Ma S (2016) Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J Funct Foods 22:463–476
Chiang MC, Nicol CJ, Cheng YC, Lin KH, Yen CH, Lin CH (2016) Rosiglitazone activation of PPARγ-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta–induced mitochondrial dysfunction and oxidative stress. Neurobiol Aging 40:181–190
Costa RO, Ferreiro E, Oliveira CR, Pereira CM (2013) Inhibition of mitochondrial cytochrome c oxidase potentiates Aβ-induced ER stress and cell death in cortical neurons. Mol Cell Neurosci 52:1–8
D’Apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Brownlee M (2010) Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest 120:203–213
Deng XY, Li HY, Chen JJ, Li RP, Qu R, Fu Q, Ma SP (2015) Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res 291:12–19
Fujita K, Yamafuji M, Nakabeppu Y, Noda M (2012) Therapeutic approach to neurodegenerative diseases by medical gases: focusing on redox signaling and related antioxidant enzymes Oxid Med Cell Longev 2012
Greenwood CE, Winocur G (2005) High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 26:42–45
Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP (2015) Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 64:3927–3936
Hao J, Chen C, Huang K, Huang J, Li J, Liu P, Huang H (2014) Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur J Pharmacol 745:152–165
Heni M, Kullmann S, Preissl H, Fritsche A, Häring HU (2015) Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol 11:701–711
Hong Y, An Z (2015) Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch Pharm Res:1–9. doi:10.1007/s12272-015-0662-z
Hoozemans JJ, Van Haastert ES, Nijholt DA, Rozemuller AJ, Scheper W (2012) Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis 10:212–215
Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV (2014) Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun 5. doi:10.1038/ncomms4496
Joshi G, Gan KA, Johnson DA, Johnson JA (2015) Increased Alzheimer’s disease-like pathology in the APP/PS1ΔE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol Aging 36:664–679
Kang S, Tsai LT, Rosen ED (2016) Nuclear mechanisms of insulin resistance. Trends Cell Biol 26:341–351
Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D (2000) Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest 105:199–205
Kwon SH, Ma SX, Hwang JY, Lee SY, Jang CG (2015) Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta 25-35 neurotoxicity. Neuroscience 304:14–28
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440:352–357
Li HH, Lin SL, Huang CN, Lu FJ, Chiu PY, Huang WN, Lai TJ, Lin CL (2016) MIR-302 attenuates amyloid-β-induced neurotoxicity through activation of Akt signaling. J Alzheimers Dis 50:1083–1098
Liang D, Li F, Fu Y, Cao Y, Song X, Wang T, Wang W, Guo M, Zhou E, Li D, Yang Z, Zhang N (2014) Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37:214–222
Lim HJ, Lee KS, Lee S, Park JH, Choi HE, Go SH, Kwak HJ, Park HY (2007) 15d-PGJ 2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol Appl Pharmacol 223:20–27
Liu J, Feng L, Ma D, Zhang M, Gu J, Wang S, Fu Q, Song Y, Lan Z, Qu R, Ma S (2013) Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neurosci Lett 549:63–68
Marín LD, Sánchez-Borzone M, García DA (2011) Comparative antioxidant properties of some GABAergic phenols and related compounds, determined for homogeneous and membrane systems. Med Chem 7:317–324
Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S (2008) Increased oxidative stress precedes the onset of high-fat diet–induced insulin resistance and obesity. Metabolism 57:1071–1077
Miladi H, Zmantar T, Chaabouni Y, Fedhila K, Bakhrouf A, Mahdouani K, Chaieb K (2016) Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb Pathog 99:95–100
Mittal K, Katare DP (2016) Shared links between type 2 diabetes mellitus and Alzheimer’s disease: a review. Diabetol Metab Syndr 10:144–149
Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, Investigators AIBL (2013) Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36:2981–2987
Moreira PI, Santos MS, Seiça R, Oliveira CR (2007) Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci 257:206–214
Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN (2010) High fat diet increases hippocampal oxidative stressand cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114:1581–1589
Najem D, Bamji-Mirza M, Yang Z, Zhang W (2016) Aβ-induced insulin resistance and the effects of insulin on the cholesterol synthesis pathway and Aβ secretion in neural cells. Neurosci Bull 32:227–238
Nicolai, A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L’Abbate A, Kappas A, Abraham NG (2009) Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53: 508–515
Phiel CJ, Wilson CA, Lee VM, Klein PS (2003) GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 423:435–439
Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci 91:409–414
Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338
Saei-Dehkordi SS, Fallah AA, Saei-Dehkordi SS, Kousha S (2012) Chemical composition and antioxidative activity of Echinophora platyloba DC. Essential oil, and its interaction with natural antimicrobials against food-borne pathogens and spoilage organisms. J Food Sci 77:631–637
Saravanan S, Pari L (2015) Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6 J mice. Eur J Pharmacol 761:279–287
Saravanan S, Pari L (2016) Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6 J mice. Chem Biol Interact 245:1–11
Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ (2012) Epidemiology of dementias and Alzheimer’s disease. Arch Med Res 43:600–608
Steculorum SM, Solas M, Brüning JC (2014) The paradox of neuronal insulin action and resistance in the development of aging-associated diseases. Alzheimers Dement 10:3–11
Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A (2014) Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 69:1212–1226
Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat Inflamm 2015:17
Wang X, Gu C, He W, Ye X, Chen H, Zhang X, Hai C (2012) Glucose oxidase induces insulin resistance via influencing multiple targets in vitro and in vivo: the central role of oxidative stress. Biochimie 94:1705–1717
Wang Y, Miao Y, Mir AZ, Cheng L, Wang L, Zhao L, Cui Q, Zhao W, Wang H (2016) Inhibition of beta-amyloid-induced neurotoxicity by pinocembrin through Nrf2/HO-1 pathway in SH-SY5Y cells. J Neurol Sci 368:223–230
Yin Z, Yu H, Chen S, Ma C, Ma X, Xu L, Ma Z, Qu R, Ma S (2015) Asiaticoside attenuates diabetes-induced cognition deficits by regulating PI3K/Akt/NF-κB pathway. Behav Brain Res 292:288–299
Youdim KA, Deans SG (2000) Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr 83:87–93
Zheng W, Huang LZ, Zhao L, Wang B, Xu HB, Wang GY, Wang ZL, Zhou H (2008) Superoxide dismutase activity and malondialdehyde level in plasma and morphological evaluation of acute severe hemorrhagic shock in rats. Am J Emerg Med 26:54–58
Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q, Zhang X, Dong M (2013) Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res 47:55–63
Acknowledgments
This study is supported by the Fundamental Research Funds for the Central Universities (Program No. JKZD2013009) and the project in our department is funded by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
FangFang, Li, H., Qin, T. et al. Thymol improves high-fat diet-induced cognitive deficits in mice via ameliorating brain insulin resistance and upregulating NRF2/HO-1 pathway. Metab Brain Dis 32, 385–393 (2017). https://doi.org/10.1007/s11011-016-9921-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9921-z