Abstract
Currently available anxiolytics cause numerous adverse effects and show craving and tolerance during long term treatment. Currently traditional medicines have been re-evaluated widely through work on various plant species. Numerous plants in traditional system show pharmacological activity with unlimited prospective for therapeutic use. Hence we planned to evaluate the effect of methanol extract of T. foenum-graecum L. seeds on anxiety, sedation and motor coordination in mice at different doses following 15 days of oral feeding. Effect on anxiety was assessed by Hole board test and Light and Dark transition models.
Phenobarbitone induced sleeping time and Rota rod test were performed to assess effect on sedation and motor coordination. In Hole board test, T. foenum-graecum L. seeds decreased the number of head dips in mice at all the three doses. In Light and Dark transition model, T. foenum-graecum L. seeds increased the period spent in the light box and the number of moves among the two compartments at 100 and 200 mg/kg as compared to control animals. In phenobarbitone induced sleeping time, T. foenum-graecum L. seeds did not reveal any sedative effect. In Rota rod test, extract exhibited significant skeletal muscle relaxant effect at 200 mg/kg (at 90 min) as compared to the control animals. Results of our study shows significant antianxiety effects of T. foenum-graecum L. seeds and may also recommend improved adverse effect profile as compared to diazepam.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anxiety is a condition of deep nervousness, doubt and fear subsequent to the expectancy of a hostile episode or state, up to a level that disrupts usual physical and emotional functions (AHMD 2007). Anxiety is one of important area of research in neuropharmacology, since almost one eighth of the world population during this decade is affected by it (Cha et al. 2005). Anxiolytic medicines generally belong to the benzodiazepine class, occupying a conspicuous position in the status of the maximum used drugs by man (Uhlenhuth et al. 1999). These drugs act via benzodiazepine receptors at Gamma amino butyric acid (GABA) pentameric complex (Prut and Belzung 2003). Currently used anxiolytic medicines cause several adverse effects and show dependency and tolerance during long term treatment (Manavi et al. 2013). Numerous plants in traditional system shows pharmacological effects with unlimited prospective for therapeutic use in the management of anxiety disorders (Carlini 2003; Faustino et al. 2010). Natural products have fundamental role in drug discovery (Harvey 2008; Hong et al. 2009; Gyawali 2010) and aided significantly towards the development of present day therapeutics. Hence there is a need to explore drugs which have better efficacy, reduced unwanted effects with least or no tolerance and addiction (Pal et al. 2010).
T. foenum-graecum L. (Fenugreek) is a regular crop that belongs to the family Fabaceae, plant was first defined about 1500 BC in Egyptian literature for several curative and nutritive uses. It is innate to Western Asia enclosing Europe, Mediterranean region, and rest of Asia (Petropoulos 2002).
Fenugreek seeds contain a wide variety of compounds with biological activity e.g. saponins (4.63 %), alkaloids (trigonelline, gentianine, carpaine), amino acids and flavonoids (Wani and Kumar 2016). Seeds are also abundant in protein, starch, natural fiber, gum, lipids and ash, while also possess ample β-carotene and vitamins (thiamine, choline). Fenugreek seeds have more proportions of minerals including Ca, P, Mg, Fe, Zn and Mn (Kan et al. 2005; Al-Jasass and Al-Jasser 2012).
Various studies have reported hypoglycemic effect of T. foenum-graecum in seeds in animals (Vats et al. 2002; Kumar et al. 2012) and humans (Madar et al. 1988; Sharma et al. 1990). Bin-Hafeez et al. (2003) reported effects of T. foenum-graecum on immune system in mice, while other reported effects in literature are hepatoprotective (Das 2014; Zargar 2014), antiallergic (Bae et al. 2012) and anticancer (Khoja et al. 2011), however no study was carried out on methanol extract of T. foenum-graecum L. seeds (METFGS) to assess its effects on anxiety, sedation and motor coordination. Hence this study was carried out precisely to evaluate the biological activity of METFGS on anxiety, sedation and motor coordination in mice considering the presence of phytochemicals (flavonoids, saponins and alkaloids) in T. foenum-graecum L. which were reported to have sedative and anxiolytic activity (Li et al. 2011; Martinez-Vázquez et al. 2012; Ibibia and Kuponiyi 2013).
Materials and methods
Preparation of extract
The T. foenum-graecum L seeds (5 kg) were purchased in August 2015 from local herbal store in Karachi, identified by Department of Pharmacognosy, University of Karachi and voucher specimen (FGS-01-14/16) was submitted to the Department of Pharmacognosy. The crude extract of methanol was prepared through cold extraction process (Hossain et al. 2010). Impurities from seeds were removed manually and ground to coarse powder, then powdered 5 kg seeds were soaked in 2500 ml of 80 % methanol for 10 days through intermittent shaking and stirring until medium brown color was developed. The solvent was filtered initially by cotton plug and then through filter paper (What-mann No.1). After filtration, solvent was vaporized under condensed pressure in a rotary evaporator at 45 °C, followed by freeze drying at −30 °C. The methanol extract so obtained was saved in petri dishes at −20 °C till further use. The resultant yield of extract was 1120 mg of dry weight.
Animals
This study was in accordance to the consent of Board of Advance Study and Research (BASR) University of Karachi, carried out at Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, University of Karachi on 35 albino mice both male and female with a weight from 18 to 24 g, were kept in plastic cages, under precise condition of temperature (22 ± 2 °C) and humidity (50 to 60 %) in a12-h light/dark cycle. Mice were provided standard diet and water frequently. The use of animals was according to guidelines of the National Institute of Health (NIH) for Care and Use of Laboratory Animals (Washington 1996).
Grouping and dosing
35 albino mice were uniformly distributed into five groups, each comprising 7 animals. Group I was labeled as control and administered distilled water 1 ml/kg body weight through oral route once daily. Group II was taken as the standard and given diazepam manufactured by Karachi Pharmaceutical Laboratories by suspending in distilled water in a dose of 1 mg/kg with the help of oro-gastric tube. Three groups were preserved as test animals and given methanol extract of T. foenum-graecum L seeds in the doses of 50, 100 and 200 mg/kg dissolved in 1 ml distill water orally once daily. Drug and extract was administered continuously for 15 days and readings for were taken during 16th – 18th day. Hole board and Light and Dark transition model tests were performed on 16th day, phenobarbitone stimulated sleeping time was assessed on day 17th and Rota rod test was performed on 18th day.
Tests for anxiety
Effect on anxiety was evaluated through Hole Board Test and Light and Dark Transition Model.
Hole board test/head dip test
The Hole-Board test measures head dip activity of experimental animals; decrease in head dip activity reflects anxiolytic behavior (Takeda et al. 1998). The device consists of an enclosed plastic rectangular box (40 cm × 40 cm × 25 cm) with 3 holes in each side of the wall (diameter 3 cm) distributed at equal space all around the four sides of the walls. The roof is made up of transparent plastic fixed in the center (Hossain and Uma Devi 2001).
Procedure
Half an hour before the start of experiment animals were familiarized with the environment in where Hole Board device was placed. The temperature was kept constant (same as in animal house). All animals were positioned in the middle of the perforated box and permitted to travel spontaneously for 5 min. The number of times mouse stuck out its snout was noted. The device was cleaned with 70 % alcohol to clear whiff of previous animal after each observation (Sandra and Ann 1975).
Light and dark transition test
The equipment consisted of two (20 cm × 10 cm × 14 cm) plastic boxes, one transparent white box well-lit with a 100 W white bulb sited 17 cm above the box and another box painted black weakly lightened by red light. The animals were permitted to travel from one box to another by an open door (door shaped hole) among two boxes. Number of crossings of animals among the two boxes and time consumed in the light box was noted. Increase in number of crossings and time consumed in the light box reflects anxiolytic behavior (Doukkali et al. 2015).
Procedure
Half an hour before the start of experiment animals were given the treatments and placed in the environment in which the Light and Dark Transition apparatus was kept to familiarize them with the environment. Each mouse was placed individually in the light box facing the open door. The moves between light and dark boxes (total number of crossings) and time consumed in the light box were noted for 5 min. The apparatus was cleaned thoroughly by ethanol swab between the experiments.
Test for sedation
Sedation was evaluated through Phenobarbitone induced sleeping time.
Phenobarbitone stimulated sleeping time
In Phenobarbitone stimulated sleeping time, sleep onset was assessed by change in time of drug administration and time of loss of righting reflex, while duration of sleep was represented by time loss to recover from righting reflex (Nugroho et al. 2012).
Procedure
Thirty minutes after giving METFGS, animals of all groups were given phenobarbitone sodium (40 mg/kg) manufactured by Atco Laboratories Limited by intraperitoneal route, immediately after this each animal was kept under observation in individual cage. The time taken for the loss of righting reflex (onset of action) and time to improve from righting reflex (duration of action) for each animal was recorded.
Test for motor coordination
Motor coordination was assessed using Rota rod test.
Rota rod test
Rota rod apparatus is comprised of a base platform and a horizontal iron rod of 3 cm diameter and 30 cm length, having a non-slippery surface. Animals were tested for their ability to hold the rod at the speed of 16 rpm for 5 minutesmin trial. The rota rod test is a performance test based on a rotating rod with forced motor activity being applied, usually by a rodent. The test estimates riding time in seconds. It can also evaluate balance, grip strength and motor coordination of the animal mainly after traumatic brain injury or to test the effect of experimental drugs (Perez et al. 1998).
Procedure
The animals were preselected through a learning period of 24 h before the test on their capacity to remain on the rod (at16 rpm) for 2 min. Thirty minutes after the treatment with extract and drug, all the test animals were individually allowed to linger on to the rod at the speed of 16 rpm and were observed for a period of 30, 60 and 90 min after dosing. Time interval between the mounting of the animal on the rotating rod and falling off were recorded as the performance time. Time spent in the apparatus was observed for 5 min duration (Perez et al. 1998).
Statistics
Data entry and analysis was done using Superior Performance Statistical Software (SPSS) version 23. Data is shown as mean ± SEM with 95 % confidence interval. ANOVA trailed by post hoc was accomplished for comparisons of values with control. Values of p ≤ 0.05 were considered significant and p ≤ 0.001 as highly significant.
Results
Table 1 reveals the comparison of anxiolytic effect after fifteen days continuous administration of the METFGS and diazepam by Hole Board test. Animals of test group I which were given 50 mg/kg of the extract revealed significant decline in the number of head dips i.e. 9± 4.2 in comparison to the control animals i.e. 26 ± 2.3. Animals of test group II which were given 100 mg/kg of the extract showed highly significant decline in the number of head dips i.e. 4 ± 7.9 as compared to control animals. Animals of test group III which were given the extract in the dose of 200 mg/kg also revealed highly significant decline in the number of head dips i.e. 4± 3.8 as compared to control animals. Animals received standard drug diazepam also revealed highly significant decline in number of head dips i.e. 3 ± 6.8 as compared to the control animals.
Table 2 reveals the result of continuous fifteen days administration of METFG and diazepam on the time consumed in the light box (seconds) and number of moves in mice during 5 min period. Test Group I animals which received extract in the dose of 50 mg/kg revealed rise in the duration of time consumed in the light box by mice i.e.98 ± 1.2 s as compared to control animals i.e. 80 ± 3 .2 s but the increase was statistically insignificant. The outcome on the number of transitions in test group I was also insignificant in comparison to the control animals. Test Group II animals which received extract in the dose of 100 mg/kg revealed substantial rise in the duration of time consumed in the light box i.e. 189 ± 3.3 s as compared to the control animals i.e.80 ± 3.2 s. The effect on number of transitions in test group II was also significant i.e. 16 ± 1.2 in comparison to the control animals i.e. 8 ± 4.4. Similarly animals of test group III which received extract in the dose of 200 mg/kg also revealed significant rise in the duration of time spent in the light box i.e. 157 ± 3.2 s as compared to control animals i.e. 80 ± 3.2 s. Increase in number of transitions were also highly significant i.e. 19 ± 3.2 as compared to control animals i.e. 8 ± 4.4.
The animals in the standard group which received diazepam in a dose of 1 mg/kg continuously for fifteen days also revealed significant increase in the duration of time consumed in the light box i.e. 204 ± 1.5 s in comparison to the animals of control group i.e. 80 ± 3.2 s. The number of transitions was also increased significantly i.e. 18 ± 7.6 in comparison to control animals i.e. 8 ± 4.4.
Table 3 reveals the effect of Sixteen days continuous administration of METFG and diazepam on phenobarbitone stimulated sleeping period. The extract exhibited no effect on Phenobarbitone induced sleeping time as neither the onset of sedation nor the duration of sedation were affected at all the three doses administered as compared to the control group. Whereas the diazepam potentiated the result of phenobarbitone in the animals of the standard group as not only the time to onset of sleep (20 ± 3.76) was decreased but also duration of sleep (180 ± 6.87) was prolonged at significant level as compared to the animals of control group i.e. onset of sleep (48 ± 3.62) and duration of sleep (55 ± 4.98).
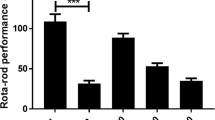
Table 4 reveals the effect of Sixteen days continuous administration of seeds of METFG and diazepam on motor coordination in mice using Rota rod test. In Rota rod test, the extract exhibited significant skeletal muscle relaxant effect at 200 mg/kg after 90 min i.e. time to fall in seconds was 203 s as compared to the animals of control group where time to fall in seconds was 319 s. However animals given the standard drug diazepam showed highly significant muscle relaxant activity after 30 min i.e. time to fall in seconds was 98 as compared to the animals of control group where time to fall in seconds was 344.
Discussion
Recently, conventional medicine has been reevaluated worldwide by extensive research works on therapeutic principles of different plant species (Harvey 2008). In this study, effect of METFGS on has been evaluated on anxiety, sedation and motor coordination.
In our study, there was significant decline in the number of head dips in mice by METFGS at 50 mg/kg while highly significant decrease at 100 and 200 mg /kg in comparison to the control animals. Head-dip activity of an animal in Hole board test represents an escape response, which declines as the animal becomes less fearful. (Gillian and Christopher 2008).
Alkaloids (Martinez-Vázquez et al. 2012), flavonoids (Li et al. 2011) and saponins (Nesterova Yu et al. 2015) are well reported in literature to have anxiolytic effect. As stated earlier, TFG seeds are rich source of flavonoids, alkaloids and saponins, hence it may be suggested that the anxiolytic effect of METFGS is due to presence of these bioactive constituents.
In Light/Dark transition model, mice received METFGS at 100 and 200 mg/kg showed significant anxiolytic effect similar to standard drug diazepam in comparison to the control group.
The Light and Dark transition model is based on the innate aversion of rodents to well-lit areas and spontaneous exploratory behavior on applying mild stressors i.e. novel environment and light. By exploiting these natural tendencies of rodents, the light and dark transition model can be employed to identify drugs that alter anxiety levels. In this model, animal is exposed to a novel environment with secure (dark compartment) and unsecure (light compartment) areas. The inherent conflict between exploratory drive and light avoidance leads to inhibition of exploratory activity (Crawley and Goodwin 1980).
Transitions between two boxes have been conveyed to be an index of movement exploration and the time consumed in each box to be a reflection of aversion. Increase in number of moves between the two compartments and the time consumed in the light box is used to evaluate anxiolytic effect (Graeff and Zangrossi 2002; Lepicard et al. 2000). In our study, both the number of moves between two boxes and duration of time consumed in the light box were increased at highly significant level as compared to the control group indicating anxiolytic effect.
Most of the anxiolytic agents exert their action by opening of activated GABA chloride channels. Flavonoids isolated from plants are found to be ligands for the GABA/ benzodiazepine (BDZ) receptors in the central nervous system (Marder et al. 2001). Anxiolytic activity of METFGS can be attributed to the presence of flavonoids in it.
The effect of METFGS on sedation and motor coordination were also assessed using Phenobarbitone induced sleeping time and Rota rod test and compared with diazepam. The extract did not exhibit any effect on Phenobarbitone stimulated sleeping time as neither the onset of sedation nor the duration of sedation was affected at any dose as compared to the control group, however diazepam potentiated the effect of phenobarbitone in the animals as not only the time onset of sleep was decreased but duration of sleep was also prolonged significantly as compared to the animals of control group.
Rota rod is a classical model to evaluate neuromuscular blockade and effect on motor coordination (Dunham and Miya 1957). In Rota rod test, the extract showed significant decrease in hand grip strength at 200 mg/kg after 90 min as compared to the animals of control group. While animals given the standard drug diazepam, however showed highly significant decrease in grip strength after 30 min. Thus it may be safe to conclude that muscle relaxant effect of the extract may be due to presence of alkaloids in fenugreek seeds, since alkaloids are known to have muscle relaxant property (Gustafson 1989; Sotnikova et al. 1997; Amirkia and Heinrich 2014; Kaur and Arora 2015).
This study suggested highly significant dose dependent anxiolytic effect of METFGS testified on two models of anxiety i.e. Hole board test and Light/Dark transition model using various doses. Anxiolytic property was almost comparable to the standard drug diazepam. Even the lowest dose used (50 mg/kg) indicated significant anxiolytic activity in hole board test with almost no sedation and mild muscle relaxant effect at highest dose. The clinical application of benzodiazepines is limited due to their adverse effects like psychomotor impairment, sedation, myorelaxation, ataxia and amnesia (Pal et al. 2010).
The limitation of study is its short dosing period which will be extended in future by increasing dosing period to assess chronic toxicity including tolerance and dependence. However it may safely be concluded that fenugreek seeds are better alternate to diazepam for its anxiolytic effect.
References
Al-Jasass FM, Al-Jasser (2012) Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci World J 859892. doi:10.1100/2012/859892 E pub 2012 Dec 24
American Heritage Medical Dictionary (2007) The American heritage medical dictionary. Houghton Mifflin Harcourt (HWH), Boston
Amirkia V, Heinrich M (2014) Alkaloids as drug leads – a predictive structural and biodiversity-based analysis. Phytochem Lett 10:xlviii–xlliii
Bae MJ, Shin HS, Choi DW, Shon DH (2012) Antiallergic effect of Trigonella foenum-graecum L. Extracts on allergic skin inflammation induced by trimellitic anhydride in BALB/c mice. J. Ethnopharmacol 144(3):514–522
Bin-Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S (2003) Immunomodulatory effects of fenugreek (Trigonella foenumgraecum L.) extract in mice. Int Immunopharmacol 3(2):257–265
Carlini EA (2003) Plants and the central nervous system. Pharmacol Biochem Behav 75(3):501–512
Cha HY, Park JH, Hong JT, Yoo HS, Song S, Hwang BY, Eun JS, KW O (2005) Anxiolytic-like effects of gensinosides on the elevated plus-maze model in mice. Biol Pharm Bull 28(9):1621–1625
Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behaviour for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13:167–170
Das S (2014) Hepatoprotective activity of methanol extract of fenugreek seeds on rats. IJPSR 5(4):1506–1513
Doukkali Z, Taghzouti K, Bouidida ELH, Nadjmouddine M, Cherrah Y, Alaoui K (2015) Evaluation of anxiolytic activity of methanolic extract of Urticaurens in a mice model. Behav Brain Funct 11:19
Dunham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc 46(3):208–209
Faustino TT, Almeida RB, Andreatini R (2010) Medicinal plants for the treatment of generalized anxiety disorder: a review of controlled clinical studies. Rev Bras Psiquiatr 32(4):429–436
Gillian RB, Christopher N (2008) The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav Process 78(3):442–448
Graeff FG, Zangrossi H (2002) Animal models of anxiety disorders. In: D’Haenen H, den Boer JA, Willner P (eds) Biological Psychiatry. Wiley Ltd, London, pp. 96–103
Gustafson T (1989) Pharmacological control of muscular activity in the sea urchin larva. I. Effects of nicotinic and muscarinic agents. Comp Biochem Physiol C: Comp Pharmacol 94:1–14
Gyawali R (2010) Natural Products in drug discovery: Current Scenario of Nepal. Bull of Nepal Pharm Association 9:35–38
Harvey AL (2008) Natural products in drug discovery. Drug Discov Today 13(19–20):894–901
Hong FJ, Xue JL, Hong Y (2009) Natural Products and drug discovery. EMBO Rep 10(3):194–200
Hossain M, Uma Devi P (2001) Effect of irradiation at the early fetal stage on adult brain function of mouse: learning and memory. Int J Radiat Biol 77(5):581–585
Hossain MS, Ahmed M, Islam A (2010) Hypolipidemic and hepatoprotective effects of different fractions of ethanolic extract of immature leaves of Mangifera indica (Linn) in alloxan induced diabetic rats. IJPSR 1(11):132–138
Ibibia T, Kuponiyi E (2013) Plant derived compounds with potential sedative and anxiolytic activities. Intern J basic. Appl Sci 02(01):63–78
Kan Y, Kan A, Ceyhan T, Sayar E, Kartal M, Altun L, Aslan S, Cevheroglu S (2005) Atomic absorption spectrometric analysis of Trigonella foenum-graecum L. seeds cultivated in Turkey. Turkish J. Pharm. Science 2(3):187–191
Kaur R, Arora S (2015) Alkaloids-important therapeutic secondary metabolites of plant origin. Jour. Crit Rev 2(3):3–8
Khoja KK, Shafi G, Hasan TN, Syed NA, Khalifa ASA, Assaf AHA, Alshatwi AA (2011) Fenugreek, a naturally occurring edible spice, kills MCF-7 human breast cancer cells via an apoptotic pathway. Asian Pacific J. Cancer Prev 12:3299–3304
Kumar P, Kale RK, Baquer NZ (2012) Antihyperglycemic and protective effects of Trigonella foenum graecum seed powder on biochemical alterations in alloxan diabetic rats. Eur Rev Med Pharmacol Sci 16(3):18–27
Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G (2000) Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6 J strains of mice. Pharmacol Biochem Behav 67:739–748
Li H, Zhou P, Yang Q, Shen Y, Deng J, Li L, Zhao D (2011) Comparative studies on anxiolytic activities and flavonoid compositions of Passifloraedulis ‘edulis’ and Passifloraedulis ‘flavicarpa. J Ethnopharmacol 133(3):1085–1090
Madar Z, Abel R, Samish S, Arad J (1988) Glucose-lowering effect of fenugreek in non-insulin dependent diabetes. Eur J Clin Nutr 42(1):51–54
Manavi C, Rajkumar V, Vijai L (2013) Anxiolytic effects of Plumeria rubra var. acutifolia (Poiret) L. flower extracts in the elevated plus-maze model of anxiety in mice. Asian J Psychiat 6:113–118
Marder M, Estiu G, Blanch LB, Viola H, Wasowski C, Medina JH, Paladini AC (2001) Molecular modeling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABA (a) receptor complex. Bioorq. Med Chem 9(2):323–335
Martinez-Vázquez M, Estrada-Reyes R, Araujo Escalona AG, Ledesma Velázquez I, Martínez-Mota L, Moreno J, Heinze G (2012) Antidepressant-like effects of an alkaloid extract of the aerial parts of Annonacherimolia in mice. J Ethnopharmacol 139(1):164–170
Nesterova Yu V, Povet’eva TN, Suslov NI, Shults EE, Ziuz’kov GN, Aksinenko SG, Afanas’eva OG, Krapivin AV, Kharina TG (2015) Anxiolytic activity of diterpene alkaloid songorine Pharmacology and Toxicology. Bull Exp Biol Med 159(5):620–622
Nugroho A, Kim MH, Choi J, Baek NI, Park HJ (2012) In-vivo sedative and gastro protective activities of Salvia Plebeia extract and its composition of polyphenols. Arch Pharm Res 35(8):1403–1411
Pal C, Phil S, Beer B, Lippa A (2010) A multicenter, placebo-controlled, double-blind, randomized study of efficacy and safety of ocinaplon (DOV 273, 547) in generalized anxiety disorder. CNS Neurosci Ther 16(2):63–75
Perez RM, Perez JA, Garcia LM, Sossa H (1998) Neuro-pharmacological activity of Solanumnigrum fruit. J Ethnopharmacol 62(1):43–48
Petropoulos GA (2002) Fenugreek-The genus Trigonella, first edn. Taylor and Francis, London, pp. 1–127
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety like behaviors: a review. Eur J Pharmacol 463:3–33
Sandra E, Ann G (1975) Validity of head dipping as measure of exploration in a modified hole board. Psychopharmacologia 44(1):53–59
Sharma RD, Raghuram TC, Rao NS (1990) Effect of fenugreek seeds on blood glucose and serum lipids in type 1 diabetes. Eur J Clin Nutr 44(4):301–306
Sotnikova R, Kettmann V, Kostalova D, Taborska E (1997) Relaxant properties of some aporphine alkaloids from Mahonia aquifolium. Methods Find Exp Clin Pharmacol 19:589–597
Takeda H, Tsuji M, Matsumiya T (1998) Changes in head-dipping behavior in the hole-board test reflects the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350:21–29
Uhlenhuth EH, Balter MB, Ban TA, Yang K (1999) Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997. Eur Neuropsychopharmacol 9:393–398
Vats V, Grover JK, Rathi SS (2002) Evaluation of antihyperglycemic and hypoglycemic effect of Trigonella foenum graecum Linn, Ocimum sanctum Linn and Pterocarpusmarsupium Linn in normal and alloxanized diabetic rats. J Ethnopharmacol 79:95–100
Wani SA, Kumar P (2016) Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. doi:10.1016/j.jssas.2016.01.007
Washington DC (1996) National research council. Guide for the care and use of laboratory animals. National Academy Press, Washington, pp 1–7
Zargar S (2014) Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J Biologic Sci 21:139–145
Acknowledgments
Authors are thankful to Dr. Mohtashim Associate Professor Department of Pharmacognosy for identifying fenugreek seeds and Department of Pharmacology, University of Karachi for providing technical and financial help to complete this piece of work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assad, T., Khan, R.A. Effect of methanol extract of Trigonella foenum-graecum L. seeds on anxiety, sedation and motor coordination. Metab Brain Dis 32, 343–349 (2017). https://doi.org/10.1007/s11011-016-9914-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9914-y