Abstract
Curcumin, the main polyphenolic component of turmeric (Curcuma longa) rhizomes has been reported to exert cognitive enhancing potential with limited scientific basis. Hence, this study sought to evaluate the effect of curcumin on cerebral cortex acetylcholinesterase (AChE) and adenosine deaminase (ADA) activities in cadmium (Cd)-induced memory impairment in rats. Animals were divided into six groups (n = 6): saline/vehicle, saline/curcumin 12.5 mg/kg, saline/curcumin 25 mg/kg, Cd/vehicle, Cd/curcumin 12.5 mg/kg, and Cd/curcumin 25 mg/kg. Rats received Cd (2.5 mg/kg) and curcumin (12.5 and 25 mg/kg, respectively) by gavage for 7 days. The results of this study revealed that cerebral cortex AChE and ADA activities were increased in Cd-poisoned rats, and curcumin co-treatment reversed these activities to the control levels. Furthermore, Cd intoxication increased the level of lipid peroxidation in cerebral cortex with a concomitant decreased in functional sulfuhydryl (−SH) group and nitric oxide (NO), a potent neurotransmitter and neuromodulatory agent. However, the co-treatment with curcumin at 12.5 and 25 mg/kg, respectively increased the non-enzymatic antioxidant status and NO in cerebral cortex with a decreased in malondialdehyde (MDA) level. Therefore, inhibition of AChE and ADA activities as well as increased antioxidant status by curcumin in Cd-induced memory dysfunction could suggest some possible mechanism of action for their cognitive enhancing properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a noxious environmental contaminant of continuing great toxicological concern worldwide (Johri et al. 2010). It is a highly accumulative toxicant with very long biological half-life of over 20 years (Johri et al. 2010). It has been reported that Cd can enter into the food chain since it is not degraded in the environment and this is one of the main route of exposure in humans (Olsson et al. 2002). In this way, the prolonged exposure to Cd has been linked to toxic effects triggered by the accumulation of this metal in a variety of structures of the central nervous system (CNS) (Sinha et al. 2009).
Studies have shown that Cd is able to cross the blood–brain barrier (BBB) and accumulate in the brain (Sinha et al. 2009) leading to cerebral edema, impairment of attention, learning and memory as well an increase in aggressive and anxiogenic-like behaviors and also worsening of the synaptic neurotransmission and the antioxidant levels (Mendez-Armenta et al. 2001, 2003; Goncalves et al. 2010; Abdalla et al. 2014). In addition, Cd can cause changes in key enzymes of the CNS involved in maintaining the levels of important neurotransmitter and neuroprotective agent (Goncalves et al. 2010, 2012; Abdalla et al. 2014).
Acetylcholine (ACh) is a neurotransmitter with an important role in many functions of both the peripheral and central nervous systems acting in the learning and memory processes as well as locomotor control and cerebral blood flow (Goncalves et al. 2010, 2012; Deiana et al. 2011; Klinkenberg et al. 2011). ACh levels in synaptic cleft are regulated by AChE activity. It has been shown that the AChE activity is implicated in cell proliferation and neurite outgrowth (Chacon et al. 2003). Interestingly, AChE responds to various insults including oxidative stress, an important event that has been related to the pathogenesis and progression of a variety of CNS disorders (Chacon et al. 2003). Thus, this enzyme is a target for the emerging therapeutic strategies to treat cognitive disorders like Alzheimer’s disease (AD) (Shen et al. 2011).
Furthermore, the enzyme adenosine deaminase (ADA) also plays an important role in synaptic neurotransmission. It catalyzes the irreversible hydrolytic deamination of adenosine to inosine thereby depleting the level of adenosine production in the brain. Adenosine, a purine ribonucleoside, exhibits neuromodulatory and neuroprotective effects in the brain and is involved in memory formation and cognitive function Recently, it has been hypothesise that inhibition of ADA activity is considered a good therapeutic approach for preventing cognitive dysfunction (Bauerle et al. 2011; Ramani et al. 2012).
To combat against Cd-induced memory impairment, antioxidant compounds have been implicated to play an important role in the neuroprotection against the establishment of oxidative stress. Curcumin is the principal natural polyphenol curcuminoid of the spice turmeric (Curcuma longa), a member of the ginger family (Zingiberaceae) (Anamika 2012). Curcumin has a wide spectrum of therapeutic properties and it has been shown to possess antioxidant, anti-inflammatory, anticancer and anti-Alzheimer’s properties (Strimpakos and Sharma 2008). In addition, a number of studies have demonstrated cognitive enhancing properties of curcumin (Pan et al. 2008; Reeta et al. 2009; Tang et al. 2009). Nevertheless, limited information is available on their mechanism of action, hence, this study evaluated the possible protective effects of this compound in memory-like behavior, antioxidative status, AChE and ADA activities in Cd exposed rats.
Materials and methods
Chemicals
Cadmium sulfate was obtained from Oxford Laboratory, Mumbai, India and solubilized in normal saline. Curcumin, acetylthiocholine iodide, adenosine and malondialdehyde were purchased from Sigma–Aldrich, St. Louis, MO, USA. All other reagents were of analytical grade and the water used was glass distilled.
Animals and experimental design
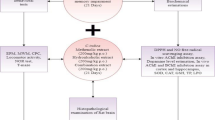
About twelve weeks old adult male albino rats (weighing 150–180 g) were obtained from the animal breeding unit at College of Medicine, Afe Babalola University, Nigeria and were housed in cages, at room temperature 25–28 °C, relative humidity 60–70 %, and 12 h light/dark cycle. Food (pellet rat chow) and water were available ad libitum. Animals were cared according to US National Institute of Health (NIH) ethical guidelines. After two weeks of acclimatization, animals were divided randomly into six groups of 6 animals each: group 1 received saline plus vehicle, group 2 received saline plus curcumin 12.5 mg/kg, group 3 received saline plus curcumin 25 mg/kg, group 4 received Cd plus vehicle, group 5 received Cd plus curcumin 12.5 mg/kg and group 6 received Cd plus curcumin 25 mg/kg. In the present study, the rats received Cd in the form of Cd sulfate at a dosage of 2.5 mg/kg, i.p (Zalups and Ahmad 2003). The choice of Cd dosage was according to Goncalves et al. (2012) where it induce memory damage while the choice of the curcumin doses (12.5 and 25 mg/kg) was made based on previous works which obtained beneficial results of this compound in brain of rats. Both solutions were administered for a period of 7 days. Curcumin was administered 30 min after Cd and the solutions were freshly prepared. Cd was diluted in saline and the curcumin in 1 % ethanol and both were administered (1 mL/kg).
It is important to note that controls were performed to correct for vehicle (1 % ethanol) interference. However, no significant differences between the results obtained to the vehicle (1 % ethanol) and to the control (saline) were observed in the parameter analyzed in this study (data not shown).
After the treatment period, animals were fasted overnight and sacrificed 24 h by cervical dislocation. The whole brains were quickly excised and washed in cold saline solution, blotted on filter papers to remove adhering blood, and the cerebral cortex was isolated and homogenized in 100 mM potassium phosphate, pH 7.5. The homogenates were centrifuged at 10,000×g for 20 min at 4 °C, and the supernatant was used for the subsequent enzymatic assays. Treatment protocol was in accordance with the ethical requirement of the Animal Use and Care Committee of Afe Babalola University, Ado - Ekiti, Nigeria.
Behavioural evaluation
Novel objects recognition (NOR) test
The novel object recognition test is a behavioural test for non-spatial working memory in rats. It consist of two sessions; first trial (T1) and the second trial (T2). The two trials were separated by an inter-trial interval of 3 h. In T1, each rat was put in the test box (22.5 × 24.5 × 11.0 cm), in which two identical objects (truncated cone: diameter, 4.5 and 6.0 cm; height, 3.5 cm) were placed at two adjacent corners, and the time spent exploring each object was measured for 5 min. During training, the animals explored between 40 and 60 % of the time on each object. Immediately after T1, rats received saline or Cd (2.5 mg/kg, i.p.). In T2, a novel object replaced the object explored less by the mouse in T1, and the time spent exploring the familiar object (F) and the novel object (N) was recorded for 5 min. Objects were made of odourless plastic and similar in size. Between each trial, the objects and the field were cleaned. The total time spent sniffing or touching each object with the nose and/or forepaws were recorded. Recognition memory index was calculated by a discrimination index (%) using the method of Adeniyi et al. (2016).
Biochemical evaluation
Determination of acetylcholinesterase (AChE) activity
The AChE enzymatic assay was determined using a modification of the spectrophotometric method of Ellman et al. (1961) as previously described by Akinyemi et al. (2016). The reaction medium (2 mL final volume) contained 100 mmol/L of K+-phosphate buffer, pH 7.5, and 1 mmol/L of 5,5′-dithiobisnitrobenzoicacid. The method is based on the formation of the yellow anion, 5,5’dithio-bis-acid-nitrobenzoic, measured by absorbance at 412 nm during 2-min incubation at 25 °C. The enzyme (40–50 μg of protein) was pre-incubated for 2 min. The reaction was initiated by adding 0.8 mmol/L of acetylthiocholine iodide. All samples were run in triplicate, and enzyme activity was expressed in micromole acetylthiocholine (AcSCh) hydrolysed per hour per milligram of protein.
Determination of adenosine deaminase (ADA) activity
ADA activity determination was performed as described by Guisti and Galanti (1984) which is based on the direct measurement of the formation of ammonia, produced when adenosine deaminase acts in excess of adenosine. In brief, 50 μL of cerebral cortex homogenate reacted with 21 mmol/L of adenosine, pH 6.5, and was incubated at 37 °C for 60 min. The protein content used for the experiment was adjusted to between 0.7 and 0.9 mg/mL. Results were expressed in units per liter (U/L). One unit (1 U) of ADA is defined as the amount of enzyme required to release 1 mmol of ammonia per minute from adenosine at standard assay conditions.
Measurement of nitric oxide (NO)
NO content in tissue homogenates was estimated in a medium containing 400 μL of 2 % vanadium chloride (VCl3) in 5 % HCl, 200 μL of 0.1 % N-(l-naphthyl)ethylene-diaminedihydrochloride, 200 μL of 2 % sulfanilamide (in 5 % HCl). After incubating at 37 °C for 60 min, nitrite levels, which corresponds to an estimative of levels of NO, were determined spectrophotometrically at 540 nm, based on the reduction of nitrate to nitrite by VCl3 (Miranda et al. 2001). Cerebral cortex nitrite and nitrate levels were expressed as nanomole of NO/mg of protein.
Determination of total thiol (TSH) content
Total thiol (TSH) content was determined according to the method previously described by Ellman (1959). Briefly, the reaction mixture consisted 40 μL of cerebral cortex homogenate, 10 μL of 10 mM DTNB and 0.1 M potassium phosphate buffer (pH 7.4) in a final volume of 200 μL. The mixture was incubated for 30 min at ambient temperature and then read the absorbance at 412 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). A standard curve was plotted for each measurement using cysteine as a standard and the results expressed as μmol/mg protein.
Determination of non-protein thiols (NPSH) content
NPSH levels were determined by the method of Ellman (1959). Briefly, an aliquot of cerebral cortex homogenate was mixed (1:1) with 10 % trichloroacetic acid (TCA). Subsequent to precipitation of protein, the resulting solution was centrifuged at 10,000×g for 5 min at 4 °C and the free SH groups were determined in the supernatant. The reaction mixture consisting 50 μL of sample, 450 μL phosphate buffer and 1.5 mL of 0.1 mM of 5,5′-dithiobis 2-nitro benzoic acid was incubated for 10 min at 37 °C. The absorbance was measured at 412 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). NPSH levels were expressed as μmol/mg of protein.
Lipid peroxidation
Lipid peroxidation was determined as the formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction according to Ohkawa et al. (1979). Briefly, the reaction mixture consisting 200 μL of tissue homogenates or standard (0.03 mM MDA), 200 μL of 8.1 % sodium dodecyl sulfate (SDS), 500 μL of 0.8 % thiobarbituric acid (TBA) and 500 μL of acetic acid solution (2.5 M HCl, pH 3.4) was heated at 95 °C for 1 h. The absorbance was measured at 532 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). TBARS tissue levels were expressed as μmol MDA produced/mg of protein.
Protein content
Protein was measured by the coomassie blue method according to Bradford (1976) using serum albumin as standard.
Statistical analysis
All data were expressed as mean ± S.E.M. The statistical analysis used was one-way and two-way ANOVA, followed by Duncan’s multiple range tests, p < 0.05 was considered to represent a significant difference in both analyses used.
Results
Effect of curcumin on non-spatial memory decline in novel object recognition (NOR) test in Cd exposed rats
The result of the effect of curcumin on memory in NOR test in Cd exposed rats is presented in Fig. 1. The result clearly showed a significant (P < 0.05) decrease in memory of rats exposed to Cd when compared with control. However, curcumin at both dosage (12.5 and 25 mg/kg) prevent a decline in memory of rats exposed to Cd. Nevertheless, curcumin did not alter memory in normal rats when compared with control.
Effect of curcumin on novel object recognition (NOR) task in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Effect of curcumin on acetylcholinesterase (AChE) and adenosine deaminase (ADA) activities in Cd exposed rats
The activities of AChE and ADA in the cerebral cortex of the experimental animals exposed to Cd and co-treated with curcumin (12.5 and 25 mg/kg) are shown in Figs. 2 and 3. The results revealed that Cd administration altered cerebral cortex AChE and ADA activities by causing a significant (P < 0.05) increase in their activities when compared to the control rats. However, co-treatment with curcumin (12.5 and 25 mg/kg) prevented these alterations by inhibiting the activities of AChE and ADA when compared to the Cd-exposed rats.
Effect of curcumin on cerebral cortex acetylcholinesterase (AChE) activity in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Effect of curcumin on cerebral cortex adenosine deaminase (ADA) activity in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Effect of curcumin on nitric oxide (NO), total and non-protein thiol and malondialdehyde (MDA) levels in Cd exposed rats
Figures 4–6 depicts the effect of curcumin on NO, total thiol and MDA levels in cadmium (Cd) induced memory impairment in rats. Exposure to Cd caused a significant decrease (p < 0.05) in cerebral cortex NO and thiol levels with a concomitant increase in MDA level when compared to the control (Figs. 4–6). However, treatment with curcumin (12.5 and 25 mg/kg) significantly (p < 0.05) reduced the elevated levels of MDA to control level as well as prevented depletion of cerebral cortex NO and thiol levels by causing a significant (p < 0.05) increase when compared with the Cd exposed rats.
Effect of curcumin on cerebral cortex nitric oxide (NO) level in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Discussion
In this study we showed that oral administration of curcumin prevented memory impairment in cadmium-induced memory deficits in rats. Our result demonstrated that exposure to Cd induced memory deficits in the novel object recognition task (Fig. 1). This is in agreement with previous studies that showed that Cd triggered impairment of memory during object recognition task (Goncalves et al. 2010, 2012; Abdalla et al. 2014). This effect has been linked to the ability of Cd to reach the brain of adult rats because of their selective permeability of the blood–brain barrier (Shukla et al. 1996; Takeda et al. 1999). Previous studies have demonstrated that significant amount of Cd reach the brains of Cd-exposed rats (Goncalves et al. 2010, 2012; Abdalla et al. 2014; Costa et al. 2015), however, this findings was in agreement with our result on Cd level in brain of Cd intoxicated rats (data not shown).
The novel object recognition task is utilized for evaluating learning and memory in rats (Gutierres et al. 2012). A significant decrease in memory index induced by Cd was observed, which suggests an impairment of learning and memory formation. This result corroborates published data where rats orally intoxicated with Cd showed impaired cognition (Goncalves et al. 2010, 2012; Abdalla et al. 2014). However, when the Cd-exposed rats were treated with curcumin, the memory index was similar to the control group. These findings indicate that co-treatment with curcumin was able to prevent learning and memory impairment induced by Cd toxicity. Previous reports showed that curcumin is able to attenuate the oxidative damage and prevent memory loss in rats exposed to other neurotoxicity models, such as aluminum chloride (Kumar et al. 2009), arsenic (Yadav et al. 2011), and cigarette smoke (Jaques et al. 2012).
Cholinergic pathways in the brain are widely known to play important roles in cognition (Costa et al. 2015). Numerous studies on Cd toxicity have found an association with behavioral disturbances and cholinergic neurotransmission since an increase or a decrease in acetylcholinesterase (AChE, E.C. 3.1.1.7) activities were verified in both animal models and humans after Cd exposure (Pari and Murugavel 2007; Goncalves et al. 2010, 2012; Abdalla et al. 2013). In the literature, both in vitro and in vivo effects of Cd on AChE activity have been described, and these results are conflicting because some studies showed activation (Carageorgiou et al. 2005; Abdalla et al. 2013) and others reported inhibition of AChE activity (Carageorgiou et al. 2005; Pari and Murugavel 2007; Goncalves et al. 2010).
In the present study, our results demonstrated that oral administration of Cd caused a significant (P < 0.05) increase in cerebral cortex AChE activity in Cd-treated rats (Fig. 2). On the basis of our results we could suggest that the mechanisms involved in the memory impairment observed in the novel object recognition test is related with the increase of AChE activity caused by Cd, leading to a reduction of cholinergic neurotransmission efficiency, i.e., reduction of acetylcholine levels in the synaptic cleft, thus contributing to progressive cognitive impairment (Soreq and Seidman 2001; Schmatz et al. 2009; Goncalves et al. 2010, 2012; Jaques et al. 2012). However, oral intake of curcumin was effective in preventing the increase in AChE activity induced by Cd in cerebral cortex of rats. The neuroprotective potential of curcumin has been studied at different doses (15 to 200 mg/kg) in animal models of neurotoxicity and disease conditions due to their ability to cross the blood–brain barrier (Kumar et al. 2009; Yadav et al. 2011; Jaques et al. 2012). In addition, several authors have demonstrated that curcumin administration inhibited AChE activity in streptozotocin-induced diabetic rats which is in agreement with our present study (Kuhad et al., 2007; Kumar et al. 2009).
Furthermore, it has been reported that the breakdown product of ATP, adenosine, acts as a CNS modulator in mammals, regulates cell metabolism, and triggers a variety of physiological effects participating in apoptosis, necrosis, and cell proliferation. Under pathological conditions, adenosine plays a protective role by modulating the release of the neurotransmitters and tropic factors (Burnstock 2006; Desrosiers et al. 2007). Therefore, we could suggest that the increase of ADA activity observed in cerebral cortex of Cd-exposed rats might lead to the conversion of adenosine into inosine, decreasing the levels of adenosine in brain synapses and consequently can contribute to the damage nervous observed in Cd-intoxication. However, it is interesting to note that curcumin-treated rats were able to prevent an increase in ADA activity in Cd-intoxicated rats (Fig. 3). This suggests that curcumin has a protective role against Cd poisoning and the probable mechanism could be due to their inhibitory effect on ADA activity thereby resulting in an increase in the level of adenosine. Previous studies have implicated curcumin and adenosine level via the modulation of the purinergic system in various pathological conditions such as Alzheimer’s disease, diabetes, stroke, hypertension and inflammation (Jaques et al. 2011, 2012; Akinyemi et al. 2016).
It has been well documented in experimental animals that nitric oxide (NO), the metabolite of L-arginine by nitric oxide synthase (NOS), is critically involved in learning and memory processes (Garthwaite and Boulton 1995; Paul and Ekambaram 2011). Three distinct NOS have been identified in the hippocampus, cortex, cerebellum, corpus striatum and medulla of mice brain (Garry et al. 2015). NOS from endothelial cells (eNOS) and neurons (nNOS) are constitutively expressed and the action of these enzymes are stimulated by an increase in intracellular calcium while the other is NO synthesized by calcium-independent induction NOS (iNOS) (Garry et al. 2015). However, NO derived from endothelial nitric oxide synthase (eNOS) appears to have neuroprotective properties whereas those derived from inducible nitric oxide synthase (iNOS) have neurotoxic effects (Garry et al. 2015).
In the present study, Cd caused a significant (P < 0.05) decreased in NO production when compared with the control. The decrease in cerebral cortex NO level suggests possible mechanism for their induction of memory dysfunction. However, curcumin prevented a decrease in NO when compared with the Cd-treated rats (Fig. 4). Curcumin have been reported to cross the blood–brain barrier (BBB) easily, and enhances the release of calcium from intracellular stores which may have been responsible for the increase in cerebral cortex NO levels observed in this study (Fukao et al. 1997; Hashiguchi et al. 2001; Paul and Subramanian, 2002). NO function as a neurotransmitter by stimulating soluble guanylyl cyclise (sGC) to form the second messenger molecule, cyclic guanosine monophosphate (cGMP) in the target cells (Garthwaite and Boulton 1995; Griffiths et al. 2008). Studies on various forms of synaptic plasticity in the brain have provided insight into the cellular and molecular mechanisms for learning and memory processes. Long-term potentiation (LTP), a homosynaptic plasticity and long-term depression (LTD), a heterosynaptic plasticity are two major forms of activity dependent synaptic plasticity in the brain (Garthwaite and Boulton 1995; Griffiths et al. 2008). NO-cGMP pathway has been implicated in the induction of cortex LTP and LTD which are known to be the predominant mechanisms of learning and memory processes (Griffiths et al. 2008).
Cadmium is considered as a metal which may induce oxidative damage by disturbing the pro-oxidant-antioxidant balance in the cell. The determination of MDA levels in living tissue is the marker for lipid peroxidation. The present study showed that MDA levels were significantly increased in cadmium treated rats indicating increased lipid peroxidation and hence increased oxidative stress following short term cadmium administration at the test dose (Fig. 5). Our results showed that behavioral dysfunction after immediate cadmium administration was accompanied by a considerable increase in lipid peroxidation which may be linked to the significant depletion of the sulfhydryl (SH)-group containing compounds (Fig. 6). Non-protein thiol (NPSH) or reduced glutathione (GSH) is part of a non-enzymatic defense system. It is a central protective antioxidant and considered the first line of defense against oxidative damage and free radical generation in the brain tissue. It can directly scavenge free radicals or act as a substrate for GPx and GST in the detoxification of hydrogen peroxide (Lu 2013).
Effect of curcumin on cerebral cortex malondialdehyde (MDA) content in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Effect of curcumin on cerebral cortex total thiol (TSH) content a and non-proteinl thiol (NPSH) content b in cadmium induced memory impairment in rats. Data are presented as the mean ± SEM (n = 6). Bars with different letters are significantly (P < 0.05) different from each other. KEY: Control – received normal saline + vehicle. Cur 12.5 – received normal saline + curcumin (12.5 mg/kg). Cur 25 – received normal saline + curcumin (25 mg/kg). Cd – received cadmium + vehicle. Cd/Cur 12.5 – received cadmium + curcumin (12.5 mg/kg). Cd/Cur 25 – received cadmium + curcumin (25 mg/kg)
Oxidative stress alters the neurotransmission and neuronal function (Bouayed et al. 2009). It is important to note that enzymes such as AChE are significant components of the biological membranes and, thus, can be important targets of oxidation of membrane caused by Cd exposure (Abdalla et al. 2014). These results allow us to infer that, these alterations may explain the changes in the activities of these enzymes found in our study and, consequently, impairment of memory observed in Cd exposed rats. However, it is noteworthy that curcumin caused a significant decreased in cerebral cortex MDA levels with a concomitant increase in the total and non-protein thiol (−SH) content of Cd exposed rats. Our results is in agreement with previous studies suggesting that alterations in the lipid membrane observed after Cd exposure could be a decisive factor in the modification of the conformational state of the AChE enzyme and memory impairment in rats (Goncalves et al. 2010, 2012).
Conclusion
The present study demonstrated that curcumin prevented memory dysfunction in Cd-induced memory impairment in rats. The inhibition of AChE and ADA activities in cerebral cortex of rats as well as prevention of increased lipid peroxidation with a concomitant increased in sulfhydryl (SH)-group containing compounds and NO level observed in this study could suggest some possible mechanism of action for their cognitive enhancing potential in Cd-treated rats. Thus, these results emphasize the close interaction between Cd-exposure and cholinergic systems in the development of cognitive impairment and point to the potential of curcumin as an adjuvant therapy for treatment of Cd-associated memory dysfunction.
References
Abdalla FH, Cardoso AC, Pereira LB, Schmatz R, Goncalves JF, Stefanello N, Fiorenza AM, Gutierres JM, Serres JDS, Zanini D, Pimentel VC, Vieira JM, Schetinger MRC, Morsch VM, Mazzanti CM (2013) Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem 381:1–8
Abdalla FH, Schmatz R, Cardoso AC, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Goncalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Goncalves JF, Mazzanti CM (2014) Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na1, K1- ATPase activities. Physiol Behav 135:152–167
Adeniyi PA, Ishola AO, Laoye BJ, Olatunji BP, Bankole OO, Shallie PD, Ogundele OM (2016) Neural and behavioural changes in male periadolescent mice after prolonged nicotine-MDMA treatment. Metab Brain Dis 30:15
Akinyemi AJ, Thome GR, Morsch VM, Stefanello N, da Costa P, Cardoso A, Goularte JF, Bello-Klein A, Akindahunsi AA, Oboh G, Schetinger MRC (2016) Effect of dietary supplementation of ginger and turmeric rhizomes on ectonucleotidases, adenosine deaminase and acetylcholinesterase activities in synaptosomes from the cerebral cortex of hypertensive rats. J Appl Biomed 14:59–70
Anamika B (2012) Extraction of curcumin. J environ Sci Toxicol. Food Technol 1:1–16
Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK (2011) Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22:14–20
Bouayed J, Rammal H, Soulimani R (2009) Oxidative stress and anxiety. Oxidative Med Cell Longev 2:63–67
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:218–254
Burnstock G (2006) Purinergic signalling. Br J Pharmacol 147:172–181
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97:320–324
Chacon MA, Reyes AE, Inestrosa NC (2003) Acetylcholinesterase induces neuronal cell loss, astrocyte hypertrophy and behavioral deficits in mammalian hippocampus. J Neurochem 87:195–204
Costa P, Goncalves JF, Baldissarelli J, Mann TR, Abdalla FH, Fiorenza AM, da Rosa MM, Carvalho FB, Gutierres JM, de Andrade CM, Rubin MA, Schetinger MRC, Morsch VM (2015) Curcumin attenuates memory deficits and the impairment of cholinergic and purinergic signaling in rats chronically exposed to cadmium. Environ Toxicol doi:10.1002/tox.22213.
Deiana S, Platt B, Riedel G (2011) The cholinergic system and spatial learning. Behav Brain Res 221:389–411
Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y (2007) Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol 179:1884–1892
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V, Jr Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A (1997) Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol 120:1328–1334
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS (2015) The role of the nitric oxide pathway in brain injury and its treatment — from bench to bedside. Exp Neurol 263:235–243
Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the nervous system. Annu Rev Physiol 57:683–706
Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60
Goncalves JF, Nicoloso FT, da Costa P, Farias JG, Carvalho FB, da Rosa MM, Gutierres JM, Abdalla FH, Pereira JS, Dias GR, Barbosa NB, Dressler VL, Rubin MA, Morsch VM, Schetinger MR (2012) Behavior and brain enzymatic changes after longterm intoxication with cadmium salt or contaminated potatoes. Food Chem Toxicol 50:3709–3718
Griffiths S, Scott H, Glover C, Bienenmann A, Ghorbel MT, Uney J (2008) Expression of long term depression underlies visual recognition memory. Neuron 58:186–194
Guisti G, Galanti B (1984) Colorimetric method. In: Bergmeyer HU (eds) Methods of Enzymatic Analysis, Verlag Chemie Weinheim, pp 315–323
Gutierres JM, Carvalho FB, Schetinger MR, Rodrigues MV, Schmatz R, Pimentel VC, Vieira JM, Rosa MM, Marisco P, Ribeiro DA, Leal C, Rubin MA, Mazzanti CM, Spanevello RM (2012) Protective effects of anthocyanins on the ectonucleotidase activity in the impairment of memory induced by scopolamine in adult rats. Life Sci 91:1221–1228
Hashiguchi W, Nagatomo I, Akasaki Y, Uchida M, Tominaga M, Takigawa M (2001) Influences of caffeine to nitric oxide production and zonisamide concentration in the brain of seizuresusceptible EL mice. Psychiat. Clin Neurosci 55:319–324
Jaques JAS, Rezer JFP, Ruchel JB, Becker LV, Rosa CS, Souza VCG, Luz SCA, Gutierres JM, Goncalves JF, Morsch VM, Schetinger MRC, Leal DBR (2011) Lung and blood lymphocytes NTPDase and acetylcholinesterase activity in cigarette smoke-exposed rats treated with curcumin. Biom Prev Nutr 1:109–115
Jaques JAS, Rezer JFP, Carvalho FB, Rosa MM, Gutierres JM, Goncalves JF, Schmatz R, Bairros AV, Mazzanti CM, Rubin MA, Schetinger MRC, Leal DBR (2012) Curcumin protects against cigarette smoke-induced cognitive impairment and increased acetylcholinesterase activity in rats. Physiol Behav 106:664–669
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. BioMetals 23:783–792
Klinkenberg I, Sambeth A, Blokland A (2011) Acetylcholine and attention. Behav Brain Res 221:430–442
Kuhad A, Pilkhwal S, Sharma S, Tirkey N, Chopra K (2007) Effect of curcumin on inflammation and oxidative stress in cisplatin induced experimental nephrotoxicity. J Agric Food Chem 55:10150–10155
Kumar A, Dogra S, Prakash A (2009) Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioral and biochemical alterations in rats. Behav Brain Res 205:384–390
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153
Mendez-Armenta M, Barroso-Moguel R, Villeda-Hernandez J, Nava-Ruiz C, Rios C (2001) Histopathological alterations in the brain regions of rats after perinatal combined treatment with cadmium and dexamethasone. Toxicol 161:189–199
Mendez-Armenta M, Villeda-Hernandez J, Barroso-Moguel R, Nava-Ruiz C, Jimenez-Capdeville ME, Rios C (2003) Brain regional lipid peroxidation and metallothionein levels of developing rats exposed to cadmium and dexamethasone. Toxicol Lett 144:151–157
Miranda KM, Espay MG,Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite.Nitric Oxide 5:62–71
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A (2002) Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ Health Perspect 110:1185–1190
Pan R, Qiu S, DX L, Dong J (2008) Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chinese. Med J 121:832–839
Pari L, Murugavel P (2007) Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicol 234:44–50
Paul V, Ekambaram P (2011) Involvement of nitric oxide in learning & memory processes. Indian J Med Res 133:471–478
Paul V, Subramanian EH (2002) Evidence for an involvement of nitric oxide and raminibutyric acid in the anticonvulsant action of L-arginine on picrotoxin induced convulsions in rats. Pharmacol Biochem Behav 72:515–519
Ramani K, Tomasi ML, Yang H, Ko K, SC L (2012) Mechanism and significance of changes in glutamate-cysteine ligase expression during hepatic fibrogenesis. J Biol Chem 287:36341–36355
Reeta KH, Mehla J, Gupta YK (2009) Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res 1301:52–60
Schmatz R, Schetinger MRC, Spanevello RM, Mazzanti CM, Stefanello N, Maldonado PA, Gutierres J, Corrêa MC, Girotto E, Moretto MB, Morsch VM (2009) Effects of resveratrol on nucleotide degrading enzymes in streptozotocin induced diabetic rats. Life Sci 84:345–350
Shen C, Yang B, Zhou T, Duan G, Yu Y (2011) Bioequivalence evaluation of two brands of rivastigmine of different salt forms, an acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease, in healthy Beagle dogs. Pharm 66:590–593
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405
Sinha M, Manna P, Sil PC (2009) Induction of necrosis in cadmium-induced hepatic oxidative stress and its prevention by the prophylactic properties of taurine. J Trace Elem Med Biol 23:300–313
Soreq H, Seidman S (2001) Acetylcholinesterase—new roles for an old actor. Nat Rev Neurosci 2:294–302
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545
Takeda A, Takefuta S, Ijiro H, Okada S, Oku N (1999) Cd transport in rat brain. Brain Res Bull 49:453–459
Tang H, Lu D, Pan R, Qin R, Xiong H, Dong J (2009) Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci 85:1–10
Yadav RS, Chandravanshi LP, Shukla RK, Sankhwar ML, Ansari RW, Shukla PK, Pant AB, Khanna VK (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicol 32:760–768
Zalups RK, Ahmad S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188
Acknowledgments
One of the authors (Ayodele Jacob Akinyemi) is a beneficiary of 2015 IBRO/ARC bursary award and wish to thank the organization for their support towards this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Rights and permissions
About this article
Cite this article
Akinyemi, A.J., Okonkwo, P.K., Faboya, O.A. et al. Curcumin improves episodic memory in cadmium induced memory impairment through inhibition of acetylcholinesterase and adenosine deaminase activities in a rat model. Metab Brain Dis 32, 87–95 (2017). https://doi.org/10.1007/s11011-016-9887-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9887-x