Abstract
Agomelatine (AGOM) as an antidepressant acts both as a melatonin-receptor agonist and a selective serotonin-receptor antagonist. As a potent melatonin derived antioxidant, AGOM might modulate depression-induced lipid peroxidation and pro-inflammatory cytokines in brain, kidney and liver. The present study explores whether AGOM protects against experimental depression-induced brain, kidney and liver oxidative stress, and plasma cytokine production in rats with chronic mild stress (CMS)-induced depression. Thirty-six rats were divided into four groups. The first group was used as an untreated control. The second group received AGOM for 4 weeks. The third group was exposed to chronic mild stress (CMS) of 4 weeks for induction depression. The fourth group received 40 mg/kg AGOM and CMS for 4 weeks. Liver and kidney lipid peroxidation levels were high in the CMS group although they were low in AGOM treatments. AGOM and AGOM + CMS treatments increased the lowered glutathione peroxidase activity and reduced glutathione levels in brain, kidney and liver of CMS group. β-carotene, vitamin A and vitamin E concentrations in the brain, kidney and liver of the four groups were not changed by CMS and AGOM treatments. However, plasma TNF-α, interleukin (IL)-1β, and IL-4 levels were high in the CMS and AGOM group and their levels were further increased by the AGOM + CMS treatment. In conclusions, AGOM induced protective effects against experimental depression-induced brain, kidney, and liver oxidative injuries through regulation of the glutathione concentrations and glutathione peroxidase activity. However, plasma cytokine productions were increased by the AGOM treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress occurs in the body cells during physiological functions including phagocytosis and mitochondrial functions. Accumulation evidences indicated that reactive oxygen species (ROS) play an important role in the pathogenesis of many diseases, particularly in neurological and psychiatric diseases due to the high content of polyunsaturated fatty acids and oxygen consumption and poor antioxidant content of central nervous system (Halliwell 2006; Nazıroğlu 2007). If the oxidative stress is not controlled by antioxidants, it can induce neuron brain damages (Halliwell 2006; Nazıroğlu 2007). ROS are scavenged by enzymatic antioxidants including glutathione peroxidase (GSH-Px) (Nazıroğlu 2009) and non-enzymatic antioxidants, such as reduced glutathione (GSH), vitamin A, and vitamin E (Halliwell 2006). Depression is characterized by activation of the inflammatory response system with increased production of pro-cytokines (Andreasson et al. 2007; Cyranowski et al. 2007). It is well known that pro-inflammatory cytokines-induced excessive oxidative stress increase ROS production (Kahya et al. 2015). Oxidative stress increases also psychological stress, which accompanies severe depression (Nazıroğlu and Demirdaş 2015). Additional reports have indicated that lipid peroxidation and antioxidants, such as GSH, GSH-Px, and vitamin E, can act as biomarkers for depression’s neuronal oxidative stress (Eren et al. 2007a, b; Kumar et al. 2015). Due to highly relationship between inflammation and intracellular oxidative stress, simultaneous investigation of anti-inflammatory and antioxidant activities of antidepressant is a worthwhile approach in the development of promising potent anti-depressive agents.
Melatonin is a strong antioxidant (Espino et al. 2011) and the central nervous system is rich for G protein-coupled melatonin receptors namely MT1 and MT2 (Ekmekcioglu 2006; Tardito et al. 2012). Agomelatine (AGOM) as a melatonin analogue is a selective melatonergic MT1/MT2 dual agonist (Demyttenaere 2011). AGOM is also a selective serotonin-receptor antagonist at 5-HT2C receptors (Millan et al. 2003). Hence, AGOM has been licensed for the treatment of major depressive disorder in adults since 2009 in the European Union (Freiesleben and Furczyk 2015). AGOM is a unique antidepressant although there are concerns on AGOM-induced liver injury in human (Voican et al. 2014). Antioxidant role of AGOM was reported in different cells (Gupta and Sharma 2014; Karakus et al. 2013; Inanir et al. 2015) although the results are conflicting.
Chronic mild stress (CMS) as an animal model is a valuable model for investigation of pathophysiological changes related to major depression and the effects of antidepressant therapies (Nestler et al. 2002; Eren et al. 2007a, b). In experimental animals, the CMS model attempts to represent most of the abnormalities associated with human depressive disorders. For example, CMS was reported to induce depression, which could be reversed by antidepressant treatment (Willner 1997). The CMS has been using in experimental animal studies for investigating interactions of antioxidant and antidepressant (Eren et al. 2007a, b). The animal model was also preferred in a study examining the effect of AGOM (Dagyte et al. 2011).
AGOM is derived from strong antioxidant melatonin (Freiesleben and Furczyk 2015) and it may induce antioxidant and anti-inflammatory effects on tissue and blood in rat. In addition concern on its hepatotoxicity effect has not been clarified yet. Thus, the we aimed to investigate whether or not AGOM has protective effects on the CMS-induced brain, liver, and kidney oxidative damage in rats. With stress and sucrose testing, the rats were given one synergic treatment of AGOM. We then assessed the brain, liver, kidney and plasma in terms of antioxidant and cytokine levels resulting from the treatments. We will discuss the obtained results with the intention of explaining AGOM’s mechanism of action on CMS-induced depression in brain, liver, kidney and blood injury.
Materials and methods
Animals and study groups
The study used 36 (10 Weeks) male Wistar albino rats weighing 180 ± 30 g were obtained from the Animal Research Laboratory at Suleyman Demirel University (SDU). The animals were kept at the laboratory and were allowed to feed ad libitum on a commercially available rat chow. The study plan was approved by the Local Experimental Animal Ethical Committee of Suleyman Demirel University (SDU) (Protocol number: 19.02.2014–01). The animals were also maintained and used according to the Animal Welfare Act and the Guide for the Care and Use of Laboratory.
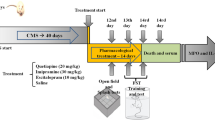
The rats were divided into four groups as follows: The control group (n = 8) had no chronic mild stress (CMS) and was not supplemented. They received DMSO (0.1 ml) via intraperitonel injection (IP) for 4 weeks. In the AGOM group (n = 10), the rats received IP 40 mg/kg/day AGOM for 4 weeks of CMS (Karakus et al. 2013; Aygün et al. 2015). The AGOM was dissolved in a DMSO solution (0.1 ml). Stock AGOM (Sigma Chemical Co., St. Louis, MO, USA) was freshly 100 times diluted to its final concentration (40 mg/kg) in 0.9 % w/v sterile saline solution. A total of 28 doses were given to the animals in the groups within 28 days. In the depression group (n = 8), experimental depression for 4 weeks was induced in the rats by CMS (Eren et al. 2007a; b). In the CMS + AGOM group (n = 10), rats were administrated with IP AGOM (same as the AGOM group) with CMS induction (same as the CMS group).
12 h after the last AGOM dose administration, all rats were killed via cardiac puncture under ether anesthesia in accordance with SDU experimental animal legislation. The blood, brain (cortex), liver and kidney samples were isolated as described in a previous study (Kahya et al. 2015). At the end of the experiments, half of the tissue and blood samples were washed with phosphate buffer (pH 7.2) and immediately used for lipid peroxidation, GSH-Px, and GSH analyses. The remaining tissue and plasma samples were then frozen at −33 °C. Antioxidant vitamins and cytokine analyses were performed within one month.
During the analyses, the tissue samples was placed on ice, cut into small pieces using scissors, and homogenized (2 min at 5000 rpm) in 5 volumes (1:5, w/v) of ice-cold Tris-HCl buffer (50 mM, pH 7.4), by a ultrasonic homogenization (SONOPULS HD 2070, Bandelin Electronic, Berlin, Germany). All preparation procedures were performed on ice.
Induction of depression with chronic mild stress (CMS)
Procedures of inducing CMS have been described previously (Eren et al. 2007a; b). The CMS group was exposed to the following stressors in random order: continuous overnight illumination, 40 °C cage tilt, paired housing, damp bedding (300 ml water spilled into bedding), exposure to an empty water bottle immediately following a period of acute water deprivation, stroboscopic illumination (300 flashes/min), and white noise (approx. 90 dB). The stressors were presented in the order shown during the first week and repeated each of the following weeks for a total of 4 weeks. Control animals were left undisturbed in the home cages.
Sucrose preference tests
Sucrose preference tests, as performed previously (Eren et al. 2007a, b) were used to operationally define anhedonia. Specifically, anhedonia was defined as a reduction in sucrose intake and sucrose preference relative to the intake and preference of the control group. A sucrose preference test consisted of first removing the food and water from each rat’s cage (both CMS and control groups) for a period of 20 h. Water and 1 % sucrose were then placed on the cages in pre-weighed glass bottles, and animals were allowed to consume the fluids freely for a period of 1 h. Two baseline preference tests were performed, separated by at least 5 days, and the results were averaged. A preference test was also conducted following the 4-week CMS period.
Lipid peroxidation analyses
Lipid peroxidation levels in the brain, liver and kidney homogenates were measured with the thiobarbituric-acid reaction by the method of Placer et al. (1966) as described in a previous study (Kahya et al. 2015). The values of lipid peroxidation in the brain, liver and kidney samples were expressed as μmol/g protein.
Reduced glutathione (GSH), glutathione peroxidase (GSH-Px) and protein assays
The GSH contents of the brain, liver and kidney homogenates were measured at 412 nm using the method of Sedlak and Lindsay (1968). GSH-Px activities of the brain, liver and kidney homogenates were assayed spectrophotometrically (UV-1800, Shimadzu, Kyoto, Japan) at 37 °C and 412 nm according to method of the Lawrence and Burk method (1976). Decrease of enzymatic reaction in absorbance at 412 nm against blank was measured spectrophotometrically (UV-1800, Shimadzu, Kyoto, Japan). GSH-Px activity and GSH level in the brain, liver and kidney homogenates were expressed as μmol/g protein. The protein contents in the brain, liver and kidney were measured by method of Lowry et al. (1951) with bovine serum albumin as the standard.
β-carotene, vitamins a and vitamin E analyses in the brain, liver and kidney samples
Vitamins A (retinol) and E (α-tocopherol) were determined in the brain cortex samples by a modification of the method described by Desai (1984) and Suzuki and Katoh (1990). About 0.25 g of the brain, liver and kidney samples were saponified by the addition of 0.3 ml of satured (60 %, w/v in water) KOH and two ml of 1 % (w/v in ethanol) pyrogallol, followed by shaking in water bath at 70 °C for 30 min. Two ml of water and 1 ml of n-hexane on ice were added and mixed with the samples that were then rested for 10 min to allow phase separation. Spectrophotometric determinations of vitamin A and vitamin E used absorbance wavelengths of 325 and 532 nm, respectively (UV-1800, Shimadzu, Kyoto, Japan). Calibrations were performed using standard solutions of all-trans retinol and α-tocopherol in hexane. Vitamin A and E concentrations in the brain, liver and kidney were expressed as μmol/g tissue.
For β-carotene analyses in the brain, liver and kidney samples, the method of Suzuki and Katoh (1990) was used. Two milliliters of hexane were mixed with 0.25 g of brain, liver and kidney samples. The value of β- carotene in hexane was measured at 453 nm in a spectrophotometer and pure hexane was used as blank. The β-carotene concentrations in the brain, liver and kidney were expressed as μmol/g tissue.
Cytokine determinations in plasma
Plasma cytokine [TNF-α, interleukin (IL)-1β and IL-4] levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s instructions as described in previous studies (Senol et al. 2014) (Multimode microplate reader, Infinite® 200 PRO series, Männedorf Switzerland). All ELISA kits were purchased from DRG Inc. (Marburg, Germany). Determinations were made in duplicate, and TNF-α, IL-1β and IL-4 results are expressed in nano gram (ng) and picogram (pg) per milliliter, respectively.
Statistical analysis
The results are presented as means ± standard deviation (SD). The SPSS Statistical program (17.0, SPSS Inc. Chicago, Illinois, USA) was used for the statistical treatment of the data. The Mann-Whitney U-test was used to establish the significance of differences among the four groups. The significance level was set at p < 0.05.
Results
Results of sucrose test
Sucrose (1 %, w/v) test results of study groups is shown in Table 1 and consumption of water with sucrose at 3rd and 4th weeks as ml/kg body weight was significantly (p < 0.05) lower in CMS-induced depression group than in basal values. In addition, consumption of water with sucrose at 3rd week as ml/kg body weight was significantly (P < 0.05) lower in CMS group than in AGOM groups.
Results of lipid peroxidation in brain, liver and kidney
Lipid peroxidation level as malondialdehyde (MDA) acts as a biomarker of oxidative stress (Placer et al. 1966). Research reports that patients with depression have high levels of lipid peroxidation due to activation of inflammatory and mitochondrial redox systems (Demyttenaere 2011) although AGOM induced antioxidant role through inhibition of melatonin receptors and mitochondrial redox systems (Kumar et al. 2015). We evaluated the inhibitory activities against oxidative stress and the scavenging activities of AGOM in the brain (Table 2), liver (Table 3) and kidney (Table 4) of rats with CMS-induced depression. The results showed that lipid peroxidation levels in the liver and kidney in the CMS group were significantly (p < 0.05) higher than the control. Hence, oxidative stress levels in the rats’ liver and kidney were increased by depression induction. However, AGOM treatment decreased the lipid peroxidation levels of the rats’ liver, and kidney. The lipid peroxidation levels in the liver (p < 0.001) and kidney (p < 0.05) in the AGOM and AGOM + CMS groups were markedly lower than in the CMS group.
Results of glutathione peroxidase (GSH-Px) activity and reduced glutathione (GSH) level in brain, liver and kidney
Tables 2, 3 and 4 present the mean GSH-Px activities and GSH results for the four groups in terms of the brain, liver and kidney, respectively. The results showed that the brain, liver and kidney GSH-Px activities (p < 0.05 and p < 0.001) and GSH (p < 0.05) levels were significantly lower in the CMS group compared to the control group. However, GSH and GSH-Px values were markedly (p < 0.05 and p < 0.001) increased in the AGOM and AGOM + CMS groups by the AGOM treatments.
Results of antioxidant vitamin concentrations in brain, liver and kidney
Tables 2, 3 and 4 also provide the mean vitamin A, β-carotene and vitamin E concentrations in the four groups’ brain, liver, and kidney. The vitamin A, β-carotene and vitamin E concentrations were not changed in the four groups by AGOM treatment and CMS exposure.
Results of cytokines in plasma
Figures 1, 2, and 3 show the mean IL-1β, IL-4, and TNF-α levels in the plasma of the four groups. Mean IL-1β levels as ng/ml in the control, AGOM, CMS, and CMS + AGOM groups were 71, 80, 78, and 90, respectively. Mean TNF-α levels as ng/ml in the four groups were 64, 92, 80, and 106, respectively. Mean IL-4 levels as pg/ml in the four groups were 69, 80, 78, and 96, respectively. The IL-1β (p < 0.05), IL-4 (p < 0.05), and TNF-α (p < 0.001) levels significantly increased in the plasma of rats with CMS-induced depression and these levels further increased with AGOM and CMS + AGOM treatments (p < 0.05 and p < 0.001).
Discussion
We found that CMS-induced depression increased lipid peroxidation levels in the liver and kidney as well as TNF-α, IL-1β, and IL-4 levels in plasma. However, GSH-Px activity and GSH level were decreased by induction of CMS depression. Therefore, CMS-induced depression in the animals was characterized by increased oxidative stress and cytokine production along with decreased antioxidant levels. AGOM administration decreased lipid peroxidation although GSH-Px activity, GSH, TNF-α, IL-1β, and IL-4 levels increased. We have thus shown that AGOM-based treatments modulated the balance of pro- and antioxidant in rats and ringdoves by down-regulating the levels of oxidative stress while up-regulating GSH redox system. To the best of our knowledge, the current study is the first to compare AGOM with particular reference to its effects on cytokine production and antioxidant redox systems in CMS-induced oxidative injury in rats.
Accumulative evidences indicate that depression is characterized by activation of the inflammatory response system with increased production of pro-cytokines (Andreasson et al. 2007; Cyranowski et al. 2007; Nazıroğlu and Demirdaş 2015). It is well known that activation of phagocytic cells by pro-inflammatory cytokines leads to excessive production of ROS, which leads to an increase the levels of lipid peroxidation as MDA through oxidation of cell membrane polyunsaturated fatty acids, and causes loss of fluidity the biological membrane. As a result of those alterations, the biological membranes induce cytokine production (Halliwell 2006). On the other hand, excessive ROS production induces phospholipase A2 activation, which changes receptor functions in the cell membranes, induces immune cells, and leads to secretion of interleukins from T cells (Sierra-Honigmann and Murphy 1992). Liver cytochrome P450 enzyme is important physiological enzyme in detoxification of ROS products in body. 90 % of AGOM is metabolized through the liver by the hepatic cytochrome P450 (Freiesleben and Furczyk 2015). Up to 80 % of AGOM is eliminated in urine as pharmacologically inactive metabolites, the main metabolites being hydroxylated and demethylated AGOM (Freiesleben and Furczyk 2015). In the current study, liver and kidney lipid peroxidation and cytokine levels are increased in CMS-induced rats due to inflammatory characterization of depression. In the current study, liver and kidney lipid peroxidation and plasma cytokine levels are increased in the rats by induction of depression although liver and kidney lipid peroxidation levels are decreased by AGOM treatment and it might be modulation of hepatic cytochrome P450 (Freiesleben and Furczyk 2015). Brain lipid peroxidation level did not change in the four groups statistically. Adaptative antioxidant responses of brain were accompanied by liver antioxidant down-regulation.
Existing evidence indicates that excessive generation of ROS might contribute to the onset of symptoms in CMS-induced depression (Eren et al. 2007a; b). This effect can be related, at least in part, to a reduction in specific endogenous antioxidant redox system, such as a decrease in GSH levels and decreased activity of antioxidant defense enzymes, including GSH-Px (Eren et al. 2007a, b; Nazıroğlu and Demirdaş 2015). Accumulation evidences support that the oxidative process significantly contributes to CMS-induced depression oxidative toxicity (Eren et al. 2007a, b). Results of recent reports indicate that the production of ROS is a key contributing factor in the pathology of depression (Demyttenaere 2011). AGOM treatment markedly, attenuates depleted antioxidant system due to excessive oxidative stress. It has been also reported that activation of melatonin receptors up-regulate gene expression of the antioxidant enzyme (Gupta and Sharma 2014). Melatonin receptor agonists have been reported to exert direct ROS scavenging activity and indirect antioxidant activity via its stimulatory actions on the antioxidant system (Kharwar and Haldar 2012). It was reported that modulation of melatonin receptor pathway through melatonin agonist induces ROS scavenger effect (Shirazi et al. 2013). Increased mitochondrial membrane depolarization through overload calcium ion (Ca2+) entry induces excessive ROS production in cells including neurons (Balaban et al. 2016). It has been reported that AGOM modulated intestinal and neuronal mitochondrial function and Ca2+ entry (de Mello et al. 2015; Kumar et al. 2015), which resulted in improvement of in antioxidant redox pathway in animals. We observed decreased lipid peroxidation levels in the liver and kidney of experimental depression-induced rats through AGOM treatment. However, GSH-Px activity and GSH levels were increased in brain, liver and kidney of the rats with CMS by AGOM treatment. Hence, AGOM treatment in the current study shows antioxidant effects through stimulation of melatonin receptors and regulation of mitochondrial function; this may be due to activation of the GSH enzyme redox system. Antioxidant vitamin concentrations in brain, liver and kidney did not change in the four groups. Adaptative antioxidant responses of antioxidant vitamins were accompanied by GSH-Px enzymatic activity up-regulation.
There are limited studies on antioxidant effects of AGOM. Gupta and Sharma (2014) reported similarly that administration of agomelatine did not show any significant effect on the levels of MDA and GSH in brain striatum of mice. Increased lipid peroxidation and decreased GSH levels were decreased in ileum of rats with prenatal valproic acid-induced autism spectrum disorder by AGOM (Kumar et al. 2015). In a study with PC-12 neuronal cell line conducted by our groups (Akpinar et al. 2014), the decreased lipid peroxidation levels were observed low in the AGOM group. In addition, the same study showed that GSH level and GSH-Px activity were high in the AGOM group, indicating an antioxidant role of AGOM in the neurons.
Accumulating evidence suggests that depression is associated with increased neuroinflammation and phagocytic activation (Cyranowski et al. 2007). A high level of pro-inflammatory cytokines, including TNF-α, IL-4, and IL-1α, exist in the plasma of patients with depression (Andreasson et al. 2007; Cyranowski et al. 2007). The generation of ROS and the activation of the NADPH oxidase redox system induced by depression play a central role in the pathogenesis of depression (Bakunina et al. 2015), and these radicals stimulate the production of cytokines (Vaváková et al. 2015). A role for TNF-α and IL-1α as mediators of the depression in cytochrome P450 activity in brain and liver during central nervous system inflammation is reported by Nicholson and Renton (2001) and they observed that the production of the cytokine production within the brain did not participate in the signaling process in the brain that leads to the concomitant loss of CYP1A activity in the liver (Nicholson and Renton 2001). AGOM is mostly metabolized through the liver by the hepatic cytochrome P450 (Freiesleben and Furczyk 2015). Reports show that TNF-α, IL-4, and IL-1α, levels contribute to brain-cell failure through depression-induced oxidative stress, microglia and phagocytic cell activations (Nicholson and Renton 2001; Bakunina et al. 2015; Vaváková et al. 2015). To our knowledge, there is no report on the effects of AGOM and melatonin in plasma TNF-α, IL-4, and IL-1α levels in rats with CMS-induced depression. In the current study, CMS-induced increase of plasma TNF-α, IL-4, and IL-1α levels was further increased through modulation of the antioxidant redox system with AGOM treatments due to activation of hepatic cytochrome P450.
In conclusion, we used an experimental animal model to demonstrate that AGOM administration can prevent depression-induced brain, liver and kidney toxicity but it stimulates cytokine production. In addition, the mechanisms underlying the protective properties of AGOM include the antioxidant. Therefore, AGOM can reduce lipid peroxidation levels in the brain, liver, and kidney of rats with depression by virtue of their inherent antioxidant properties. Toxic effect of AGOM on liver might be induced by increase of plasma cytokine production. In consequence, AGOM treatment may have a beneficial effect in managing depression as well as their oxidant complications.
Abbreviations
- AGOM:
-
agomelatine
- CMS:
-
chronic mild stress
- GSH:
-
reduced glutathione
- GSH-Px:
-
glutathione peroxidase
- ROS:
-
reactive oxygen species
References
Akpinar A, Uğuz AC, Nazıroğlu M (2014) Agomelatine and duloxetine synergistically modulates apoptotic pathway by inhibiting oxidative stress triggered intracellular calcium entry in neuronal PC12 cells: role of TRPM2 and voltage-gated calcium channels. J Membr Biol 247:451–459
Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M (2007) A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun 21:147–152
Aygün H, Aydın D, İnanır S, Ekici F, Ayyıldız M, Ağar E (2015) The effects of agomelatine and melatonin on ECoG activity of absence epilepsy model in WAG/Rij rats. Turk J Biol 39:904–910
Bakunina N, Pariante CM, Zunszain PA (2015) Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144:365–373
Balaban H, Nazıroğlu M, Demirci K (2016) The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: The involvement of TRPM2 and TRPV1 channels. doi:10.1007/s12035-016-9835-0
Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA (2007) Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 21:229–237
Dagyte D, Crescente I, Postema F, Seguin L, Gabriel C, Mocaer E, Den Boer JA, Jaap Koolhaas JM (2011) Agomelatine reverses the decrease in hippocampal cell survival induced by chronic mild stress. Behav Brain Res 218:121–128
de Mello AH, da Rosa SL, Moreira Cereja AC, de Bona SR, Florentino D, Modolon Martins M, Petronilho F, Quevedo J, Tezza Rezin G (2015) Effect of subchronic administration of agomelatine on brain energy metabolism and oxidative stress parameters in rats. Psychiatry Clin Neurosci. doi:10.1111/pcn.12371
Demyttenaere K (2011) Agomelatine: a narrative review. Eur Neuropsychopharmacol 21: S703–S709.
Desai ID (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Ekmekcioglu C. (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 60:97–108.
Eren I, Nazıroğlu M, Demirdaş A (2007a) Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res 32:1188–1195
Eren I, Naziroğlu M, Demirdaş A, Celik O, Uğuz AC, Altunbaşak A, Ozmen I, Uz E (2007b) Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem Res 32:497–505
Espino J, Bejarano I, Paredes SD, Barriga C, Rodríguez AB, Pariente JA (2011) Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J Pineal Res 51:195–206
Freiesleben SD, Furczyk K (2015) A systematic review of agomelatine-induced liver injury. J Mol Psych 3:4
Gupta S, Sharma B (2014) Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol Biochem Behav 122:122–135
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Inanir S, Copoglu US, Kokacya H, Dokuyucu R, Erbas O, Inanır A (2015) Agomelatine protection in an LPS-induced psychosis-relevant behavior model. Med Sci Monit 21:3834–3839
Kahya MC, Naziroğlu M, Çiğ B. (2015) Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj 29:1490–1406.
Karakus E, Halici Z, Albayrak A, Polat B, Bayir Y, Kiki I, Cadirci E, Topcu A, Aksak S (2013) Agomelatine: An antidepressant with new potent hepatoprotective effects on paracetamol-induced liver damage in rats. Hum Exp Toxicol 32:846–857
Kharwar RK, Haldar C (2012) Daily variation in antioxidant enzymes and lipid peroxidation in lungs of a tropical bird Perdicula asiatica: role of melatonin and nuclear receptor RORα. Comp Biochem Physiol A Mol Integr Physiol 162:296–302
Kumar H, Sharma BM, Sharma B (2015) Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem Int 91:34–45
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin- Phenol reagent. J Biol Chem 193:265–275
Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D (2003) The novel melatonin agonist Agomelatine (S20098) Is anantagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306:954–964
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Nazıroğlu M, Demirdaş A (2015) Psychiatric disorders and TRP channels: Focus on psychotropic drugs. Curr Neuropharmacol 13:248–257
Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. (2002) Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528
Nicholson TE, Renton KW (2001) Role of cytokines in the lipopolysaccharide-evoked depression of cytochrome P450 in the brain and liver. Biochem Pharmacol 62:1709–1717
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann’ s reagent. Anal Biochem 25:192–205
Senol N, Nazıroğlu M, Yürüker V (2014) N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res 39:685–692
Shirazi A, Mihandoost E, Ghobadi G, Mohseni M, Ghazi-Khansari M (2013) Evaluation of radioprotective effect of melatonin onwhole body irradiation induced liver tissue damage. Cell J 14:292–297
Sierra-Honigmann MR, Murphy PA (1992) Suppression of interleukin–1 action by phospholipase-A2 inhibitors in helper T lymphocytes. Pept Res 5:258–261
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Tardito D, Molteni R, Popoli M, Racagni G (2012) Synergisticmechanisms involved in the antidepressant effects of agomelatine. Eur Neuropsychopharmacol 22:S482–S486
Vaváková M, Ďuračková Z, Trebatická J (2015) Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Med Cell Longev 12:898393
Voican CS, Corruble E, Naveau S, Perlemuter G (2014) Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry 171:404–415
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329.
Acknowledgments
The abstract of the study was submitted to the 6th World Congress of Oxidative Stress, Calcium Signaling and TRP Channels, held 24 and 27 May 2016 in Isparta, Turkey (www.cmos.org.tr). The authors wish to thank researcher Bilal Çiğ and technician Muhammet Şahin (Neuroscience Research Center, SDU, Isparta, Turkey) for helping with the cytokine, lipid peroxidation and antioxidant analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
AD and MN formulated the hypothesis and was responsible for writing the report. GÖÜ was responsible for the animal experiments.
Compliance with ethical standards
ᅟ
Financial disclosure
There is no financial disclosure for the current study.
Conflict of interest
None of the authors have any conflicts to disclose. All authors approved the final manuscript.
Rights and permissions
About this article
Cite this article
Demirdaş, A., Nazıroğlu, M. & Ünal, G.Ö. Agomelatine reduces brain, kidney and liver oxidative stress but increases plasma cytokine production in the rats with chronic mild stress-induced depression. Metab Brain Dis 31, 1445–1453 (2016). https://doi.org/10.1007/s11011-016-9874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9874-2