Abstract
Alchemilla viridiflora Rothm., Rosaceae is a herbaceous plant widespread in central Greece, Bulgaria, North Macedonia and Serbia with Kosovo. Liquid chromatography-mass spectrometry analysis leads to the identification of 20 compounds in methanol extract, mainly ellagitannins and flavonoid glycosides. Given that various plant extracts have traditionally been used to treat hypertension and that some of the analyzed methanol extract constituents have beneficial cardiovascular effects, we hypothesized that some of these effects are achieved by inhibiting angiotensin I-converting enzyme (ACE). The dose-dependent ACE inhibitory activities of A. viridiflora and miquelianin were observed with an IC50 of 2.51 ± 0.00 µg/mL of A. viridiflora extract compared to the IC50 of 5.4139 ± 0.00 µM for miquelianin. The contribution of the single compounds to the tested activity was further analyzed through the in silico experimental approach. Computational docking results showed that tiliroside, ellagic acid pentose and galloyl-hexahydroxydiphenoyl-glucose exhibited even better binding affinity for the ACE active site than miquelianin, for which ACE activity was confirmed by an in vitro assay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic arterial hypertension is a common disorder and the most important risk factor for general morbidity and mortality. It is also associated with an increased risk of developing cardiovascular disease. The number of people worldwide with high blood pressure has reached 1.13 billion, according to new research. If not treated properly, hypertension can increase the risk of heart thrombosis, heart attack or renal failure [1, 2]. According to the latest recommendations, angiotensin I-converting enzyme (ACE) inhibitors, alone or combined with other antihypertensive drugs, are the drugs of choice to treat patients with hypertension due to their ability to effectively lower the mean, systolic and diastolic pressures in hypertensive patients [3].
However, serious safety concerns regarding synthetic ACE inhibitors, such as hypotension, renal insufficiency, and hyperkalaemia, have led to a growing interest in searching for other sources of potent ACE inhibitors [3]. Various completed and ongoing studies have shown that many plants, plant extracts and secondary plant metabolites possess ACE inhibitory activity. Among secondary plant metabolites, flavonoids, as strong antioxidants, are considered to be the most active inhibitors [4]. Regarding essential oils, a few studies have also demonstrated their ability to inhibit ACE inhibition [5,6,7,8]. Also, tannins are found to be non-specific inhibitors of ACE, through sequestration of the enzyme metal cofactor (Zn2+), precipitation of protein or as non-specific enzyme (trypsin and chymotrypsin) inhibitors [9]. It has been reported that the extracts obtained from different parts of the plants from various families (leaves, stems, stem barks, roots, fruits, aerial parts or even the whole plant) can inhibit ACE. Most of the plant species exhibiting ACE inhibition belong to the family Fabaceae, but only a few to the family Rosaceae [4]. However, species from the genus Alchemilla L., Rosaceae, although known as good sources of phenolic compounds, tannins and flavonoid glycosides, have not been studied before concerning ACE inhibitory activity [10,11,12], regardless of their ethnopharmacological use for treatment of hypertension in the form of herbal tea [13, 14]. Likewise, it has been previously shown that methanol extract of the most common used Alchemilla vulgaris L., exhibits a vasorelaxant effect on isolated rat aorta precontracted with noradrenaline and potassium [15], followed by reduction of increased systolic blood pressure in N (gamma)-nitro-l-arginine methyl ester (L-NAME) hypertensive rats after oral administration of 300 mg/kg/day for 2 weeks [16]. These effects are considered to be due to the high content of flavonoids in the extract, mainly quercetin, but the mechanism of actions of the extract has remained unknown.
Considering the abovementioned traditional data and recent in vitro studies, Alchemilla species could be good candidates for discovering new natural inhibitors of the angiotensin I-converting enzyme.
As a result, the goal of this study was to chemically characterize a methanol extract of the Balkan endemic species A. viridiflora and evaluate its potential to inhibit ACE activity. The study workflow is presented in Fig. 1. The workflow consists of three main parts: preparation and characterization of the methanol extract, enzyme assay and in silico evaluation of individual extract constituents’ contributions to this activity.
Material and methods
Plant material
Aerial parts of A. viridiflora were collected in July 2013 at Mt. Suva Planina in subalpine pastures in the spruce zone at 1750 m s.m., on carbonate soil. This is also a novel location for the species in its range's far north. Voucher specimens (20130708/1-2,) were deposited in the Natural History Museum (Belgrade, Serbia) and the identification of plant material was done by Dr. Marjan Niketić.
Preparation of extracts
The plant material was air-dried at room temperature. The methanol extract (80.94 g) was obtained by solvent extracting the air-dried, powdered material (320 g of A. viridiflora) for 2 days.
Chemical composition of A. viridiflora methanol extract
The content of total polyphenols was determined spectrophotometrically [17]. The content of polyphenols was expressed as µg of the standard substance gallic acid, (GA)/mg of dry extract. The assays for determining tannin and flavonoid concentration were carried out according to the European Pharmacopoeia's appropriate monographs (Ph. Eur. 10.0) [18].
The methanol extract solution (5 mg/mL) was further studied using the liquid chromatography-mass spectrometry (LC–MS) method. The analysis was performed on liquid-mass chromatograph Agilent Technologies HPLC 1260 Infinity with an automatic injector, diode array and single quadrupol mass detectors (SinglequadMSdetector6130). Separation was carried out on a Zorbax SB Aq-C18 column (3.0 × 150 mm; 3.5 m) at 25 °C with a binary mobile phase (A: 0.1% solution of formic acid in water; B: acetonitril). The following gradient program was employed with a flow rate of 0.3 mL/min: 0–30 min from 10 to 25% B; 30–35 min from 25 to 70% B; 35–40 min -return to 10% B.The injection volume was 3 μL. Detection wavelengths were at 280 and 350 nm and in negative mode in a range of 50–2000 m/z. Electrospray ionisation under atmospheric pressure was done under a pressure of 40 psi, temperature of 350 °C and a nitrogen flow of 10 L/min. The deprotoned molecule signals and fragmented ions were obtained under fragmentation voltages of 100 V and 250 V in full-scan.Compounds were identified tentatively by comparison with literature data, based on the comparison of their ultraviolet (UV) and mass (MS) spectra to those of commercially available standards or previously isolated compounds. All organic solvents were HPLC grade and were purchased from J.T.Baker (Deventer, The Netherlands).
In vitro ACE inhibitory activity
To investigate the ACE inhibitory activity of methanol extract of A. viridiflora ACE Kit-WST (Dojindo, Japan) was used. Briefly, the sample was dissolved in absolute ethanol and diluted with a pH 8.3 borate buffer. Five different concentrations of sample solution (0.0016, 0.008, 1.00, 2.50 and 5.00 mg/mL) were added to the 96-well microplate together with 20 μL of borate buffer and 20 μL of deionised water. The enzymatic reaction started by adding the enzyme solution 3-hydroxybutyrylglycylglycyl-glicine and aminoacylase. After the incubation period of 60 min at 37 °C, 200 µL of indicator solution was added to each sample well and once more incubated at room temperature for 10 min. Absorbance was measured at 450 nm using an Evolution 300 absorbance microplate reader. In parallel, ACE inhibitory activity of different concentrations of standard miquelianin (0.0007, 0.0167, 0.4181, 1.0452 and 2.0903 mM) was investigated following the same method. The miquelianin standard was purchased from Sigma-Aldrich.
The inhibitory activities of extract and standard were calculated according to the following equation: Inhibitory activity (%) = ((absorbance without an inhibitor − absorbance with an inhibitor)/(absorbance without an inhibitor − blank absorbance)) × 100.
The half-maximal inhibitory concentration (IC50), the concentration of the sample solution that gives 50% ACE inhibition, were calculated in Microsoft Excel by fitting the dose–response curve using the sample concentration for the x-axis and percentage inhibition of ACE for the y-axis.
ACE inhibitory activity in silico
Prediction of the identified compounds from the studied A.viridiflora methanol extract potential for ACE inhibition was performed by an in silico experimental approach. The molecular docking calculations were prepared using the Yet Another Scientific Artificial Reality Application (Yasara) Structure package (v. 20.4.24). A rectangular box with dimensions 30 Å × 30 Å × 30 Å was centered around a lisinopril molecule complexed with ACE (crystal structure PDB:1O86). Some ligand molecules were downloaded as 3D molecules from Pubchem (brevifolin carboxylic acid, ellagic acid 4-O-xylopyranoside, galloyl-hexahydroxydiphenoyl (HHDP)-glucose, HHDP-hexoside, miquelianin and tiliroside), while others were downloaded as 2D molecules (agrimoniin, pedunculagin, sanguiin H-10, tellimagrandin I and II) which were later converted into 3D molecules by the CORINA online service. All ligands were energetically optimized by Yasara Structure software. The ligand-receptor pairs with the lowest binding energy were considered to have the best docking conformations. The binding energy values of each tested ligand were compared with the results of lisinopril (a confirmed ACE inhibitor) and only better results than lisinopril were presented and further discussed. The visualization software (Discovery Studio Visualizer v.20.1.0.19295) was used to examine the output files of the most stable complexes and to generate 2D interaction illustrations.
Results and discussion
Chemical composition of A. viridiflora methanol extract
Alchemilla viridiflora Rothm. (A. sect. Calycinae), Rosaceae is an herbaceous plant widespread in mountainous areas of central Greece, Bulgaria, North Macedonia and Serbia with Kosovo. This is up to 80 cm tall plant with suborbicular leaves and greenish flowers with epicalyx-segments are as long as sepals. The whole plant is dense patent hairy [19, 20].
This species has not been studied before concerning ACE inhibitory or any other biological activity except in-vitro anti-Helicobacter pylori activity by virtue of the ability of ellagic acid and flavonoid isoquercitrin from methanol extract which exhibited strong activity at very low concentrations (0.125–0.5 μg/mL and 2–16 μg/mL, respectively) [21]. Likewise, studies on the chemical composition of A. viridiflora. are not available in the literature thus these results represent novel findings.
LC–MS analysis of methanol extract of A. viridiflora has lead to the identification of 20 compounds, mainly ellagitannins and flavonoid glycosides (Fig. 2; Table 1). As mentioned, these chemical constituents were identified mostly tentatively by comparison with literature data, commercially available standards or previously isolated compounds, based on the comparison of their ultraviolet (UV) and mass (MS) spectra. As it is known, ellagitannins are hydrolysable tannins esterified with hexahydroxydiphenic acid (HHDP) and the most often glucose. Their characteristic UV–Vis spectra are at 254 nm and 360–368 nm. Typical neutral losses of ellagitannins during MS fragmentation are galloyl (152 Da), gallic acid (170 Da), hexahydroxydiphenic acid, HHDP, (302 Da), galloylglucose (332 Da), HHDP-glucose (482 Da), and galloyl-HHDP-glucose (634 Da) residues. Among flavonoids, quercetin and its derivatives have absorption maximum at 354 nm and characteristic MS fragmentation ion at m/z 301 (negative mode), while kaempferol and its derivate have λmax at 348 nm and characteristic MS fragment ion at m/z 285 (negative mode) [11, 12, 22,23,24].
HPLC chromatogram of methanol extract of Alchemilla viridiflora (5 mg/mL) analyzed by the LC–MS and recorded at 280 and 350 nm. Legend: (1) HHDP-hexoside, (2) pedunculagin I isomer, (3) pedunculagin I isomer, (4) galloyl – HHDP-glucose and quercetin-hexoside- glucuronide, (5) brevifolin carboxylic acid, (6) tellimagrandin I, (7) sanguiin H-10 isomer, (8) galloyl-bis-HHDP-glucose, (9) sanguiin H-10 isomer, (10) tellimagrandin II, (11) miquelianin, (12) agrimoniin, (13) ellagic acid pentose, (14) pentagalloylglucose, (15) quercetin methyl ether glucuronide, (16) quercetin dimethyl ether glucuronide, (17) formate adduct of triterpene acid hexoside, (18) formate adduct of triterpene acid hexoside, (19) tiliroside, (20) formate adduct of triterpene acid hexoside
Peak 1 produced a [M−H]− ion at m/z 481 and generated fragment ion m/z 301 [M−180−H]− (loss of hexose) corresponding to an HHDP residue, and m/z 275 by decarboxylation of the HHDP moiety [25]. This compound has been identified as HHDP-hexoside. Peaks 2 and 3 correspond to isomeric compounds, with the [M−H]− ion at m/z 783, yielding main fragment ions at m/z 481 [M−302−H]− (loss of HHDP) and 301 [M−482−H]− (loss of HHDP-glucose), whose fragmentation pattern corresponds to a bis-HHDP-glucose structure presumably pedunculagin I isomers [26]. Peak 4 had [M−H]− at both m/z 633 and 639 with main fragments at m/z 463 [M−170−H]− (loss of gallic acid) and 463 [M−176−H]− (loss of glucuronide unit) and m/z 301 [M−332−H]− (loss of galloylglucose) and 301 [M−338−H]− (loss of hexoside-glucuronic unit). The same mass [M−H]− at m/z 633 can be seen in m-galloyl-HHDP-glucose, but this compound has a main fragment at m/z 481 [M−152−H]− (loss of galloyl moiety) which suggests that the galloyl unit is probably bonded via an m-depside bond, and not attached directly to the glucose core. On the contrary, loss of gallic acid in Peak 4 indicates that the galloyl unit is attached directly to the glucose [24]. Accordingly, peak 4 is identified as galloyl-HHDP-glucose (presumably a corilagin isomer). In the case of the compound with an ion at m/z 639 it clearly indicates that the compound can be identified as quercetin-hexoside-glucuronide [26]. Peak 5 exhibited the [M−H]− ion at m/z 291 and fragment ions at m/z 247 [M−45−H]− (loss of carboxylic acid) which corresponds to brevifolin, ion m/z 219 [M−73−H]− which corresponds to the loss of C=OCOOH and m/z 191, which are characteristic fragment ions for brevifolin carboxylic acid according to literature [27]. Peak 6 [M−H] − ion at m/z 785 with fragment 392 [M—2H] 2− was identified as tellimagrandin I using a standard compound previously isolated from flowers of Filipendula vulgaris Moench. [28]. Peaks 7 and 9 were tentatively identified as sanguiin H-10 isomers, with a fragment ion at m/z 783. This dimeric ellagitanninis composed of galloyl-bis-HHDP-glucose (m/z 935) and galloyl-HHDP-glucose (m/z 783). The proposed fragment ions at m/z l265 [M-302-H] − (loss of HHDP), 1103 [M−464−H]− (loss of HHDP-glucose), 933 [M−634−H]− (loss of galloyl-HHDP-glucose), 631 [M-936-H]− (loss of HHDP-glucose-galloyl-HHDP) and 301 [M−1266−H]− (loss of galloyl-HHDP-glucose-galloyl-HHDP-glucose) [26]. Peak 8 [M−H]− ion at m/z 935 and fragment ions at m/z 783 [M−152−H]− (loss of galloyl unit), 633 [M−302−H]− (loss of HHDP) and 301 [M−634−H]− (loss of galloyl-HHDP-glucose) is identified as galloyl-bis-HHDP-glucose [26]. Peak 10 is identified as tellimagrandin II using a standard compound previously isolated from flowers of Filipendula vulgaris [M−H]− ion at m/z 937 with fragment ions m/z 767 [M – 170−H]– (loss of galloyl unit), m/z 468 [M-2H] 2– and 301[M-634-H]– (loss of galloyl-HHDP-glucose) [29]. Peak 11 is identified compared to the mass spectra of used standard substance quercetin-3-O-β-glucuronide (miquelianin) with [M−H]− ion at m/z 477, with a fragment ion at m/z 301 [M−176−H]− (loss of glucuronide), which corresponds to quercetin. Peak 12 corresponds to agrimoniin. It showed a [M−H]− ion at m/z 1869 and fragment ions at m/z 1567 [M−302−H]– (loss of an HHDP unit), 1265 [M−604−H]– (loss of bis-HHDP), 1085 [M−784−H]– (loss of bis-HHDP-glucose) and the main fragment at m/z 935 [M−2H]2–, corresponding to one galloyl-bis-glucose unit, followed then by fragmentation ions at m/z 633 (935−302, loss of HHDP unit) and 301 (633−332, loss of galloyl-glucose residue) [30]. Peak 13 has [M−H]− ion at m/z 433 and fragment ion at m/z 301 [M−132−H]− (loss of a pentose), corresponding to the both ellagic acid and quercetin. As mentioned before, ellagic acid and it derivatives show characteristic UV spectra with λmax at 254 nm and 360–368 nm, while quercetin and its glycosides have an absorption maximum at about 354 nm. Hence, this compound is proposed to be ellagic acid pentose [24]. Peak 14 shows [M−H]− at m/z 939 and a fragment ion at m/z 769 [M−170−H]− corresponding to loss of gallic acid moiety. In addition, the appearance of fragment ions at m/z 787 and 635 corresponds to the loss of the first galloyl moiety [M−152−H]−, and the second one [M−304−H]−, respectively, thus this compound can be interpreted as pentagalloylglucose [31]. Peak 15 and 16 exhibit [M−H]− ion at m/z 491 and 505, respectively, with fragment ions at m/z 329 and m/z 315 [M−176−H]− which clearly indicates the loss of glucuronide unit, while the fragment of m/z 301 in both peaks indicates to presence of quercetin aglycone ([M−190−H]− loss of glucuronide and methylene moiety; [M−204−H]− loss of glucuronide and two methylene units). The proposed compounds are tentatively identified as quercetin methyl ether glucuronide [26], and quercetin dimethyl ether glucuronide. Peaks 17, 18 and 20 exhibit an [M−H]− ion at m/z 711, 709 and 695, respectively. This form of fragmentation may correspond to triterpene components found in raspberry, Rubus ideus [32]. Namely, all the compounds show proposed loss of formate [M-46-H]−, giving fragments at m/z 665, 663 and 649, respectively, followed by loss of hexose moiety [M−162−H]−to form the fragments at m/z 503, 501 and 487, respectively. These fragments are presumably aglycones of pentacyclic triterpenoids, ursane type (asatic and madecassic acid) [33] and the olean type (serjanic acid) [34]. Therefore, these peaks could be tentatively identified as formate addicts of triterpene acid-O-hexoside. Peak 19 exhibits an [M−H] − ion at m/z 593 and a fragment ion at m/z 285 [M−308−H] − (loss of a coumaroyl glucoside moiety) which corresponds to kaempferol, in addition to fragment ions at m/z 447 [M−146−H] − (loss of p-coumaroyl) which corresponds to kaempferol glucoside. This compound is identified based on the comparison of its UV and an MS spectrum to those of commercially available standard as kaempferol-3-O-(6-p-coumaroyl)-glucoside, tiliroside.
The major phenolic compounds found in the genus Alchemilla are tannins and flavonoids. The aerial parts of Alchemilla vulgaris, the common lady’s mantle are reported to contain up to 15% of tannins, of which 5–8% of ellagitannins, followed by flavonol glycosides (2.2–2.5% in leaves, 1.0–1.9% in flowers) [35]. As expected, results of this study showed that methanol extract of A. viridiflora is rich in phenolic compounds (233.41 ± 3.29 µg GA/mg of dry extract); thereof the content of total flavonoids was 0.30% ± 0.05 of and 3.74% ± 0.98 of tannins. Regarding the qualitative characterization of phytochemical compounds from A. viridiflora methanol extracts some compounds are commonly identified in this genus, like hydrolysable tannins, in particular ellagitannins. Acetone–water extract of aerial parts of both A. vulgaris, A. mollis and methanol extract of aerial parts of A. persica have been reported to possess the dimer agrimoniin, since it has been recognized as a marker compound of the Rosaceae family [26, 36]. Monomers, such as pedunculagin and sanguiin H-10 and its derivatives are also frequent constituents found in mentioned extracts of these three species [26, 36]. Galloyl-HHDP-glucose and galloyl-bis-HHDP-glucose, commonly present units in the structure of many ellagitannins, have also been previously identified in various Alchemilla species [26, 36]. On the contrary, substances such as HHDP-hexoside, brevifolin carboxylic acid, tellimagrandin I and II and ellagic acid pentose were identified for the first time in Alchemilla species. In the Rosaceae family, HHDP-monohexosides have been previously identified in methanol extracts of wild blackberries, Rubus grandifolius L. [37]. Ellagic acid is a common constituent of Alchemilla species, while its conjugates are reported for the first time in this paper. These metabolites can also be found in other species of the Rosaceae family, such as in acetone extract of strawberry fruits (Fragaria × ananassa) [24]. Brevifolin carboxylic acid is considered as an end product of ellagitannin hydrolyses, and it can be commonly found in the fruits, leaves, flowers or heartwood of pomegranate, Punica granatum [27]. This ellagitannin has previously been reported in species from Rosaceae family. It has been isolated from raspberry (Rubus idaeus L.) juice and whole plant of Duchesnea indica (Andrews.) Focke. [38, 39]. The presence of formate adducts of triterpene acid hexoside was not detected in Alchemilla species before. Nevertheless, triterpenes, such as ursolic acid, 2-a-hydroxyursolic acid, tormentic acid, euscophic acid, and oleanolic acid, have been identified in aerial parts of A. vulgaris, A. alpine L. and A. faroënsis (Lange) Buser [40]. Flavonoids are also often found in the genus Alchemilla. These are dominantly derivatives of flavonols, quercetin and kaempferol. Miquelianin, quercetin-3-O-glucuronide, is a commonly found flavonoid in Alchemilla species. It was detected in acetone–water extracts of A. vulgaris and A. mollis leaves and stalks, as well as its methyl derivative [26], in A. coriacea Buser, A. filicaulis Buser, A. glabra Neygenf., A. xanthochlora Rothm. [22], A. speciosa Buser [41] and A. achtarowii Pawł.[11]. Quercetin-hexoside-glucuronide and quercetin methyl ether glucuronide were both previously found in Alchemilla species [26], while quercetin dimethyl ether glucuronide has never been reported neither in Alchemilla species or Rosaceae family. Other quercetin derivates, quercetin-3-glucoside (isoquercitrin), quercetin-3-O-galactoside (hyperoside), quercetin 3-O-rutinoside (rutin) were also indentified in A. mollis, A. speciosa, and A. vulgaris and other various Alchemilla species from the north-eastern Black Sea region of Turkey [10, 23, 41, 42]. Kaempferol derivatives, kaempferol-3-O-β-d-(6″-p-coumaroyl)-glucopyranoside (tiliroside) has been isolated before from other Alchemilla species, such as from aerial parts of A. vulgaris, A. barbatiflora Juz., A. mollis and A. achtarowii [10,11,12, 43]. Kaempferol 3-O-β-d-glucoside (astragalin), kaempferol 3-O-β-d-glucuronide, kaempferol 3-O-β-(2″-O-α-L-rhamnopranosyl)-glucopyranoside uronic acid were found in A. speciosa and additionally kaempferol 3-O-(4″-E-p-coumaroyl)-robinobioside (variabiloside G) in A. achtarowii [11, 15]. Phenolic acids, such as gallic, 3, 4-dixydroxy-benzoic acid, chlorogenic and caffeic acid are also commonly present [44].
ACE inhibitory activity
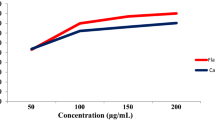
Molecules obtained from various plant isolates have gained great interest as ACE inhibitors recently. The most promising compounds based on their structure differences could be divided in tannins, flavonoids, and essential oil [5, 45]. Although structurally different, all these compounds have in common the presence of functional groups which serve as hydrogen (H) bond acceptors or donors, such as phenolic and carboxylic. Different concentration ranges of the A. viridiflora methanol extract rich in flavonoids and ellagitannins and miquelianin standard were evaluated in vitro for ACE activity inhibition. The results of the ACE inhibitory activity of the tested samples at the concentration range of 0.0016–5 mg/mL are presented in Fig. 3 and ACE inhibitory activity of miquelianin at the concentration range of 0.0007–2.0903 mM in Fig. 4.
The dose-dependent activities of A. viridiflora and miquelianin were observed in all tested concentration ranges. The result obtained from in vitro investigation of ACE inhibitory activity of methanol extract of A. viridiflora showed IC50 of 2.51 ± 0.00 µg/mL compared to IC50 of 5.4139 ± 0.00 µM for miquelianin. According to the manufacturer of ACEKit-WST (Dojindo, Japan), IC50 of Alacepril and Captopril are 3.62 μM and 2.14 nM, respectively. Our extract had lower IC50 than standard substance miquelianin, whose activity was significantly lower than captopril, but in range with alacepril, potent synthetic ACE inhibitor. These result suggested that beside miquelianin, some other extract constituents also contributed to this activity.
The results obtained from the molecular docking simulation study showed that 6 out of 20 compounds from methanol extracts obtained from A. viridiflora, formed more stable complexes with amino acid residues in receptor binding site than lisinopril, a well-known synthetic ACE inhibitor (positive control) under the same experimental conditions (Table 2). Furthermore, three of them formed more stable complexes with the active site residues than miquelianin, whose ACE activity was confirmed by an in vitro assay.
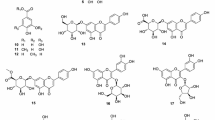
These compounds are as follows: tiliroside, ellagic acid pentose and galloyl-HHDP-glucose. The structures of these compounds are shown in Fig. 5. Among them, only tiliroside shows certain structure similarities with miquelianin and it is also the ligand which exhibited the best affinity for ACE binding site. Tiliroside is a flavonoid found in certain food plants that has been shown to have some favorable biological effects. One of them, which are important to this study, has been shown to have significant antihypertensive activity in a previous report [46].
Visualization software generated (Discovery Studio Visualizer v.20.1.0.19295) 2D illustrations of key receptor amino acid residues interactions with selected ligands from Alchemilla viridiflora sample: a brevifolin carboxylic acid, b ellagic acid pentose, c galloyl-HHDP-glucose, d miquelianin, e tellimagrandin I and f tiliroside
Ellagic acid in combination with the sugar moiety is a constituent unit of ellagitannins, which are known for their cardiovascular benefits [47]. Ellagic acid, as a constituent and metabolite of ellagitannins, has been shown to have considerable vasorelaxant effects in in vitro animal models via an endothelial-dependent mechanism [48]. In addition, Looi et al. (2020) verified its inhibitory effect on ACE activity in vitro. According to their in silico research, the discovered ACE inhibitory activity is likely due to interactions of ellagic acid with histidine and glutamic acid residues, which prevented the conversion of angiotensin I to angiotensin II in the presence of captopril. In our computational work, interaction with His 513 is also implicated in the stability of the ellagic acid pentose complex with ACE. [49]
Investigation of potential inhibitory effects on ACE activity of eleven Cuphea spp. crude extracts along with pure compounds showed that polyphenol miquelianin exhibited inhibitory activity comparable to captopril; a well-known ACE inhibitor which was the case in our study also [50].
According to subsequent interaction analysis, all complexes were stabilized by conventional H bond interactions between the receptor and chosen ligands. Furthermore, complexes were also stabilized through Van der Waals and π-interactions (Fig. 5).
Interactions with amino acid residues: Glu 162, His 353, Ala 354, Asp 377, Glu 384, Lys 511, His 513, Tyr 520, Tyr 523 in ACE active site is considered as crucial for ACE enzyme activity inhibition [51]. Tiliroside, tellimagrandin I, galloyl-HHDP and ellagic acid pentose from A. viridiflora methanol extract stabilized the most favorable molecule orientation through conventional H-bonds interactions with the above mentioned residues.
Conclusion
To summarize, the investigation of the chemical composition of methanol extract of Alchemilla viridiflora proposed a high content of total polyphenols, and 20 of them were identified. Tannins, with a content of 3% of total polyphenols, were identified mainly as ellagitannins, while flavonoids (0.30%) were identified as hexosides of quercetin and kaempferol. The extract showed dose-dependent in vitro ACE inhibitory activity, which was mainly attributed to the presence of flavonoids and tannins. The A. viridiflora extract and miquelianin showed dose-dependent in vitro ACE inhibitory activity with IC 50 values of 2.51 µg/mL and 5.4139 ± 0.00 µM, respectively.
Tiliroside, a flavonoid, had higher receptor affinity than miquelianin, which had its ACE inhibition activity verified in vitro. Among ellagitannins, galloyl-HHDP and ellagic acid pentose also showed better affinity for receptor than miquelianin. Nevertheless, further studies are required to find out whether the use of extract of A. viridiflora is related to blood pressure lowering activity, and whether the proposed mechanism of ACE inhibition is mainly responsible for that effect.
Data availability
Available upon responsible request.
Code availability
Not applicable.
References
WHO (2019) Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 13 Sept 2021
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K (2016) Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387:957–967. https://doi.org/10.1016/S0140-6736(15)01225-8
Netherlands Pharmacovigilance Center Lareb (2015). https://www.lareb.nl/en/news/ace-inhibitors-and-hallucinations. Accessed 13 Sept 2021
Siddesha J, D’Souza C, Vishwanath BS (2010) Inhibition of Angiotensin Converting Enzyme (ACE) by medicinal plants exhibiting antihypertensive activity. In: Govil JN, Singh VK (eds) Recent progress in medicinal plants. Drug plants III, vol 29. Studium Press, New Delhi, pp 269–308
Suručić R, Kundaković T, Lakušić B, Drakul D, Milovanović SR, Kovačević N (2017) Variations in chemical composition, vasorelaxant and angiotensin I-converting enzyme inhibitory activities of essential oil from aerial parts of Seseli pallasii Besser (Apiaceae). Chem Biodivers 14(5):e1600407. https://doi.org/10.1002/cbdv.201600407
Ben Mansour M, Balti R, Rabaoui L, Bougatef A, Guerfel M (2013) Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from south Tunisian Ajuga pseudoiva Rob Lamiaceae. Process Biochem 48:723–729. https://doi.org/10.1016/j.procbio.2013.02.022
Zouari N, Fakhfakh N, Zouari S, Bougatef A, Karray A, Neffati M, Ayadi MA (2011) Chemical composition, angiotensin I-converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae). Food Bioprod Process 89(4):257–265. https://doi.org/10.1016/j.fbp.2010.11.006
Hajji M, Masmoudi O, Souissi N, Triki Y, Kammoun S, Nasri M (2010) Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem 121(3):724–731. https://doi.org/10.1016/j.foodchem.2010.01.021
Liu JC, Hsu FL, Tsai JC, Chan P, Liu JY, Thomas GN, Tomlinson B, Lo MY, Lin JY (2003) Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci 73(12):1543–1555. https://doi.org/10.1016/S0024-3205(03)00481-8
Trendafilova A, Todorova M, Nikolova M, Gavrilova A, Vitkova A (2011) Flavonoid constituents and free radical scavenging activity of Alchemilla mollis. Nat Prod Commun 6(12):1851–1854. https://doi.org/10.1177/2F1934578X1100601216
Trendafilova A, Todorova M, Gavrilova A, Vitkova A (2012) Flavonoid glycosides from Bulgarian endemic Alchemilla achtarowii Pawl. Biochem Syst Ecol 43:156–158
D’Agostino M, Dini I, Ramundo E, Senatore F (1998) Flavonoid glycosides of Alchemilla vulgaris L. Phytother Res 12:S162–S163. https://doi.org/10.1002/(SICI)1099-1573(1998)12:1+/3CS162::AID-PTR284/3E3.0.CO;2-P
Nihoul-Ghenne L (1950) Presence of Alchemilla alpina L. Together with Alchemilla vulgaris L. in a tea for high blood pressure. J Pharm Belg 5:335–338
Pieroni A, Giusti ME, Quave CL (2011) Cross-cultural ethnobiology in the Western Balkans: medical ethnobotany and ethnozoology among Albanians and Serbs in the Pešter Plateau, Sandžak, South-Western Serbia. Hum Ecol 39:333–349. https://doi.org/10.1007/s10745-011-9401-3
Takir S, Sezgi B, Süzgeç-Selçuk S, Eroğlu-Özkan E, Beukelman KJ, Mat A, Uydeş-Doğan BS (2014) Endothelium-dependent vasorelaxant effect of Alchemilla vulgaris methanol extract: a comparison with the aqueous extract in rat aorta. Nat Prod Res 28(23):2182–2185. https://doi.org/10.1080/14786419.2014.926352
Takir S, Altun IH, Sezgi B, Suzgeç-Selçuk S, Mat A, Uydeş-Doğan BS (2015) Vasorelaxant and blood pressure lowering effects of Alchemilla vulgaris: a comparative study of methanol and aqueous extracts. Pharmacogn Mag 11(41):163–169
Kolundžić M, Stanojković T, Radović J, Tačić A, Dodevska M, Milenković M, Sisto F, Masia C, Farronato G, Nikolić V, Kundaković T (2017) Cytotoxic and antimicrobial activities of Cantharellus cibarius Fr. (Cantarellaceae). J Med Food 20:790–796. https://doi.org/10.1089/jmf.2016.0176
Council of Europe (2019) European pharmacopoeia. Council of Europe, Strasbourg
Tutin TG, Burges NA (2010) Flora Europaea, Rosaceae to Umbelliferae, vol 2. Cambrige University Press, Cambridge
Kurtto A, Fröhner SE, Lampinen R (2007) Atlas Florae Europaeae. Distribution of vascular plants in Europe 14. Rosaceae (Alchemilla and Aphanes). The Committee for Mapping the Flora of Europe & Societas Biologica Fennica Vanamo, Helsinki
Krivokuća M, Niketić M, Milenković M, Golić N, Masia C, Scaltrito MM, Sisto F, Kundaković T (2015) Anti-helicobacter pylori activity of four Alchemilla species (Rosaceae). Nat Prod Commun 10(8):1369–1371. https://doi.org/10.1177/2F1934578X1501000814
Fraisse D, Carnat A, Carnat AP, Lamaison JL (1999) Standardization of the aerial parts of Alchemilla. Ann Pharm Fr 57(5):401–405
Kaya B, Menemen Y, Saltan FZ (2012) Flavonoid compounds identified in Alchemilla L. species collected in the north-eastern black sea region of Turkey. Afr J Tradit Complement Altern Med 9(3):418–425. https://doi.org/10.4314/2Fajtcam.v9i3.18
Aaby K, Ekeber D, Skrede G (2007) Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem 55:4395–4406. https://doi.org/10.1021/jf0702592
Singh A, Bajpai V, Kumar S, Sharma KR, Kumar B (2016) Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat Prod Commun 11(2):239–244. https://doi.org/10.1177/2F1934578X1601100227
Duckstein SM, Lotterm EM, Meyer U, Lindequist U, Stintzing FC (2012) Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z Naturforsch C J Biosci 67:529–540
Wu S, Tian L (2017) Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 22(10):1606. https://doi.org/10.3390/molecules22101606
Samardžić S (2018) Comparative chemical and pharmacological investigation of lyophilized flower infusions of reprezentatives of the genus Filipendula Miller in Serbia. Dissertation, University of Belgrade
Samardžić S, Arsenijević J, Božić D, Milenković M, Tešević V, Maksimović Z (2018) Antioxidant, anti-inflammatory and gastroprotective activity of Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench. J Ethnopharmacol 213:132–137. https://doi.org/10.1016/j.jep.2017.11.013
Grochowski DM, Skalicka-Woźniak K, Orhan IE, Xiao J, Locatelli M, Piwowarski JP, Granica S, Tomczyk M (2017) A comprehensive review of agrimoniin. Ann N Y Acad Sci 1401(1):166–180. https://doi.org/10.1111/nyas.13421
Ghareeb MA, Sobeh M, El-Maadawy WH, Mohammed HS, Khalil H, Botros S, Wink M (2019) Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato-renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants 8(9):415. https://doi.org/10.3390/antiox8090415
McDougall GJ, Allwood JW, Pereira-Caro G, Brown EM, Latimer C, Dobson G, Stewart D, Ternan NG, Lawther R, O’Connor G, Rowland I, Crozier A, Gill CIR (2017) The composition of potentially bioactive triterpenoid glycosides in red raspberry is influenced by tissue, extraction procedure and genotype. Food Funct 8(10):3469–3479. https://doi.org/10.1039/C7FO00846E
Xia B, Bai L, Li X, Xiong J, Xu P, Xue M (2015) Structural analysis of metabolites of asiatic acid and its analogue madecassic acid in zebrafish using LC/IT-MSn. Molecules 20(2):3001–3019. https://doi.org/10.3390/molecules20023001
Mad T, Sterk H, Mittelbach M, Rechberger GN (2006) Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J Am Soc Mass Spectrom 17(6):795–806. https://doi.org/10.1016/j.jasms.2006.02.013
ESCOP Monograph of Alchemillae herba (2013) ESCOP monographs: the scientific foundation for herbal medicinal products. European Scientific Cooperative on Phytotherapy, Exeter
Afshar FH, Maggi F, Ferrari S, Peron G, Acqua SD (2015) Secondary metabolites of Alchemilla persica growing in Iran (East Azarbaijan). Nat Prod Commun 10:1705–1708. https://doi.org/10.1177/2F1934578X1501001018
Spínola V, Pinto J, Llorent-Martínez EJ, Tomás H, Castilho PC (2019) Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem Toxicol 123:443–452. https://doi.org/10.1016/j.fct.2018.11.006
Sójka M, Janowski M, Grzelak-Błaszczyk K (2019) Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur Food Res Technol 245:1113–1122. https://doi.org/10.1007/s00217-018-3212-3
Zhu M, Dong X, Guo M (2015) Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 20(12):22463–22475. https://doi.org/10.3390/molecules201219859
Olafsdottir ES, Omarsdottir S, Jaroszewski JW (2001) Constituents of three Icelandic Alchemilla species. Biochem Syst Ecol 29(9):959–962. https://doi.org/10.1016/s0305-1978(01)00038-2
Felser C, Schimmer O (1999) Flavonoid glycosides from Alchemilla speciosa. Planta Med 65(7):668–670. https://doi.org/10.1055/s-2006-960845
Tasić-Kostov M, Arsić I, Pavlović D, Stojanović S, Najman S, Naumović S, Tadić V (2019) Towards a modern approach to traditional use: in vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J Ethnopharmacol 238:111789. https://doi.org/10.1016/j.jep.2019.03.016
Renda G, Özel A, Barut B, Korkmaz B, Šoral M, Kandemir Ü, Liptaj T (2018) Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec Nat Prod 12(1):76–85. https://doi.org/10.25135/rnp.07.17.07.117
Denev P, Kratchanova M, Ciz M, Lojek A, Vasicek O, Blazheva D, Nedelcheva P, Vojtek L, Hyrsl P (2014) Antioxidant, antimicrobial and neutrophil-modulating activities of herb extracts. Acta Biochim Pol 61:359–367. https://doi.org/10.18388/abp.2014_1907
Barbosa-Filho JM, Martins VKM, Rabelo LA, Moura MD, Silva MS, Cunha EVL, Souza MFV, Almeida RN, Medeiros IA (2006) Natural products inhibitors of the angiotensin converting enzyme (ACE): a review between 1980–2000. Rev Bras Farmacogn 16(3):421–446. https://doi.org/10.1590/S0102-695X2006000300021
Silva GC, Pereira AC, Rezende BA, da Silva FJP, Cruz JS, de Souza MDFV, Gomes RA, Teles YCF, Cortes SF, Lemos VS (2013) Mechanism of the antihypertensive and vasorelaxant effects of the flavonoid tiliroside in resistance arteries. Planta Med 79(12):1003–1008. https://doi.org/10.1055/s-0032-1328765
Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA (2010) Ellagitannins, ellagic acid and vascular health. Mol Aspects Med 31(6):513–539. https://doi.org/10.1016/j.mam.2010.09.005
Yılmaz B, Usta C (2013) Ellagic acid-induced endothelium-dependent and endothelium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism. Phytother Res 27(2):285–289. https://doi.org/10.1002/ptr.4716
Looi D, Goh BH, Khan SU, Ahemad N, Palanisamy UD (2021) Metabolites of the ellagitannin, geraniin inhibit human ACE; in vitro and in silico evidence. Int J Food Sci Nutr 72(4):470–477. https://doi.org/10.1080/09637486.2020.1830263
Santos MC, Toson NSB, Pimentel MCB, Bordignon SAL, Mendez ASL, Henriques AT (2020) Polyphenols composition from leaves of Cuphea spp. and inhibitor potential, in vitro, of angiotensin I-converting enzyme (ACE). J Ethnopharmacol 255:112781. https://doi.org/10.1016/j.jep.2020.112781
Fang L, Geng M, Liu C, Wang J, Min W, Liu J (2019) Structural and molecular basis of angiotensin-converting enzyme by computational modeling: Insights into the mechanisms of different inhibitors. PLoS ONE 14(4):e0215609. https://doi.org/10.1371/journal.pone.0215609
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia through Grant Agreement with University of Belgrade-Faculty of Pharmacy No: 451-03-9/2021-14/200161.
Author information
Authors and Affiliations
Contributions
All authors JR, RS, MN and TK contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radović, J., Suručić, R., Niketić, M. et al. Alchemilla viridiflora Rothm.: the potent natural inhibitor of angiotensin I-converting enzyme. Mol Cell Biochem 477, 1893–1903 (2022). https://doi.org/10.1007/s11010-022-04410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04410-7