Abstract
Apoptosis is upregulated in all forms of diabetes, and the mitochondria act as target in diabetes pathophysiology. Quercetin and vitamin E have both shown usefulness in the delay of progression of diabetes-induced complications. However, their effect on the apoptotic process in diabetes mellitus is unknown. We hypothesize that quercetin treatment in diabetes may decrease the propensity for cardiomyocytic death via regulation of the mitochondria permeability transition (mPT) pore opening. Hearts from normal and streptozotocin-induced diabetic rats were used for the study. Low ionic strength heart mitochondria were used for swelling assay and mitochondrial lipid peroxidation (mLPO) activity was spectrophotometrically assessed. Levels of cytochrome c and caspase 3 and 9 were determined by immunohistochemistry, while lesions assessed by histology. Diabetic heart mPT pore showed larger amplitude swelling than control, while mLPO levels were increased in diabetic rats relative to control, this resulted in cytochrome c release. This initiated increased caspase 3 and 9 activity in diabetic rats (p < 0.05). Histology showed hemorrhagic lesions in diabetic rat hearts. Quercetin and vitamin E treatment reversed these effects, suggestive of their anti-apoptotic effect. Quercetin and vitamin E protection in diabetes is mediated by mPT pore inhibition and modulation of mitochondrial-mediated apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is an epidemic that has affected approximately 382 million people worldwide and expected to increase to about 592 million of the world population in 2035, generating a major world public health burden [1]. Sadly, the occurrence of diabetes mellitus is affecting a number of organs, including the kidney, eyes, and heart [2,3,4]. Diabetes is a major risk factor in the development of various cardiovascular diseases, including heart failure. It has been shown that hyperglycemia associated with this pathological condition causes severe oxidative stress to the cardiomyocytes and thus leads to diabetic cardiomyopathy [5]. The direct association of hyperglycemia with macro- and micro-vascular complications of Type 1 and 2 diabetes has been reported [6]. Cardiovascular disease occurrence in diabetes is significantly high, with studies showing an increased risk of heart failure due to inadequate hyperglycemia control [7]. It is now well established that myocardial cell death by apoptosis is a major determinant in the pathogenesis of cardiomyopathies, including ischemia–reperfusion, toxic exposure, and various other chronic diseases [8].
It has been demonstrated that apoptosis, a major form of programmed cell death, plays an important role in several pathological disorders involving the heart. The inhibition of this process in myocytes is emerging as a potential therapeutic strategy [9]. Virtually all the apoptotic signaling pathways which take place in non-cardiac cell types have been shown to also occur during the induction of apoptosis in the heart via the oxidative stress mechanism [10]. It is now clear that apoptosis may take place via at least two pathways, including the extrinsic and intrinsic or mitochondrial-mediated pathways [10]. In the mitochondrial-mediated pathway, the opening of the mitochondrial permeability transition (mPT) pore or outer mitochondrial membrane permeabilization causes the release of cytochrome c into the cytoplasm thus resulting in the activation of the initiation and executioner caspases, leading to cell death [11]. As in other cells, the molecular triggers of cardiomyocytic apoptosis center mainly on the balance between the concentrations of the pro-apoptotic and anti-apoptotic proteins, such as bcl 2, bax, cytochrome c, and the caspases [11]. It has been revealed that the intrinsic pathway is activated in myocytes by a number of cellular stimuli, including hypoxia, ischemia–reperfusion, and oxidative stress, which all enhance the mitochondrial permeability transition and thus causes an increased permeability of the outer and inner mitochondrial membranes [12]. It has been shown that reactive oxygen species (ROS)-induced oxidative stress causes an imbalance in the redox potential of the cell, thereby resulting in a number of pathological features [13]. However, there is no known mechanism for the full elucidation of the exact site of ROS generation in the mitochondria, but complexes I and III are recognized as the main sites of generation. This event potentiates the mPT pore opening with the feedback mechanism impacting pro-apoptotic proteins that could eventually damage nuclear DNA and initiate cardiac death [14, 15]
Observations of Gu et al. demonstrated that the attenuation of early cardiac cell death via suppression of mitochondrial oxidative stress by metallothionein results in a prevention of diabetic cardiomyopathy [16]. Also, Liu et al. reported that the protective effects of N-acetyl-l-cysteine on the heart of streptozotocin (STZ)-induced diabetic rats may be attributable to its protection of the heart against oxidative damage [17]. Furthermore, Kumar et al. showed that multiple antioxidants improve cardiac complications and inhibit cardiac cell death in STZ-induced diabetic rats [18]. Despite the fact that the etiology of ischemic and reperfusion injury is arguably multifactorial, available evidence indicate clearly that opening of the mPT pore is involved in cardiomyopathy [19]. It has been demonstrated that diabetic heart mitochondria display an enhanced susceptibility to mPT pore opening both in humans and animal models of diabetes [20, 21].
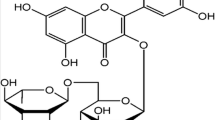
Although the exact nature of the component of the mPT pore is still under debate, it is generally accepted that apoptosis, being an orderly regulated process, is a logical therapeutic target [22, 23]. In this regard, certain bioactive agents have been shown to have the ability to modulate the opening or inhibition of the pore and as such may be useful in chemoprevention and chemotherapy [24]. In view of the fact that cardiomyocyte apoptosis has been documented as a pivotal form of cell death in ischemia and reperfusion damage, which are characteristic features of cardiovascular complications in diabetes, it becomes imperative to investigate the influence of certain dietary factors on mitochondrial-mediated apoptosis of the diabetic heart [25]. Vitamin E and quercetin are important dietary antioxidants and we have previously shown that they inhibit mitochondrial-mediated apoptosis in the liver of STZ-induced diabetic rats [26]. Low-dose quercetin and vitamin E have been shown to be safe for consumption, demonstrating promising pharmacokinetic properties [27, 28]. Furthermore, these have been shown to reduce the severity of pancreatic β-cell dysfunction in diabetes through their antioxidant and anti-inflammatory properties [29, 30]. The present study was therefore designed to gain insight into the modulatory or protective effects of these dietary components on the heart of STZ-induced diabetic rats via the inhibition of the mPT pore and mitochondrial-mediated apoptosis.
Materials and methods
Chemicals and reagents
Unless otherwise indicated, all reagents were of the highest purity grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA). Caspases 9 and 3 and cytochrome c antibodies were purchased from Elabscience laboratory (China).
Experimental animals
Animal procedure received approval by the Research Ethics Committee for the Animal Care and Use of the University of Ibadan, Nigeria. Male Wistar rats (100–120 g) used in the study were obtained from the Preclinical Animal House, College of Medicine, University of Ibadan, Nigeria. The rats were kept in temperature-controlled (25 °C) ventilated cages with 12-h light/dark cycling and given food and fresh water ad libitum.
Body weight determination
The body weight of rats was determined after acclimatization and recorded as the initial weight. The weekly weight obtained for each animal was used to adjust the doses of quercetin for standardization throughout the study period.
Experimental design
Subsequent to 2-week acclimatization, diabetes was induced by a single intraperitoneal injection of 45 mg/kg STZ dissolved in 100 mM sodium citrate (pH 4.5) after an overnight fast for rats [31]. Animals which had consistent 72-h fasting blood glucose concentrations of ≥ 250 mg/dL were considered diabetic.
Forty-eight rats were used for the study and these were randomly distributed into eight (8) groups with six (6) animals in each group.
Group I: Normal control rats (NC) that received no special treatment for 28 days.
Group II: NC + oral gavage of 10 mg/kg quercetin (Q10) for 28 days.
Group III: NC + oral gavage of 30 mg/kg quercetin (Q30) for 28 days.
Group IV: NC + oral gavage of 10 mg/kg vitamin E (V) for 28 days.
Group V: Diabetic control rats (DC) that were induced with 40 mg/kg STZ and received no special treatment for 28 days.
Group VI: DC + oral gavage of Q10 for 28 days.
Group VII: DC + oral gavage of Q30 for 28 days.
Group VIII: DC + oral gavage of V for 28 days.
These experiments were repeated twice. The number of days used in the study was based on previous studies [32,33,34]. The doses of quercetin and vitamin E were based on their earlier protective reports in rats [35,36,37].
Biochemical assays
After 28 days, rats were sacrificed by cervical dislocation and blood samples were obtained through cardiac puncture. However, blood sampling for glucose determination was obtained through ocular puncture after 72 h by a trained technologist.
The blood was collected into Ethylenediaminetetraacetic acid (EDTA) and glucose tubes (sodium fluoride tubes) and used for biochemical analysis and analysis of Glucose, Cholesterol, and Triacylglycerol.
Plasma was obtained from blood samples by centrifugation at 3000 rpm for 10 min and the supernatant was carefully pipetted. Aliquots of the samples were used for glucose determination using glucose oxidase method, while cholesterol and triacylglycerol levels were determined using commercially available kits.
Isolation of rat heart mitochondria
Low ionic strength mitochondria were isolated essentially according to the previous methods with slight modifications using differential centrifugation [38, 39]. The heart was aseptically removed and washed with the isolation buffer (220 mM Mannitol, 70 mM Sucrose, 1 mM EDTA, 10 mM Tris; pH 7.4). This was weighed and cut into pieces with a pair of scissors. A 10% suspension was prepared and homogenized in a Teflon glass cup. The homogenate was centrifuged twice at 3000 rpm for 5 min in MSE-refrigerated centrifuge. The nuclear debris was discarded and the supernatant was collected. This was centrifuged again at 11,000 rpm for 10 min to obtain the mitochondrial fraction. Mitochondrial pellets were washed twice with washing buffer [220 mM Mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM Tris, and 0.2% Bovine Serum Albumin (BSA)] at 10500 rpm for 10 min. The mitochondria obtained were re-suspended in buffer [220 mM Mannitol, 70 mM Sucrose and 10 mM Tris–HCl], dispensed as aliquots into Eppendorf tubes and kept on ice until use. All procedures were carried out at 4 °C.
Measurement of mitochondrial swelling
Mitochondria (0.4 mg/mL) were pre-incubated in the presence of 0.8 µM rotenone and swelling buffer [220 mM mannitol, 70 mM sucrose, and 10 mM Tris(hydroxymethyl)aminomethane (Tris)–HCl (pH 7.4)] for 3½ minutes. This was prior to the addition of 5 mM sodium succinate in the absence of a triggering agent (Ca2+) at 25 °C. The absorbance change at 540 nm was monitored over a period of 12 min at 30-s interval [40].
Estimation of mitochondrial lipid peroxidation (mLPO) determination
The mLPO levels were determined according to previous methods [41]. This was estimated by incubating the mitochondria in a mixture containing 20% (v/v) acetic acid, 0.8% (w/v) thiobarbituric acid, and 1.1% (w/v) sodium dodeocylsulfate (SDS) in (1:1). The reaction tubes were placed in a water bath and heated at 95 °C for 1 h. The tubes were cooled and butanol was added. These were vortex mixed before centrifugation at 3000 rpm for 10 min. The organic supernatant was pipetted carefully into clean, dry test tubes and absorbance readings were taken at 532 nm against a reagent blank.
Immunohistochemical determination of release of cytochrome c and activation of caspases 3 and 9
Immunostaining was done on heart tissues sliced to 5 µM thick. These were embedded in paraffin and rehydrated using methanol and xylene. Citrate (pH 6.0) was used for heat-induced epitope retrieval for 20 min, followed by immersion in cold water for 10 min. Thereafter, the activities of endogenous peroxidase in the heart sections were blocked with 5% H2O2 for 5 min. These were incubated overnight at 4 °C with 1:200 anticytochrome c and caspase 3 and 9 primary monoclonal antibodies. They were washed and incubated again with horseradish peroxidase-labeled anti-rabbit monoclonal secondary antibodies. The peroxidase-binding sites were detected by 0.05% 3′-3′-diaminobenzidine tetrahydrochloride. Counterstaining was performed using Mayer’s hematoxylin. Slides were viewed by microscopy and photographed with a digital camera.
Protein determination
The protein content of the heart mitochondria was determined using BSA as standard [42].
Statistical analysis
Data were analyzed by one-way Analysis of Variance using GraphPad prism. Significance was estimated by Tukey post hoc test and statistical difference of less than p < 0.05 was considered significant. The normal distribution was conducted by Shapiro–Wilk test and power analysis was calculated. The appropriate sample size was determined using an alpha of 0.05 and a power of 80% obtained with a medium effect size of d = 0.5. The amount of stains in immunohistochemical analysis were quantified using Fiji Software package, version 5.
Results
Quercetin and vitamin E treatment lowered blood glucose, cholesterol, and triglyceride levels
Increased blood glucose is a key marker in diabetes pathophysiology. In the study, the result (Fig. 1A) shows that the blood glucose in diabetic rats was increased relative to control rats (p < 0.05). Oral administration of 10 and 30 mg/kg quercetin decreased the blood glucose by 65 and 61%, respectively, relative to diabetic rats. Similarly, vitamin E treatment decreased the blood glucose level by 70% in comparison to diabetic rats. It was observed that there was no statistical difference between the treatment regimens. In Fig. 1B, the plasma cholesterol and triglyceride levels were significantly higher in diabetic animals compared with normal animals. Again, treatment with quercetin and vitamin E for 28 days significantly lowered the levels of both parameters in diabetic rats after 28 days of treatment, with no significant difference in the treatment options.
Effect of quercetin and vitamin E on certain biochemical parameters. A Effects of quercetin and vitamin E treatment on blood glucose levels in STZ-induced diabetic rats. B Triglycerides and cholesterol levels in STZ-induced diabetic rats previously exposed to quercetin and vitamin E. C Effect of vitamin E and quercetin on mitochondrial lipid peroxidation in normal and diabetic rat heart after 28 days of oral administration. NC Normal control, DC Diabetes control, NC + Q10 Normal control that received 10 mg/kg quercetin, NC + Q30 Normal control that received 30 mg/kg quercetin, NC + V Normal control that received 10 mg/kg vitamin E, DC + Q10 Diabetic control that received 10 mg/kg quercetin, DC + Q30 Diabetic control that received 30 mg/kg quercetin, DC + V Diabetic control that received 10 mg/kg vitamin E. Assays were carried out in triplicates, and values are expressed as mean ± SD. a = p < 0.05 compared to DC, b = No statistical difference between DC + Q10 and DC + Q30, c = No statistical difference between DC + Q10, DC + Q30 and DC + V, d = p < 0.05 statistical difference between DC + Q10, DC + Q30 and DC + V, e = No statistical difference between NC + Q10, NC + Q30, NC + V. a# = No statistical difference between normal control and NC + Q10, NC + Q30 and NC + V. d# = No statistical difference between blood glucose at 72 h and 28 days in NC + Q10, NC + Q30, and NC + V, * = p < 0.05 compared to blood glucose at 72 h. BG Blood glucose, Trig Triglyceride level, Chol Cholesterol level
Effect of quercetin and vitamin E on mitochondrial lipid peroxidation
Mitochondria are prime targets of lipid peroxides in diseases, and the free radicals generated compromise the integrity of the membrane. To assess the impact of diabetes on the mitochondrial membrane, the heart mLPO levels were determined in normal and diabetic control rats. The results in Fig. 1C showed an elevation of mLPO levels in diabetic rats relative to normal rats. Furthermore, an investigation on the effect of quercetin administration on normal rat for 28 days showed no significant effect in mLPO levels (Fig. 1C). Treatment of diabetic rats with 10 and 30 mg/kg quercetin and 10 mg/kg vitamin E reduced the mLPO levels by 87, 73, and 58%, respectively (Fig. 1C).
Quercetin and vitamin E reduced mitochondrial permeability transition pore opening in STZ-induced diabetic rat heart
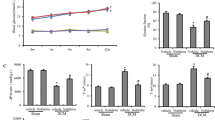
Previous studies from our laboratory have shown that quercetin reverses mPT pore opening in the liver of STZ-induced diabetic rats. Therefore, in this study, we determined the intactness of the normal rat heart mitochondria. This was evaluated by incubating 0.4 mg/mL mitochondria in a suspension buffer containing 0.8 µM, 5 mM succinate at 25 °C (pH 7.4) and the rate of decrease in absorbance was monitored at 540 nm over a period of 12 min. The results (Fig. 2A) showed that there was no significant change in absorbance of normal rat heart mitochondria but addition of 12 mM Ca2+ to the buffer solution caused significant increase in mitochondrial swelling or mPT pore opening which was reduced by 5 mM spermine, a standard inhibitor of the mPT pore opening thus indicating that the mitochondria used in the study were intact. In contrast, the heart mitochondria of STZ-induced diabetic rats showed significant opening of the mPT pore than mitochondria from normal rats (Fig. 2B) which showed no appreciable pore opening. The results of the effects of various doses of quercetin and vitamin E on the status of the mPT pore in heart mitochondria of control animals are shown in Fig. 2C. Here, the results show clearly that quercetin and vitamin E had no significant effect whatsoever on the integrity of mPT pore following oral administration of these substances for 28 days. In order to determine the potency of quercetin and vitamin E in reducing mPT pore opening in diabetic rat heart, 10 and 30 mg/kg quercetin and 10 mg/kg vitamin E were administered for 28 days. Results in Fig. 2D showed that mPT pore opening was observed in diabetic rat heart and was reduced by 42, 83, and 50% in animals that received 10 and 30 mg/kg quercetin and 10 mg/kg vitamin E, respectively.
Effects of quercetin and vitamin E on the mitochondrial membrane permeability transition pore. A Representative profile of the change in absorbance of heart mitochondria respiring on succinate in the presence of rotenone with triggering agent (TA) or without TA (NTA) or Spermine: Inhibitor. B Assessment of the Integrity of heart mitochondrial membrane permeability transition pore in STZ-induced diabetic rat respiring on succinate in the presence of rotenone. C Effects of quercetin and vitamin E on mitochondrial permeability transition pore of rat heart after 28 days of oral administration. D Effects of vitamin E and quercetin on heart mitochondrial permeability transition pore of STZ-induced diabetic rats after 28 days of oral administration. NC Normal control, DC Diabetes control, NC + Q10 Normal control that received 10 mg/kg quercetin, NC + Q30 Normal control that received 30 mg/kg quercetin, NC + V Normal control that received 10 mg/kg vitamin E, DC + Q10 Diabetic control that received 10 mg/kg quercetin, DC + Q30 Diabetic control that received 30 mg/kg quercetin, DC + V Diabetic control that received 10 mg/kg vitamin E
Quercetin and vitamin E reduced cytochrome c release in diabetic rats
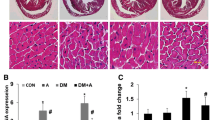
The results of the immunohistochemical analysis of cytochrome c release in hearts of normal and STZ-induced diabetic rats following administration of quercetin and vitamin E are presented in Fig. 3. As shown in the figure, increased cytochrome c release was observed in diabetic rats compared with the normal control rats. Treatment of diabetic rats with 10 and 30 mg/kg quercetin and vitamin E showed reduction in the levels of cytochrome c release by 1.1-, 1.3-, and 1.5-fold, respectively, compared with normal control or diabetic rats.
Extent of cytochrome c release by the mitochondria from STZ-induced diabetic rat orally exposed to quercetin and vitamin E for 28 days. A NC—Normal control, B DC—Diabetic control, C DC + Q10—Diabetic control that received 10 mg/kg quercetin, D DC + V—Diabetic control that received 10 mg/kg vitamin E, E DC + Q30—Diabetic control that received 30 mg/kg quercetin. ImageJ software was used to quantity the intensities of the stains. Scale bar = 5 µM. a = p < 0.05 compared to DC, b = No statistical difference between DC + Q10 and DC + Q30, c = No statistical difference between DC + Q10, DC + Q30, and DC + V
Quercetin and vitamin E downregulate caspase 9 and 3 activity in diabetic rats
To investigate the anti-apoptotic effect of quercetin on diabetic rats, we determined the effects of quercetin treatment of diabetic rats on downstream caspase 3 and 9 specific for mitochondrial-mediated apoptosis. Here, the results showed that there was a larger extent of caspase 3 and 9 activation in diabetic rats compared with normal control. Treatment with 30 mg/kg quercetin and vitamin E decreased activation of caspase 9 by 1.6- and 2.4-fold, respectively, while 10 and 30 mg/kg quercetin and vitamin E decreased caspase 3 activation by 1.2-, 1.3-, and 2.6-fold, respectively (Fig. 4).
Caspases 9 and 3 activity in STZ-induced diabetic rat heart following administration of vitamin E and quercetin for 28 days. A NC—Caspase 9 activity in normal control, B DC—Caspase 9 activity in diabetic control, C DC + Q10—Caspase 9 activity in diabetic control that received 10 mg/kg quercetin, D DC + V—Caspase 9 activity in diabetic control that received 10 mg/kg vitamin E, E DC + Q30—Caspase 9 activity in diabetic control that received 30 mg/kg quercetin, F NC—Caspase 3 activity in normal control, G DC—Caspase 3 activity in diabetic control, H DC + Q10—Caspase 3 activity in diabetic control that received 10 mg/kg quercetin, I DC + V—Caspase 3 activity in diabetic control that received 10 mg/kg vitamin E, J DC + Q30—Caspase 3 activity in diabetic control that received 30 mg/kg quercetin. ImageJ software was used to quantity the intensities of the stains. Scale bar = 5 µM. a = p < 0.05 compared to DC, b = No statistical difference between DC + Q10 and DC + Q30, c = No statistical difference between DC + Q10, DC + Q30, and DC + V
Quercetin and vitamin E reduce hemorrhagic lesions and congestions of coronary vessels in diabetic rats
While normal rat heart showed no visible lesions, diabetes caused hemorrhagic lesions and congestion of coronary vessels (Fig. 5A, B). Treatment with 10 and 30 mg/kg reduced these complications to mild inflammation (Fig. 5C, E). Furthermore, 10 mg/kg Vitamin E reduced the lesions to multi-focal areas of moderate inflammation (Fig. 5D).
Photomicrographs of sections of the hearts of STZ-induced diabetes rats after oral exposure to quercetin and vitamin E for 28 days. A NC—Normal control, B DC—Diabetic control, C DC + Q10—Diabetic control that received 10 mg/kg quercetin, D DC + V—Diabetic control that received 10 mg/kg vitamin E, E DC + Q30—Diabetic control that received 30 mg/kg quercetin. Scale bar = 5 µM. Blue arrow shows congestion of coronary vessels, green arrow shows hemorrhagic lesion, black arrow shows focal area of inflammation, and orange arrow shows multi-focal areas of moderate inflammation involving the myocardium and pericardium
Discussion
The observation that apoptotic cardiomyocyte loss is the most important determinant of mortality and morbidity in various cardiovascular diseases such as heart failure put credence to postulate that inhibition of cardiomyocyte apoptosis holds promise as an effective strategy for treatment of cardiovascular diseases [43]. Data from work on animal models on the inhibition of mPT pore opening by either cyclosporine A or genetic ablation of cyclosporine D provide strong protection from both reperfusion injury and congestive heart failure show clearly that the mPT pore is a promising drug target in human cardiovascular diseases [44]. Diabetic cardiomyopathy is known to be triggered by severe oxidative stress factors brought about mainly by uncontrolled hyperglycemia in diabetic patients and this effect directly affects the mitochondria [45]. The results obtained in this study show that quercetin and vitamin E sustained glycemic control in diabetic rats during the period of the study. This finding is consistent with the recent reports that dietary supplement ameliorated hyperglycemia in diabetes [46, 47]. Several reports have shown that increased triglyceride and cholesterol in diabetes lipidemia would cause cardiovascular disease [48]. The present study confirmed the potency of quercetin treatment in reducing hyperlipidemia, therefore probably may reduce cardiotoxic risk. We have previously shown that the mPT pore is significantly opened in liver mitochondria of STZ-induced diabetic rats and that quercetin and vitamin E inhibited the mPT pore in diabetic rats [26]. The effects of the antioxidants have also been shown to be relevant in testicular protection in diabetes [37]. These antioxidants significantly improved the status of the liver mPT pore and the diabetic complications. This study investigated the effect of quercetin and vitamin E on rat heart mitochondrial-mediated apoptosis via the mPT pore opening in STZ-induced diabetic rats. Our results show that the mPT pore of hearts of STZ-induced diabetic rats is significantly opened as previously reported [49]. The results obtained from experiments on the oral administration of either quercetin or vitamin E showed that the antioxidants significantly inhibited pore opening in diabetic rats. This suggests their cardio-protective role in excessive apoptosis during diabetes, confirming that inhibition of mPT pore restore cardio-protection in diabetic hearts [50]. Our findings from the study also showed that the effect of 30 mg/kg quercetin was the most outstanding in inhibiting heart mPT pore opening in diabetic rats.
Sequel to the fact that quercetin and vitamin E inhibited the mPT pore opening in diabetic condition, effects of quercetin and vitamin E on mLPO were investigated via determination of MDA levels. In this study mLPO level was elevated in diabetic rats, while treatment with the antioxidants inhibited the alteration of membrane lipid contents. It has been shown that the mitochondria are prime target and source of lipid peroxides in diseases. Therefore, rescuing this situation as shown by quercetin and vitamin E treatment could help maintain the integrity of cardiomyocytes in diabetic condition.
Studies have shown that induction of oxidative stress is a determinant of apoptosis in diabetes with flavonoids performing remedial roles [10, 51, 52]. These studies give credence to the fact that quercetin and vitamin E show antioxidant role in diabetes. Disturbance of membrane fluidity caused by peroxidation of membrane phospholipid and sensitization of mPT pore enhances cytochrome c release. This is an irreversible event in the progression to cell death [10]. Elevated cytochrome c release in the heart sections of diabetic rats confirmed mPT pore opening in the hearts of diabetic rats. Treatment of diabetic rats with quercetin and vitamin E showed reduction in cytochrome c release. The data obtained in this study showed that the presence of cytosolic cytochrome c activated the initiator procaspase 9 and subsequently the executioner caspase 3 and caused cell death. Taken together, the present study showed that quercetin and vitamin E decreased the extent of excessive apoptosis in diabetic rats by reversing the opening of the mPT pore and reducing the release of cytochrome c to the cytosol and thereby decreasing caspase 9 and 3 activation and preventing the death of the cardiomyoctes.
Furthermore, histopathological examinations of the sections of the heart showed reduced lesions as a result of quercetin and vitamin E treatment in STZ-induced diabetic rats.
Researches have shown that over 66% of the active agents in drugs have their natural sources from plant origin; more of these are currently undergoing clinical trials [53, 54]. The mechanism of actions of these is by lowering glycemic status. It has been reported that Trigonella foenum-graecum, Ipomea batata, and Silybum mariamum among others have improved glycemic status in diabetes [54]. Therefore it is unsurprising that vitamin E and quercetin obtained from dietary sources could modulate glycemic status in diabetes. Inhibition of intrinsic apoptotic proteins and the mPT pore opening by the antioxidants has shown repression of membrane fluidity and conservation of apoptotic proteins in DM. This partly explains the molecular basis of the cardio-protective effects, therefore addressing an unmet need in diabetes pathophysiology. It is suggested that these dietary molecules should be used as adjuvant therapy for pharmacologic management of cardiovascular disease in diabetes.
Conclusion
The study showed the anti-apoptotic role of quercetin and vitamin E in STZ-induced diabetes, therefore these could be probable pharmacologic agent to target downstream intrinsic apoptotic pathway leading to excessive cardiomyocytic death in diabetes.
Data availability
All data used in the study are included in the manuscript.
References
Ojieabu WA, Odusan O, Ojieabu NI, Oku LM (2017) Evaluation of prevalence of micro- and macrovascular complications among elderly type 2 diabetes patients in a health facility. Afr J Biomed Res 20:131–135
Cannon MV, Sillje HHW, Sijbesma JWA et al (2016) LXRα improves myocardial glucose and reduces cardiac hypertrophy in a mouse model of obesity-induced type 2 diabetes. Diabetologia 59:634–643
Lee VK, Hosking BM, Holeniewka J et al (2018) BTBR ob/ob mouse model of type 2 diabetes exhibits early loss of retinal function and retial inflammation followed by late vascular changes. Diabetologia 61:2422–2432
Zhao Y, Wenting Z, Jia Q et al (2019) High dose of vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front physiol 9:1–13
Volpe CMO, Villar-Delfino PH, dos Anjos PMF et al (2018) Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9:119. https://doi.org/10.1038/s41419-017-0135-z
Chawla A, Chawla R, Jaggi S (2016) Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocr Metab 20(4):546
Kasznicki J, Drzewoski J (2013) Heart failure in the diabetic population-pathophysiology, diagnosis and management. Arch Med Sci 10(3):546–556
Kim NH, Kang PM (2010) Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J40(7):299–305. https://doi.org/10.4070/kcj.2010.40.7.299
Teringova E, Tousek P (2017) Apoptosis in ischemic heart disease. J Trans Med 15(1):87. https://doi.org/10.1186/s12967-017-1191-y
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163. https://doi.org/10.1152/phyrev.00013.2006
Riswana NA, Bhuvaneshwari P (2019) Apoptotic cell death in heart failure associated with diabetes. Int J Clinicopathol Correl 3:12–18
Kwong JQ, Molkentin JD (2015) Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab 21(2):206–214. https://doi.org/10.1016/j.cmet.2014.12.001
Checa J, Aran JM (2020) Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res 13:1057–1073
Peoples JN, Saraf A, Ghazal N et al (2019) Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med 51:1–13. https://doi.org/10.1038/s12276-019-0355-7
Kent AC, El Baradie KBY, Hamrick MW (2021) Targeting the mitochondrial permeability transition pore to prevent age-associated cell damage and neurodegeneration. Oxid Med Cell Longev. https://doi.org/10.1155/2021/6626484
Gu J, Chen Y, Wu H et al (2017) Metallothionein is downstream of Nrf 2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes 66(2):529–542. https://doi.org/10.2337/db15-1274
Liu C, Lu XZ, Shen MZ et al (2015) N-acetyl cysteine improves the cardiac function: possible role of fibrosis inhibition. BMC Cardiovasc Disord 15:84. https://doi.org/10.1186/s12872-015-0076-3
Kumar S, Prasad S, Sitasawad SL (2013) Multiple antioxidants improve cardiaccomplications and inhibit cardiac cell death in steptozocin-induced diabetic rats. PLoS ONE 7:e67009
Kalogeris T, Baines CP, Krenz M et al (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Williamson CL, Dabkowski ER, Baseler WA et al (2010) Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol 298:H633–H642
Anderson EJ, Rodriguez E, Anderson CA et al (2011) Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol 300:H118–H124
Alvian KN, Beutner G, Lazrove E et al (2014) An uncoupling channel within the c-subunit ring of the F1F0-ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 111:10580–10585
Bonora M, Wieckowski MR, Chinopoulos C et al (2014) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. https://doi.org/10.1038/onc.2014.96
Ojo OO, Rotimi S, Adegbite OS et al (2019) BrideliaferrugineaInhibit rat heart and liver mitochondrial membrane permeability transition pore opening following myocardial infarction. Int J Pept Res Ther. https://doi.org/10.1007/s10989-019-09950-z
Javadov S, Karmazyn M (2007) Mitochondrial permeability as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem 20:1–22
Daniel OO, Adeoye AO, Ojowu J et al (2018) Inhibition of liver mitochondrial membrane permeability transition pore opening by quercetin and vitamin E in streptozotocin-induced diabetic rats. BBRC 504:460–469
Mahipa A, Klapman J, Vignesh S, Yang CS, Neuger A, Chen D et al (2016) Pharmacokinetics and safety of vitamin E δ-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites. Cancer ChemotherPharmacol 78(1):157–165
Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, Abd El-Hack ME, Taha AE et al (2020) The pharmacological activity, biochemical properties and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods 9(3):374. https://doi.org/10.3390/foods9030374
Carvalho KMMB, Morais TC, de Melo TS et al (2010) The natural flavonoid quercetin ameliorates cerulin-induced acute pancreatitis in mice. Biol Pharm Bull 33(9):1534–1539
Xu R, Zhang S, Tao A et al (2014) Influence of vitamin supplementation on glycaemic control: a meta-analysis of randomized controlled trials. PLoS ONE 4:e95008. https://doi.org/10.1371/journal.pone0095008
Saravanan G, Leelavinothan P (2005) Effect of SyzygiumCumini bark on blood glucose, plasma, insulin and C-peptide in streptozotocin induced diabetic rats. Int J Endocrinol Metab 4(2):96–105
Ayepola OR, Cerf ME, Brooks NL et al (2014) Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine 21:1785–1793
Gardic C, Bauerova K, Stringa B et al (2015) Quercetin reduced-inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch Biochem Biophys 583:150–157
Rashid K, Sil PC (2015) Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol 282(3):297–310
Calfee-Mason KG, Spear BT, Glauert HP (2002) Vitamin E inhibits NF-kappa B in rats administered the hepatic tumor promoter, phenobarbital. J Nutr 132(10):3178–3185
Roslan J, Giribabu N, Karim K et al (2017) Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother 86:570–582
Ojo OO, Olorunsogo OO (2021) Quercetin and vitamin E attenuate diabetes-induced testicular anomaly in Wistar rats via the mitochondrial-mediated apoptotic pathway. Andrologia. https://doi.org/10.1111/and.14185
Mela L, Seitz S (1979) Isolation of mitochondria with emphasis on heart mitochondria from small amounts of tissue. Methods Enzymol 55:39–46. https://doi.org/10.1016/0076-6879(79)55006-X
Sanz AA, Hiona GC, Kujoth AY et al (2007) Evaluation of gender differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol 42(3):173–182. https://doi.org/10.1016/j.exger.2006.10.003
Lapidus RG, Sokolove PM (1993) Inhibition by spermine of the inner membrane permeability transition of isolated rat heart mitochondria. FEBS Lett 313(3):314–318
Ruberto G, Baratta MT, Deans SG et al (2000) Antioxidant and antimicrobial activity of foeniculum vulgare and Crithmum maritimum essential oils. Planta Med 66(8):687–693
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Javadov S, Karmazyn M, Escobales N (2009) Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther 330(3):670–678
Halestrap AP, Pasdios P (2009) The role of the mitochondrial permeability transition pore in heart disease. Biochem Biophys Acta 1789:1402–1415
Li J, Yu S, Ying J et al (2017) Resvaratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt 1/PGC-1α pathway. Oxid Med Cell Longev. https://doi.org/10.1155/2017/7584691
Takemoto K, Doi W, Noriyoshi M (2016) Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Biochim Biophys Acta 1862:647–650
Dhanya R, Arya AD, Nisha P (2017) Quercetin, a lead compound against type 2 diabetes ameliorate glucose uptake via AMPK pathway in skeletal muscle cell line. Frontiers in Pharmcol 8(336):1–9
Ameida DAT, Braga CP, Novelli ELB et al (2012) Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidants vitamin. Braz Arch Biol Technol 55(4):527–536
Sloan RC, Moukdar F, Frasier CR et al (2012) Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol 52:1009–1018
Najafi M, Farajnia S, Mohammadi M et al (2014) Inhibition of mitochondrial permeability transition pore restores the cardioprotection by postconditioning in diabetic hearts. J Diabetes Metab Disord 13:106
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
Sarian MN, Ahmed QU, So’ad SZM et al (2017) Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study. Biomed Res Int. https://doi.org/10.1155/2017/8386065
Harvey AL (2008) Natural products in discovery. Drug Discov Today 13:894–901
Tabatabaei-Malazy O, Larijani B, Abdollahi M (2015) Targeting metabolic disorders by natural products. J Diabetes Metab Disord 14:57
Acknowledgements
The authors acknowledge the contributions of the Director, Biomembrane and Biotechnology Laboratory, Department of Biochemistry, College of Medicine, University of Ibadan, Nigeria.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
OOj and OOl assisted in conceptualization, methodology, data curation, investigation, writing and editing of the manuscript, visualization, resources, and funding; IO assisted in data curation, investigation, resources, and funding; OOb involved in writing and editing of the manuscript; OOl assisted in conceptualization, supervision, and project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed using experimental animals were in accordance with the ethical standards of the procedures of the University of Ibadan Ethics Committee, Ibadan, Nigeria.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ojo, O.O., Obaidu, I.M., Obigade, O.C. et al. Quercetin and vitamin E ameliorate cardio-apoptotic risks in diabetic rats. Mol Cell Biochem 477, 793–803 (2022). https://doi.org/10.1007/s11010-021-04332-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04332-w