Abstract
Bone morphogenetic protein 9 (BMP9) is a recently discovered cytokine mainly secreted by the liver and is a member of the transforming growth factor β (TGF-β) superfamily. In recent years, an increasing number of studies have shown that BMP9 is associated with liver diseases, including nonalcoholic fatty liver disease (NAFLD), liver fibrosis and hepatocellular carcinoma (HCC), and BMP9 signaling may play dual roles in liver diseases. In this review, we mainly summarized and discussed the roles and potential mechanisms of BMP9 signaling in NAFLD, liver fibrosis and HCC. Specifically, this article will provide a better understanding of BMP9 signaling and new clues for the treatment of liver diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone morphogenetic proteins (BMPs) are multifunctional cytokines that include over 30 members to date and constitute the largest subgroup of the transforming growth factor β (TGF-β) superfamily [1]. According to their sequence homology and functional diversity, the BMP/growth differentiation factor (GDF) subfamily can be further divided into smaller subgroups: BMP2/4, BMP5/6/7/8, BMP9/BMP10, GDF1/3, GDF5/6/7, GDF8/11, GDF9/BMP15 and GDF10/BMP3 [2]. BMPs were initially discovered in the context of bone and cartilage formation and fracture repair. In addition, BMPs are known to be involved in structures and processes throughout the entire body, ranging from embryo development to tissue homeostasis and regeneration [3, 4].
BMP9, also known as GDF2, is a newly discovered member of the BMPs family. BMP9 is mainly expressed by the liver and is secreted in both autocrine and paracrine ways [5, 6]. BMP9 was initially regarded as a hematopoietic [7], osteogenic and chondrogenic factor [8]. It has also been shown to regulate glucose and lipid metabolism [9], iron balance [10], cholinergic neuron differentiation [11], angiogenesis [12], lymphangiogenesis [13], liver regeneration and liver fibrosis [14, 15]. This article summarizes and discusses the roles and potential mechanisms of BMP9 signaling in nonalcoholic fatty liver disease (NAFLD), liver fibrosis and hepatocellular carcinoma (HCC), and may provide potential targets for the treatment of liver diseases.
Biochemistry of BMP9
BMP9 and its receptors
Nonparenchymal cells, such as hepatic stellate cells (HSCs), Kupffer cells (KCs) and endothelial cells, are regarded as the main BMP9-producing cell types in healthy rat livers [5]. While another study reported that cholangiocytes and hepatocytes were producers of BMP9 in mice and humans. As the number of hepatocytes is much more than that of cholangiocytes, hepatocytes are considered the major source of BMP9 [6]. However, a recent study demonstrated that HSCs were the major producers of BMP9, and BMP9 expression was further increased when HSCs were activated [14].
BMP9 is synthesized as a 429 amino acid (aa) precursor protein (Pre-pro-BMP9) composed of a signal peptide (22 aa), a prodomain (297 aa) and a mature protein (110 aa). The Pre-pro-BMP9 homodimerizes is then cleaved by serine endoprotease to produce two active forms: the short mature form and the complexed form, in which the prodomain remains noncovalently bound to the mature short form [1] (Fig. 1). Distinguishing from other BMPs, the mature form of BMP9 and the BMP9 pro-region complex have equal biological activity, whereas the pro-region alone is inactive [16].
Synthesis of BMP9. BMP9 is formed as a precursor protein (pre-pro-BMP9) composed of 429 amino acids (aa), including a signal peptide (22 aa), a prodomain (297 aa, 33 kDa) and a mature protein (110 aa, 12.5 kDa). Then, pre-pro-BMP9 homodimerizes and is cleaved by serine endoprotease to produce two active forms: the short mature form (25 kDa) and the complexed form (100 kDa)
BMPs transduce their signals by binding to transmembrane serine-threonine kinase receptors (type I and type II receptors). There are four known BMP type I receptors (ALK1, ALK2, ALK3 (BMPRIA) and ALK6 (BMPRIB)) and three type II receptors (BMPR-II, ActR-IIA and ActR-IIB). The heterotetrameric receptor complexes consist two molecules of type I and II receptors each. Upon BMP ligands binding to receptor complexes, constitutively active receptor kinases are phosphorylated that in turn phosphorylate downstream signaling molecules [1, 17, 18]. In contrast to TGF-β, which binds efficiently to type II receptors, BMPs bind to the type I receptor with high affinity. The preassembled type I receptor-ligand complex has increased binding affinity for type II receptors [19]. Several BMP receptors have been implicated in BMP9 signaling, including ALK1, ALK2, BMPR-II, ActR-IIB and the co-receptor endoglin. BMP9 and BMP10 were shown to be two specific high-affinity physiological ligands for ALK1 [20]. The affinity of BMP9 to its receptors may vary in different conditions, in cells with low levels of ALK1 or ActR-IIB, signal transduction can be initiated by forming a complex with lower affinity receptors [21, 22]. In endothelial cells, BMP9 has a high affinity for ALK1 and the co-receptor endoglin [20].
BMP9 signaling pathway
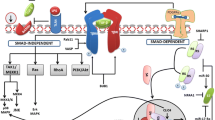
BMP9-receptor complexes activate the canonical Smad pathway and some Smad-independent signaling pathways to directly affect target gene transcription. Upon type I receptor activation, Smad1/5/8 (R-Smads) are phosphorylated, form complexes with Smad4 (co-Smad) and translocate to the nucleus. In the nucleus, these factors bind to and initiate the transcription of their target genes, interact with other DNA-binding proteins and recruit transcriptional coactivators or corepressors [1, 18] (Fig. 2). Notably, some research found that BMP9 also stimulated Smad2 and Smad3 phosphorylation, which is classically associated with TGF-β signaling. However, this pathway has only been reported in certain endothelial cell lines [23, 24].
BMP9 signaling pathway. Smad-dependent signaling: BMP9 binds to BMP receptors located on the cell membrane. The coreceptor endoglin can regulate signal transduction via BMP receptors. The complex acts on Smad1/5/8 (R-Smads) and phosphorylates it, and then R-Smads combine with Smad4, bind to the target genes and induce gene expression. Smad-independent signaling: BMPs initiate Smad-independent pathways, including MAPKs (p38, ERK and JNK), Wnt, PI3K/AKT, Rho-GTPase, NF-κB and modulate microRNAs in certain cell types and conditions
In certain cell types and conditions, BMPs initiate Smad-independent pathways, including MAPKs (p38, ERK and JNK), Wnt, PI3K/AKT, Rho-GTPase, NF-κB and modulate microRNAs. BMPs can trigger different signaling simultaneously or selectively. However, the mechanisms involved in these pathways are not completely understood [25]. Some studies demonstrated that MAPKs were activated in BMP9-induced osteogenesis and differentiation of mesenchymal stem cells (MSCs) and appeared to modulate the Smad pathway [26,27,28]. JNKs [28] and p38 inhibition [27] resulted in reductions in BMP9-induced Smad signaling and the osteogenic differentiation of MSCs. Various interactions occur between different intracellular signal transduction pathways. Whether the canonical Smad pathway and the Smad-independent pathway interact with each other remains to be fully elucidated.
BMP9 in NAFLD
NAFLD has been considered to be the most common chronic liver disease in the world. It is also known as metabolic-associated fatty liver disease (MAFLD) and is defined as a clinicopathological syndrome [29,30,31]. With the increased incidence of type 2 diabetes mellitus (T2DM) and obesity, the incidence of NAFLD is increasing each year [32]. NAFLD comprises nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), which may develop into cirrhosis, HCC or even death. Multiple parallel hits, including oxidative stress, insulin resistance (IR), environmental elements, cytokines, intestinal microbiota and genetic differences, account for the development of NASH [33, 34].
BMP9 and T2DM
T2DM is a significant risk factor for NAFLD. The prevalence of NAFLD in patients with T2DM exceeds 60% [32]. Furthermore, the occurrence of T2DM appears to accelerate the progression of NAFLD and is a predictor of advanced fibrosis and mortality [35].
The literature shows that BMP9 is involved in regulating glucose homeostasis. In a functional genomics screening analysis of diabetes, BMP9 was identified as the first hepatic anti-diabetic factor in vitro and in vivo. Similar to insulin, BMP9 regulated directional glucose metabolism in hepatocytes by inhibiting hepatic glucose production, promoting glycogen synthesis and activating the expression of key lipid metabolism enzymes. However, unlike insulin, BMP9 reduced glycemia in not only diabetic but also healthy mice. It was hypothesized that BMP9 may be a hypoglycemic factor due to its potential therapeutic implications [36]. Subsequently, a study showed that BMP9 played a physiological role in glucose homeostasis as a hepatic insulin-sensitizing substance. The expression of hepatic BMP9 was decreased in three distinct rat models of insulin resistance, and could be upregulated by oral glucose challenge. In addition, the researchers found that serum BMP9 neutralization with anti-BMP9 antibodies induced glucose intolerance and IR in fasting rats [37]. It was also indicated that the transcription factor Smad5, which is activated by BMP9 and Akt2, regulated glucose uptake in skeletal muscle. This process may be a mechanism by which BMP9 modulates the effects of insulin [38]. Consistent with these data in animals, Luo et al. reported that circulating BMP9 levels correlated with glucose homeostasis and insulin sensitivity in humans. Circulating BMP9 levels were significantly decreased in newly diagnosed T2DM patients and negatively correlated with some clinical indexes of T2DM. Acute IR induced by hyperinsulinemia and lipid infusion could reduce circulating BMP9 levels [39]. In a clinical study, circulating BMP9 levels were measured in newly diagnosed metabolic syndrome (MetS) patients and healthy controls, and the levels of BMP9 were obviously lower in MetS patients than in healthy controls. Circulating BMP9 levels were associated with the main components of MetS and IR. In a multiple linear regression analysis, BMP9 was independently associated with T2DM, free fatty acids (FFA) and the homeostasis model assessment of insulin resistance (HOMA-IR). Furthermore, circulating BMP9 levels gradually decreased as the number of MetS components increased [40]. Similarly, circulating BMP9 concentrations were negatively associated with the HOMA-IR [41]. Under high fat diet (HFD) feeding, mice administered the sh-BMP9 gene exhibited worsened glucose tolerance and IR [35]. Yang et al. found that hepatic BMP9 expression was reduced in IR mice and in diabetic patients. In HFD-induced mice, the overexpression of hepatic BMP9 could mitigate glucose tolerance and IR. In mice treated with adenovirus-BMP9 and glucosamine-treated liver cells, the phosphorylation of insulin signaling molecules were increased [9]. Overall, we can conclude that BMP9 plays a positive role in IR and T2DM. In addition, BMP9 can exert a leptin-like effect to reduce food intake and lipid accumulation, as well as increase energy metabolism [36, 42], and is expected to become a new target for the treatment of NAFLD.
BMP9 and obesity
Obesity increases the risk of NAFLD, and the prevalence of NAFLD is positively correlated with an increase in body mass index (BMI). The prevalence of NAFLD is approximately 25% in the general population but is increased to more than 90% in very obese individuals [32, 34]. This finding highlights the significance of including weight management in any NAFLD treatment.
Adipose tissue is closely associated with energy homeostasis and could be a target against obesity, IR and T2DM. There are two distinct types of fat, white adipose tissue (WAT) and brown adipose tissue (BAT). WAT usually serves as a lipid reservoir, and BAT dissipates energy as heat via thermogenesis [43]. WAT is also a remarkable endocrine organ that secretes adipokines. The secretion of proinflammatory adipokines from WAT due to obesity-mediated dysfunction has been reported to cause IR and T2DM [44, 45]. In addition, BAT was reported to regulate glucose homeostasis and improve insulin sensitivity [46]. Recently, Sooho and colleagues found that BMP9 induced WAT browning, suppressed HFD-induced obesity, and improved obesity-mediated IR and NAFLD, which was mediated by enhancing the expression of FGF21 [47, 48]. It was also shown that MB109 (a recombinant derivative of human BMP9), could upregulate FGF21 expression in obese mice [48]. By enhancing the expression of FGF21, MB109 reduced pathological liver manifestations caused by obesity, including reducing serum ALT, AST and total cholesterol levels and inhibiting lipid accumulation in the liver. Furthermore, MB109 enhanced glucose and lipid metabolism in obese mice in a dose-dependent manner. The results showed that BMP9 may have an important therapeutic effect on obesity-related NAFLD. Yong et al. found that BMP9 knockout could decrease peroxisome proliferator–activated receptor α (PPARα) expression, which further resulted in a reduction in fatty acid oxidation gene expression, thereby exacerbating triglyceride accumulation and expediting obesity, IR, and hyperglycemia and promoting liver steatosis [49]. Consistent with this finding, Sun et al. revealed that BMP9 played important roles in both lipid and glucose metabolism and inflammatory responses in the progression of NAFLD. The researchers found that BMP9 could alleviate obesity, liver steatosis and macrophage infiltration by decreasing promoter chromatin accessibility of Cers6, Fabp4, Fos and Tlr1 [50]. These findings above indicated that BMP9 could improve NAFLD. However, our recent study showed that overexpression of BMP9 promoted methionine choline deficiency (MCD)‑induced NASH in nonobese mice. We demonstrated that BMP9 overexpression enhanced MCP‑1 expression and promoted the recruitment and polarization of hepatic macrophages, thus leading to worsened NASH [51]. The conflicting results from different studies may be due to the use of different animal models. The HFD-induced obese model is usually used to evaluate the IR associated with fatty liver; however, the MCD-induced lean model has been widely used to evaluate the mechanisms underlying NASH in rodents. Similarly, a study indicated that BMP9 increased the number of F4/80-positive cells in the liver and modulated the lipopolysaccharide (LPS)-mediated inflammatory response [52]. Therefore, we conclude that BMP9 may play a negative role in lean NAFLD.
BMP9 in liver fibrosis
Liver fibrosis is the consequence of nearly all chronic liver diseases and is a major determinant of prognosis in patients with liver disease. Advanced liver fibrosis can lead to cirrhosis, liver failure, and even multiorgan dysfunction and can become a medical burden worldwide [53, 54]. HSCs are the main executors of fibrogenesis. Liver cytokines activate quiescent HSCs and induce their transformation to myofibroblasts [55], which are highly proliferative, migratory, and contractile cells that secrete extracellular matrix (ECM) upon activation. Then, HSCs synthesize ECM components and inhibit ECM degradation, thereby leading to excess ECM deposition and the occurrence of liver fibrosis [56]. To date, multiple signaling pathways have been shown to modulate the progression of liver fibrosis, including TGF-β, BMPs and Smad [57,58,59,60].
BMP9 has been widely studied in liver fibrosis, because it is mainly produced in the liver [6]. Recently, Breitkopf-Heinlein et al. reported the role of BMP9 as a profibrogenic factor in the liver [14]. In that study, the researchers found that BMP9 was predominately synthesized by HSCs and acted on HSCs, thereby resulting in the proliferation and transformation of HSCs into myofibroblasts, which could accelerate the synthesis of ECM. However, the team also reported that there was no significant change in BMP9 expression in patients with hepatitis B virus-associated liver fibrosis [14]. As the main type I receptor in liver nonparenchymal cells [25], ALK1/Smad1/Id1 signaling could induce HSCs to transform into fibroblasts [61]. The BMP9 antagonist ALK1-Fc can reduce collagen accumulation and improve fibrosis [14]. In addition, the promoting effect of BMP9 on liver fibrosis may be the result of cellular interactions between HSCs and liver sinusoidal endothelial cells (LSECs), since LSECs have been observed to directly control the activation of HSCs [62] and ALK1 is highly expressed in LSECs [14]. It was proposed that epithelial to mesenchymal transition (EMT) may have an important effect on liver fibrosis [63, 64]. Research has demonstrated that BMP9/ALK1/Smad1 signaling is involved in EMT by downregulating of E-cadherin and upregulating of the mesenchymal cell marker vimentin [65]. Similarly, Li et al. demonstrated that BMP9 directly activated HSCs via Smad signaling to induce hepatic fibrosis. Furthermore, serum BMP9 levels increased with the stage of liver fibrosis in some patients [15]. Munoz-Felix et al. found that besides Smad1/5/8 signaling, BMP9 can also activate Smad2/3 and Erk1/2, inducing the expression of fibrosis-related molecules in cultured mouse fibroblasts [66]. Deletion of BMP9 reduced liver damage and fibrosis and improved the hepatic regenerative response through overactivation of the ERK-MAPK, PI3K/AKT and c-Met signaling pathways in a 3,5 diethoxycarbonyl-1,4 dihydrocollidine-induced cholestatic liver injury model [67]. These studies reveal that BMP9 levels were positively correlated with the progression of hepatic fibrosis. However, Desroches-Castan et al. found that BMP9 had a protective effect on perivascular liver fibrosis in a 129/Ola mouse model, and BMP9 deletion controlled LSECs fenestration and terminal differentiation, thereby inducing the capillarization of LSECs and causing fibrosis, suggesting that BMP9 might protect against liver fibrosis [68]. This is opposite to the function of BMP9 in hepatic fibrosis. The team also found no difference in LSECs fenestration and differentiation markers between wild-type and BMP9-knockout mice on a C57BL/6 background [69]. Overall, it can be concluded that the role of BMP9 in LSECs fenestration and hepatic perivascular fibrosis depends on the genetic background.
In general, BMP9 is an important cytokine in the development of hepatic fibrosis. The above evidence indicates that BMP9 plays a positive role in the activation of HSCs and promotes fibrosis. However, BMP9 controls the differentiation and capillarization of LSESs and improves perivascular liver fibrosis. The different functions of BMP9 may be due to the different liver microenvironments, and more studies should be done to explore the mechanisms of BMP9 in liver fibrosis.
BMP9 in HCC
HCC is the major primary liver malignancy and has been considered the sixth most common cancer and the fourth most cancer-related death in the world; its morbidity and mortality rates have increased in recent years [70]. HCC is very intractable to treatment. Even after surgery or percutaneous ablation, 70% of patients experience recurrence within five years [71]. Once tumors progress to an advanced stage, current medical therapies offer only a marginal survival benefit. Except for some patients who are diagnosed early and receive treatment, such as liver transplantation or surgical resection, the prognosis remains poor. The poor prognosis and high recurrence rate of HCC patients are associated with the tendency of HCC to exhibit tumor invasiveness and form intrahepatic and extrahepatic metastases. EMT is a process in which cells gain mesenchymal characteristics by losing their epithelial characteristics, such as reductions in cell adhesion and polarity [63, 72]. EMT is associated with a variety of processes, including tumor initiation, malignant progression, tumor cell migration, venous invasion, metastasis and treatment resistance [72]. Epithelial plasticity has become a hot topic in the study of HCC, and TGF-β signaling is considered to be a key inducer of the EMT phenotype in malignant liver cells. TGF-β plays dual roles in tumor: in normal and early tumor cells, TGF-β inhibits cell growth and promotes apoptosis; in late tumor cells, it promotes the growth, invasion, EMT and metastasis of tumors [73,74,75].
In recent years, BMP9 has been found to play different roles in the progression of different tumors. BMP9 promotes the proliferation of ovarian cancer cells and inhibits the proliferation, migration and invasion of osteosarcoma cells, prostate cancer cells and breast cancer cells [76,77,78]. However, the role of BMP9 signaling in HCC is still unclear. BMP9 has been found to stabilize healthy liver cells and help maintain the polarization state and function of cells. Furthermore, physiological levels of BMP9 inhibit the proliferation of mature (healthy) vessels and exert stabilizing effects, but high levels can promote angiogenesis in different tumors. It was reported that BMP9 was an EMT-inducing factor in HCC. BMP9 enhanced cell migration and was positively correlated with the invasiveness of the tumor [65]. In HepG2 cells, BMP9 triggered Smad1/5/8 phosphorylation and upregulated Id1 expression. Importantly, HepG2 cells generate autocrine BMP9 to support their own proliferation and growth. BMP9 also triggered cell cycle progression and had a prominent antiapoptotic effect on HepG2 cells, which suggests that BMP9 may have a protumorigenic effect [79]. Garcia-Alvaro et al. found that BMP9 induced Smad-dependent and Smad-independent signaling, specifically that of p38 MAPK and PI3K/AKT. However, only the p38 MAPK pathway participated in BMP9-induced promotion of HepG2 cell growth. The researchers demonstrated that p38 MAPK activation was required for the protective effect of BMP9 on serum detoxification-induced apoptosis [80]. These findings help us understand BMP9 signaling in the protumorigenic process in the liver. Given that HCC is a hypervascularized tumor [81], ALK1 is highly expressed in the blood vessels of liver tumors. ALK1 inhibition may be a good therapeutic strategy for HCC. In this context, some drugs based on the antiangiogenic properties of ALK1 inhibitors have been produced [82, 83]. Furthermore, the results presented in this study showing protumorigenic effects of BMP9 on HCC cells provide further evidence for the use of ALK1 inhibitors in HCC treatment.
However, a study showed that MB109 inhibited the proliferation of HCC cells via p38 MAPK/ID3/p21 signaling, which induced survivin suppression and G0/G1 cell cycle arrest. In addition, persistent MB109 treatment restrained the expression of liver cancer stem cell (LCSC) markers. This finding also confirmed the ability of MB109 to suppress LCSCs and exert antitumor effects in vivo [84]. Contrary to the previous research findings, these results demonstrated the important therapeutic potential of MB109 to activate BMP9 signaling for antitumor therapies.
Overall, these results indicate that the effect of BMP9 signaling on liver cancer remains dubious, and BMP9 signaling may be a potential target for HCC treatment. Besides, further research should be implemented to determine how BMP9 signaling regulates HCC and why BMP9 signaling has different effects on different HCC cell types.
Conclusion
BMP9 is a multifunctional cytokine that plays an important role in many cellular processes, including bone and cartilage formation, glucose and lipid metabolism, iron balance, cholinergic neuron differentiation, angiogenesis and lymphangiogenesis. The liver is the main organ that expresses and secretes BMP9; thus, BMP9 may be involved in the progression of liver diseases. In this review, we described the function and complex mechanism of BMP9 signaling in NAFLD, liver fibrosis and HCC (Table 1 and Fig. 3). As mentioned previously, BMP9 can regulate glucose and lipid homeostasis by inhibiting liver gluconeogenesis, transforming WAT to BAT, increasing insulin sensitivity, inhibiting liver lipid deposition and playing a leptin-like role; thus, it has positive effects on T2DM and obesity. T2DM and obesity are two major and independent risk factors for NAFLD. In HFD-induced obese mice, Yong et al. found that BMP9 knockout promoted liver steatosis by decreasing the expression of the fatty acid oxidation gene PPARα [49]. Sun et al. revealed that BMP9 plays important roles both in lipid and glucose metabolism and in inflammatory responses in the progression of NAFLD [50]. In contrast to these results, BMP9 overexpression enhanced MCP‑1 expression and promoted the recruitment and polarization of hepatic macrophages, thus leading to worsened NASH in MCD-induced lean mice [51]. Therefore, the role of BMP9 in NAFLD needs further study.
NAFLD comprises NAFL and NASH, which may develop into advanced fibrosis, HCC or even death. In patients with NASH, 39.1–40.8% will progress to advanced fibrosis [85]. In terms of metabolic syndrome factors, BMI and the occurrence of diabetes have been associated with worsened fibrosis stage [86]. Since BMP9 has a positive effect on T2DM and obesity, we hypothesize that BMP9 may improve NASH-related fibrosis. However, in an MCD-induced mouse model, BMP9 overexpression induced an increase in TGF‑β1 and PAI‑1 and downregulated MMP‑2, which are both profibrotic features, although HSCs activation, as indicated by α‑SMA positivity in the liver, was not promoted by BMP9 [51]. To date, mouse models of the effect of BMP9 on liver fibrosis are usually induced by chemical reagents, such as carbon tetrachloride, and BMP9 has rarely been studied in NASH-related fibrosis. Therefore, additional experiments are needed to investigate the role of BMP9 in NASH-associated liver fibrosis.
Obesity is believed to be a significant risk factor for the development of several malignancies, including HCC. Multiple obesity-mediated mechanisms are thought to play roles in the development of HCC with and without NAFLD [87]. T2DM and IR may also contribute to the development of HCC, and up to 70% of diabetic patients also have NAFLD, which is itself a risk factor for HCC. However, a prospective cohort study showed that the increased risk of HCC in diabetic patients persisted even after excluding patients diagnosed with NAFLD [88], suggesting an independent effect. As mentioned previously, BMP9 may inhibit HCC by ameliorating T2DM and obesity. However, BMP9 could induce HCC by enhancing EMT, promoting proliferation and inhibiting apoptosis in HepG2 cells. In addition, no NAFLD-HCC research has been conducted on the effect of BMP9 on NAFLD-HCC until now. Thus, further research needs to be done in the future.
In summary, we found that BMP9 may have dual roles in different conditions and diseases and may be a biomarker for the diagnosis, monitoring and management of liver diseases. Knowledge of BMP9 signaling will expand our understanding of the development of NAFLD, liver fibrosis, and HCC. Further experiments should be carried out to investigate the role of BMP9 in liver disease.
References
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620. https://doi.org/10.1016/j.cellsig.2010.10.003
Kim M, Choe S (2011) BMPs and their clinical potentials. BMB Rep 44(10):619–634. https://doi.org/10.5483/BMBRep.2011.44.10.619
Sanchez-Duffhues G, Hiepen C, Knaus P, Ten Dijke P (2015) Bone morphogenetic protein signaling in bone homeostasis. Bone 80:43–59. https://doi.org/10.1016/j.bone.2015.05.025
Hiepen C, Yadin D, Rikeit P, Dörpholz G, Knaus P (2016) Actions from head to toe: an update on bone/body morphogenetic proteins in health and disease. Cytsokine Growth Factor Rev 27:1–11. https://doi.org/10.1016/j.cytogfr.2015.12.006
Miller AF, Harvey SAK, Thies RS, Olson MS (2000) Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem 275(24):17937–17945. https://doi.org/10.1074/jbc.275.24.17937
Bidart M, Ricard N, Levet S, Samson M, Mallet C, David L et al (2012) BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell Mol Life Sci 69(2):313–324. https://doi.org/10.1007/s00018-011-0751-1
Ploemacher RE, Engels LJ, Mayer AE, Thies S, Neben S (1999) Bone morphogenetic protein 9 is a potent synergistic factor for murine hemopoietic progenitor cell generation and colony formation in serum-free cultures. Leukemia 13(3):428–437. https://doi.org/10.1038/sj.leu.2401363
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT et al (2004) Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 11(17):1312–1320. https://doi.org/10.1038/sj.gt.3302298
Yang M, Liang Z, Yang M, Jia Y, Yang G, He Y et al (2019) Role of bone morphogenetic protein-9 in the regulation of glucose and lipid metabolism. FASEB J 33(9):10077–10088. https://doi.org/10.1096/fj.201802544RR
Truksa J, Peng H, Lee P, Beutler E (2006) Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A 103(27):10289–10293. https://doi.org/10.1073/pnas.0603124103
Schnitzler AC, Mellott TJ, Lopez-Coviella I, Tallini YN, Kotlikoff MI, Follettie MT et al (2010) BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. J Neurosci 30(24):8221–8228. https://doi.org/10.1523/jneurosci.5611-09.2010
Garcia de Vinuesa A, Abdelilah-Seyfried S, Knaus P, Zwijsen A, Bailly S (2016) BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev 27:65–79. https://doi.org/10.1016/j.cytogfr.2015.12.005
Yoshimatsu Y, Lee YG, Akatsu Y, Taguchi L, Suzuki HI, Cunha SI et al (2013) Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A 110(47):18940–18945. https://doi.org/10.1073/pnas.1310479110
Breitkopf-Heinlein K, Meyer C, Konig C, Gaitantzi H, Addante A, Thomas M et al (2017) BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 66(5):939–954. https://doi.org/10.1136/gutjnl-2016-313314
Li P, Li Y, Zhu L, Yang Z, He J, Wang L et al (2018) Targeting secreted cytokine BMP9 gates the attenuation of hepatic fibrosis. Biochim Biophys Acta Mol Basis Dis 1864(3):709–720. https://doi.org/10.1016/j.bbadis.2017.12.008
Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L et al (2005) Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem 280(26):25111–25118. https://doi.org/10.1074/jbc.M503328200
Yadin D, Knaus P, Mueller TD (2016) Structural insights into BMP receptors: specificity, activation and inhibition. Cytokine Growth Factor Rev 27:13–34. https://doi.org/10.1016/j.cytogfr.2015.11.005
Nickel J, Mueller TD (2019) Specification of BMP Signaling. Cells 8(12):1579. https://doi.org/10.3390/cells8121579
Lawera A, Tong Z, Thorikay M, Redgrave RE, Cai J, Mv D et al (2019) Role of soluble endoglin in BMP9 signaling. Proc Natl Acad Sci U S A 116(36):17800–17808. https://doi.org/10.1073/pnas.1816661116
David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109(5):1953–1961. https://doi.org/10.1182/blood-2006-07-034124
Herrera B, van Dinther M, Ten Dijke P, Inman GJ (2009) Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res 69(24):9254–9262. https://doi.org/10.1158/0008-5472.can-09-2912
Herrera B, Dooley S, Breitkopf-Heinlein K (2014) Potential roles of bone morphogenetic protein (BMP)-9 in human liver diseases. Int J Mol Sci 15(4):5199–5220. https://doi.org/10.3390/ijms15045199
Star GP, Giovinazzo M, Langleben D (2010) Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: a potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc Res 80(3):349–354. https://doi.org/10.1016/j.mvr.2010.05.010
Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L et al (2007) BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 120(6):964–972. https://doi.org/10.1242/jcs.002949
Herrera B, Sánchez A, Fabregat I (2012) BMPS and liver: more questions than answers. Curr Pharm Des 18(27):4114–4125. https://doi.org/10.2174/138161212802430503
Xu DJ, Zhao YZ, Wang J, He JW, Weng YG, Luo JY (2012) Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep 45(4):247–252. https://doi.org/10.5483/bmbrep.2012.45.4.247
Zhao Y, Song T, Wang W, Wang J, He J, Wu N et al (2012) P38 and ERK1/2 MAPKs act in opposition to regulate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS ONE 7(8):e43383. https://doi.org/10.1371/journal.pone.0043383
Zhao YF, Xu J, Wang WJ, Wang J, He JW, Li L et al (2013) Activation of JNKs is essential for BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep 46(8):422–427. https://doi.org/10.5483/bmbrep.2013.46.8.266
Younossi ZM (2019) Non-alcoholic fatty liver disease—A global public health perspective. J Hepatol 70(3):531–544. https://doi.org/10.1016/j.jhep.2018.10.033
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73(1):202–209. https://doi.org/10.1016/j.jhep.2020.03.039
Eslam M, Sanyal AJ, George J, International Consensus P (2020) MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158(7):1999–2014. https://doi.org/10.1053/j.gastro.2019.11.312
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E et al (2019) Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69(6):2672–2682. https://doi.org/10.1002/hep.30251
Takaki A, Kawai D, Yamamoto K (2013) Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci 14(10):20704–20728. https://doi.org/10.3390/ijms141020704
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24(7):908–922. https://doi.org/10.1038/s41591-018-0104-9
Jia Y, Niu D, Li Q, Huang H, Li X, Li K et al (2019) Effective gene delivery of shBMP-9 using polyethyleneimine-based core-shell nanoparticles in an animal model of insulin resistance. Nanoscale 11(4):2008–2016. https://doi.org/10.1039/c8nr08193j
Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q et al (2003) An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol 21(3):294–301. https://doi.org/10.1038/nbt795
Caperuto LC, Anhe GF, Cambiaghi TD, Akamine EH, Do Carmo Buonfiglio D, Cipolla-Neto J et al (2008) Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: possible candidate for hepatic insulin-sensitizing substance. Endocrinology 149(12):6326–6335. https://doi.org/10.1210/en.2008-0655
Anhê FF, Lellis-Santos C, Leite AR, Hirabara SM, Boschero AC, Curi R et al (2010) Smad5 regulates Akt2 expression and insulin-induced glucose uptake in L6 myotubes. Mol Cell Endocrinol 319(1–2):30–38. https://doi.org/10.1016/j.mce.2010.01.003
Luo Y, Li L, Xu X, Wu T, Yang M, Zhang C et al (2017) Decreased circulating BMP-9 levels in patients with type 2 diabetes is a signature of insulin resistance. Clin Sci 131(3):239–246. https://doi.org/10.1042/CS20160543
Xu X, Li X, Yang G, Li L, Hu W, Zhang L et al (2017) Circulating bone morphogenetic protein-9 in relation to metabolic syndrome and insulin resistance. Sci Rep 7(1):17529. https://doi.org/10.1038/s41598-017-17807-y
Huang H, Wang W, Yang G, Zhang Y, Li X, Liu H et al (2018) Circulating bone morphogenetic protein-9 levels are associated with hypertension and insulin resistance in humans. J Am Soc Hypertens 12(5):372–380. https://doi.org/10.1016/j.jash.2018.02.007
Morioka T, Emoto M, Yamazaki Y, Kurajoh M, Motoyama K, Mori K et al (2018) Plasma soluble leptin receptor levels are associated with pancreatic β-cell dysfunction in patients with type 2 diabetes. J Diabetes Investig 9(1):55–62. https://doi.org/10.1111/jdi.12657
Cristancho AG, Lazar MA (2011) Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12(11):722–734. https://doi.org/10.1038/nrm3198
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. https://doi.org/10.1038/nature05482
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127(1):1–4. https://doi.org/10.1172/jci92035
Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F (2015) Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol 6:4. https://doi.org/10.3389/fphys.2015.00004
Kuo MM, Kim S, Tseng CY, Jeon YH, Choe S, Lee DK (2014) BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials 35(10):3172–3179. https://doi.org/10.1016/j.biomaterials.2013.12.063
Kim S, Choe S (1862) Lee DK (2016) BMP-9 enhances fibroblast growth factor 21 expression and suppresses obesity. Biochim Biophys Acta 7:1237–1246. https://doi.org/10.1016/j.bbadis.2016.04.006
Yang Z, Li P, Shang Q, Wang Y, He J, Ge S et al (2020) CRISPR-mediated BMP9 ablation promotes liver steatosis via the down-regulation of PPARa expression. Sci Adv 6(48):eabc5022. https://doi.org/10.1126/sciadv.abc5022
Sun QJ, Cai LY, Jian J, Cui YL, Huang CK, Liu SQ et al (2021) The Role of bone morphogenetic protein 9 in nonalcoholic fatty liver disease in mice. Front Pharmacol 11:605967. https://doi.org/10.3389/fphar.2020.605967
Li Q, Liu B, Breitkopf-Heinlein K, Weng H, Jiang Q, Dong P et al (2019) Adenovirus-mediated overexpression of bone morphogenetic protein-9 promotes methionine choline deficiency-induced no-nalcoholic steatohepatitis in non-obese mice. Mol Med Rep 20(3):2743–2753. https://doi.org/10.3892/mmr.2019.10508
Gaitantzi H, Karch J, Germann L, Cai C, Rausch V, Birgin E et al (2020) BMP-9 modulates the hepatic responses to LPS. Cells 9(3):617. https://doi.org/10.3390/cells9030617
Hernandez-Gea V, Friedman SL (2011) Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456. https://doi.org/10.1146/annurev-pathol-011110-130246
Tacke F, Trautwein C (2015) Mechanisms of liver fibrosis resolution. J Hepatol 63(4):1038–1039. https://doi.org/10.1016/j.jhep.2015.03.039
Higashi T, Friedman SL, Hoshida Y (2017) Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 121:27–42. https://doi.org/10.1016/j.addr.2017.05.007
Lee YA, Wallace MC, Friedman SL (2015) Pathobiology of liver fibrosis: a translational success story. Gut 64(5):830–841. https://doi.org/10.1136/gutjnl-2014-306842
Fabre T, Molina MF (2018) Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci Immunol 3(28):7754. https://doi.org/10.1126/sciimmunol.aar7754
Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO (2018) Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 68–69:435–451. https://doi.org/10.1016/j.matbio.2018.04.006
Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN (2019) TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 8(11):1419. https://doi.org/10.3390/cells8111419
Herrera B, Addante A, Sanchez A (2017) BMP signalling at the crossroad of liver fibrosis and regeneration. Int J Mol Sci 19(1):39. https://doi.org/10.3390/ijms19010039
Muñoz-Félix JM, González-Núñez M, López-Novoa JM (2013) ALK1-Smad1/5 signaling pathway in fibrosis development: friend or foe? Cytokine Growth Factor Rev 24(6):523–537. https://doi.org/10.1016/j.cytogfr.2013.08.002
Maretti-Mira AC, Wang X, Wang L, DeLeve LD (2019) Incomplete differentiation of engrafted bone marrow endothelial progenitor cells initiates hepatic fibrosis in the rat. Hepatology 69(3):1259–1272. https://doi.org/10.1002/hep.30227
Nieto MA, Huang RY, Jackson RA, Thiery JP (2016) Emt: 2016. Cell 166(1):21–45. https://doi.org/10.1016/j.cell.2016.06.028
Kulik L, El-Serag HB (2019) Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156(2):477–491. https://doi.org/10.1053/j.gastro.2018.08.065
Li Q, Gu X, Weng H, Ghafoory S, Liu Y, Feng T et al (2013) Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci 104(3):398–408. https://doi.org/10.1111/cas.12093
Munoz-Felix JM, Cuesta C, Perretta-Tejedor N, Subileau M, Lopez-Hernandez FJ, Lopez-Novoa JM et al (2016) Identification of bone morphogenetic protein 9 (BMP9) as a novel profibrotic factor in vitro. Cell Signal 28(9):1252–1261. https://doi.org/10.1016/j.cellsig.2016.05.015
Addante A, Roncero C, Almale L, Lazcanoiturburu N, Garcia-Alvaro M, Fernandez M et al (2018) Bone morphogenetic protein 9 as a key regulator of liver progenitor cells in DDC-induced cholestatic liver injury. Liver Int 38(9):1664–1675. https://doi.org/10.1111/liv.13879
Desroches-Castan A, Tillet E, Ricard N, Ouarné M, Mallet C, Belmudes L et al (2019) Bone morphogenetic protein 9 is a paracrine factor controlling liver sinusoidal endothelial cell fenestration and protecting against hepatic fibrosis. Hepatology 70(4):1392–1408. https://doi.org/10.1002/hep.30655
Desroches-Castan A, Tillet E, Ricard N, Ouarne M, Mallet C, Feige JJ et al (2019) Differential consequences of Bmp9 deletion on sinusoidal endothelial cell differentiation and liver fibrosis in 129/Ola and C57BL/6 Mice. Cells 8(9):1079. https://doi.org/10.3390/cells8091079
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Kanwal F, Singal AG (2019) Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 157(1):54–64. https://doi.org/10.1053/j.gastro.2019.02.049
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29(3):212–226. https://doi.org/10.1016/j.tcb.2018.12.001
Ikushima H, Miyazono K (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10(6):415–424. https://doi.org/10.1038/nrc2853
Colak S, Ten Dijke P (2017) Targeting TGF-β signaling in cancer. Trends Cancer 3(1):56–71. https://doi.org/10.1016/j.trecan.2016.11.008
Batlle E, Massagué J (2019) Transforming growth factor-β signaling in immunity and cancer. Immunity 50(4):924–940. https://doi.org/10.1016/j.immuni.2019.03.024
Ye L, Kynaston H, Jiang WG (2008) Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res 6(10):1594–1606. https://doi.org/10.1158/1541-7786.mcr-08-0171
Ouarné M, Bouvard C, Boneva G, Mallet C, Ribeiro J, Desroches-Castan A et al (2018) BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer. J Exp Clin Cancer Res 37(1):209. https://doi.org/10.1186/s13046-018-0885-1
Varadaraj A, Patel P, Serrao A, Bandyopadhay T, Lee NY, Jazaeri AA et al (2015) Epigenetic regulation of GDF2 suppresses anoikis in ovarian and breast epithelia. Neoplasia 17(11):826–838. https://doi.org/10.1016/j.neo.2015.11.003
Herrera B, Garcia-Alvaro M, Cruz S, Walsh P, Fernandez M, Roncero C et al (2013) BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS ONE 8(7):e69535. https://doi.org/10.1371/journal.pone.0069535
Garcia-Alvaro M, Addante A, Roncero C, Fernandez M, Fabregat I, Sanchez A et al (2015) BMP9-induced survival effect in liver tumor cells requires p38MAPK activation. Int J Mol Sci 16(9):20431–20448. https://doi.org/10.3390/ijms160920431
Morse MA, Sun W (2019) The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res 25(3):912–920. https://doi.org/10.1158/1078-0432.ccr-18-1254
Simonelli M, Zucali P, Santoro A, Thomas MB, de Braud FG, Borghaei H et al (2016) Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase-1, in patients with hepatocellular carcinoma. Ann Oncol 27(9):1782–1787. https://doi.org/10.1093/annonc/mdw240
Abou-Alfa GK, Miksad RA, Tejani MA, Williamson S, Gutierrez ME, Olowokure OO et al (2019) A phase Ib, open-label study of dalantercept, an activin receptor-like kinase 1 ligand trap, plus sorafenib in advanced hepatocellular carcinoma. Oncologist 24(2):161-e170. https://doi.org/10.1634/theoncologist.2018-0654
Jung JW, Yoon S-M, Kim S, Jeon Y-H (2016) Bone morphogenetic protein-9 is a potent growth inhibitor of hepatocellular carcinoma and reduces the liver cancer stem cells population. Oncotarget 7(45):73754–73768. https://doi.org/10.18632/oncotarget.12062
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R (2015) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13(4):643–654. https://doi.org/10.1016/j.cgh.2014.04.014
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34(3):274–285. https://doi.org/10.1111/j.1365-2036.2011.04724.x
Margini C, Dufour JF (2016) The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 36(3):317–324. https://doi.org/10.1111/liv.13031
El-Serag HB, Tran T, Everhart JE (2004) Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 126(2):460–468. https://doi.org/10.1053/j.gastro.2003.10.065
Funding
The work was supported by the National Natural Science Foundation of China (Grant No.81670515).
Author information
Authors and Affiliations
Contributions
QQJ: conceptualization, reviewed the literature, writing of original draft; BBL: conceptualization, revised the manuscript; KSX: critical revision and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, QQ., Liu, BB. & Xu, KS. New insights into BMP9 signaling in liver diseases. Mol Cell Biochem 476, 3591–3600 (2021). https://doi.org/10.1007/s11010-021-04182-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04182-6