Abstract
Background: Transplantation of bone marrow-derived mesenchymal stem cells (BMSCs) is a potential therapy for cerebral ischemia. However, the underlying protective mechanism remains undetermined. Here, we tested the hypothesis that transplantation of BMSCs via intravenous injection can alleviate neurological functional deficits through activating PI3K/AKT signaling pathway after cerebral ischemia in rats.
Methods: A cerebral ischemic rat model was established by the 2 h middle cerebral artery occlusion (MCAO). Twenty-four hours later, BMSCs (1 × 106 in 1 ml PBS) from SD rats were injected into the tail vein. Neurological function was evaluated by modified neurological severity score (mNSS) and modified adhesive removal test before and on d1, d3, d7, d10 and d14 after MCAO. Protein expressions of AKT, GSK-3β, CRMP-2 and GAP-43 were detected by Western-bolt. NF-200 was detected by immunofluorescence.
Results: BMSCs transplantation did not only significantly improve the mNSS score and the adhesive-removal somatosensory test after MCAO, but also increase the density of NF-200 and the expression of p-AKT, pGSK-3β and GAP-43, while decrease the expression of pCRMP-2. Meanwhile, these effects can be suppressed by LY294002, a specific inhibitor of PI3K/AKT.
Conclusion: These data suggest that transplantation of BMSCs could promote axon growth and neurological deficit recovery after MCAO, which was associated with activation of PI3K/AKT /GSK-3β/CRMP-2 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) have been found in various tissues such as bone marrow, adipose tissue, and umbilical cord blood [1], and can differentiate into multiple mesodermal lineages including osteoblasts, adipocytes and neurons in vitro [2,3,4]. Due to their self-renewal and potential multi-lineage properties, MSCs have been extensively studied in animal models of stroke [5]. The beneficial effects of MSC treatment may be due to their direct differentiation and paracrine effect. Many inflammatory cytokines and hypoxic conditions can stimulate the release of growth factors from MSCs, many of which are known activators of the AKT signal transduction pathway [6]. These growth factors promote restorative processes by activating AKT in the post-ischemic brain, such as angiogenesis [7], neurogenesis [8] and neuroprotection [9]. The evidence generated over the past years suggests that transplanted MSCs can reduce infarct volume and improve functional behavioral outcome following MCAO in rats [10]. But whether it could improve axon growth and the mechanism of transplanted MSC-induced functional benefit after MCAO remain unclear. Here, we tested the hypothesis that transplantation of BMSCs via intravenous injection can promote axon growth and alleviate neurological functional deficits through activating PI3K/AKT signaling pathway after cerebral ischemia in rats.
Methods
Ethics statement
All animals used in this study were provided by Animal Center of Fujian Medical University (Fuzhou, China). Animals were cared for in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). All study procedures were approved by Fujian Medical University Institutional Animal Care and Use Committee. The investigators responsible for molecular, cytological, histological and functional studies were blinded to the treatment groups. We make all efforts to minimize the number of animals used as well as their suffering.
Isolation culture and identification of BMSCs
We prepared primary BMSCs from the bone of male Sprague–Dawley rats weighted 60 to 80 g as described in our former study [11]. Rats were anesthetized with 10% chloral hydrate (3 ml/kg). The bilateral femurs and tibias were aseptically dissected and cut off. The marrows cells were extruded with Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma) containing 10% fetal bovine serum, 2 mmol/L glutamate and 100 U/ml penicillin/streptomycin, and cultured in chamber (37 °C, 5% CO2). After 24 h, the non-adherent cells were removed by changing the medium. Then culture medium was replaced approximately every 3 days. When dense colonies of spindle-shaped cells covered greater than 80% in the flask, the cells were passed into the secondary culture. They were lifted by 0.05% Trypsin-EDTA (Gibco). The 3 rd passage BMSCs were applied to the following experiments.
The differentiation of BMSCs towards the osteogenic and adipogenic lineage was carried out as previously described [12, 13]. For osteoblast differentiation, BMSCs were cultured for three weeks with osteogenic medium, containing 10−7 M dexamethasone, 50 μg/ml ascorbic acid and 10 mM β- glycerophosphate (Sigma). For adipocyte differentiation, BMSCs were cultured for 3 weeks with adipogenic medium, containing 10−6 M dexamethasone, 10 μg/ml insulin and 100 μg/ml 3-isobutyl-1-methylxantine (Sigma). Von kossa dyes and oil-red-O were employed to identify adipocytes and osteoblasts respectively.
Animal model
Male adult SD rats weighted 260 to 280 g were randomly divided into 4 groups: sham-operated group (Sham group), transient right middle cerebral artery occlusion group (MCAO group), MCAO+BMSCs-treated group (MCAO+BM group) and MCAO+BMSCs+LY294002 group (MCAO+BM + LY group) (n = 12). Male adult SD rats were subjected to 2 h middle cerebral artery occlusion (MCAO) [11]. Rats were anesthetized with 10% chloral hydrate (3 ml/kg). MCAO was induced by advancing a 4–0 surgical nylon suture (18.5 to 19.5 mm) with an round tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCAO. Two hours later, we restored the blood flow by withdrawing the nylon suture. The sham-operated group underwent the same procedure without inserting the suture. The rectal temperature was controlled at 37 °C with a homeothermic blanket. After recovering from anesthesia, the animals were allowed free access to food and water. All rats were killed on d14 after MCAO.
Cell and drug administration
SD rats received BMSCs (1 × 106 in 1 mL PBS) with ice bath by injecting into the tail vain 24 h after ischemia, which were harvested after culture for 3 or 4 passages and suspended in phosphate buffered saline (PBS). To investigate the role of the PI3-kinase pathway after MCAO, LY294002 (a PI3-kinase inhibitor, Cell Signaling) dissolved in the dimethyl sulfoxide and phosphate-buffered saline was injected into intracerebroventricularly according to atlas (3 μL, bregma; 1.8 mm lateral, 1.0 mm posterior, 3.5 mm deep) 1 h before MCAO.
Behavioral tests
To confirm whether the BMSCs could promote neurological deficit recovery following stroke, two behavioral tests, the modified neurological severity scores (mNSS) test [14] and the adhesive-removal somatosensory test [15], were performed before MCAO (baseline) and on d1, d3, d7, d10 and d14 after MCAO .
mNSS test
The mNSS test is a composite of motor, sensory, balance and reflex tests [16]. Neurological function is graded on a scale of 0 to 18 (normal score = 0, maximal deficit score = 18). In the severity scores of injury, 1 point is awarded for a specific abnormal behavior or for the lack of a tested reflex; 13 to 18 indicates severe injury; 7 to 12, moderate injury; 1 to 6, mild injury. Thus, the higher the score indicates a more severe injury.
Adhesive-removal somatosensory test
For the adhesive-removal somatosensory test, somatosensory deficit was measured both before and after surgery [17]. Before surgery, the animals were trained for 3 days. Once the rats were able to remove the dots within 120 s, they were subjected to MCAO. All rats were familiarized with the testing environment. In the initial test, two pieces of adhesive-backed paper dots (of each size, 113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The time to remove each stimulus from forelimbs was recorded on 5 trials per day. Individual trials were separated by at least 120 s.
Western blot analysis
For brain extracts, the animals were sacrificed with 10% chloral hydrate and their brain were removed on d14 after MCAO (n = 6). The ipsilateral hemispheres of MCAO and normal control rat brain were dissected on ice. And then the wet weight was rapidly measured. According to the instruction of RIPA (Beyotime) lysis buffer, total protein was extracted from cortex tissue. The sample was centrifuged at 13,000 r/min for 15 min at 4 °C. And then the supernatant was used for protein analysis. Protein concentration was determined with the BCA protein assay (Beyotime). Total protein (15 μg/lane) was separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (PVDF, Millipore) by semi-dry blotter (BIO-RAD, USA). The immunoblot was blocked for 2 h at room temperature, incubated by following primary antibodies overnight at 4 °C, washed and incubated for 2 h by secondary antibody with a 1:2000 dilution of horseradish peroxidase (HRP)-conjugated IgG secondary antibody, accordingly. Signals on membranes were visualized by an ECL western blotting detection kit (Beyotime) on Kodak XTB-01 films. The primary antibodies used in this study were rabbit anti-pAKT monoclonal antibody (1:1000, Cell signaling Technology), rabbit anti-AKT polyclonal antibody (1:1000, Cell signaling Technology), rabbit anti-pGSK-3β(Ser9) monoclonal antibody (1:1000, Cell signaling Technology), rabbit anti-GSK-3β polyclonal antibody (1:1000, Cell signaling Technology), rabbit anti- pCRMP-2(Thr514) monoclonal antibody (1:700, Cell signaling Technology), rabbit anti-CRMP-2 polyclonal antibody (1:1000, Cell signaling Technology), rabbit anti-GAP-43 monoclonal antibody (1:1000, Cell signaling Technology), mouse anti-GAPDH monoclonal antibody (1:500, Wuhan boster). All experiments were repeated three times. All bands from western blot were analyzed by Image J software (version 1.6 NIH).
Immunofluorescence
In order to detect the change of the axon, immunofluorescence was performed. Rats were euthanized with 10% chloral hydrate on d14 after MCAO. For preparation of frozen sections, rats were perfused transcardially with normal saline and paraformaldehyde and the brain samples were removed immediately. Blocks corresponding to coronal coordinates from bregma −1 to 1 mm were obtained and frozen rapidly in liquid nitrogen. A series of 8-um-thick sections were obtained. Antibodies used for fluorescent detection consisted of the following: rabbit anti-NF-200 (1:100; Sigma-Aldrich). Secondary antibodies used were Cy3 donkey anti-rabbit IgG (1:400, Jackson Immunoresearch). Negative controls were labeled in the absence of primary antibody. Finally, the sections were used to detect by a laser scanning confocal microscope (Zeiss, LSM510).
Statistical analysis
All the data were presented as mean ± SD. Statistical analysis was evaluated with SPSS19.0 software by Independent sample test and one way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test were used to measure statistical significance. Statistical significance was accepted at P < 0.05.
Results
Morphological and differentiation capacity of BMSCs
24 h after culture, there were many of lipid droplets and debris. After replacing the medium, minority adherent growing spindle cells could be recognized (Fig. 1a). On the third day, little small cell colony formation was observed. On the fifth day, the cells increased significantly in number and were arranged in bundles or whorls, and the colony formation became bigger. During the seventh to ninth day, more than 80% cell fused. Cell morphology and growth pattern were similar to the primary cells. The cell morphology was single fusiform (Fig. 1b). Three weeks after osteogenic induction, the cell morphology disappeared and black calcium mineralization deposition could be observed in the intercellular (Fig. 1c). Three weeks after adipogenic induction, lots of orange lipid droplets were visible (Fig. 1d).
Morphological characteristics and differentiation of BMSCs. (a) P0, BMSCs grow as a morphologically homogeneous population of fibroblast-like cells; (b) P3, BMSCs grow as whorls of densely packed spindle-shaped cells. (c) Cell stained with Von kossa dyes show that BMSCs differentiated into osteocyte of calcium deposits (black). (d) Cells stained with Oil-red-O dyes show that BMSCs differentiated into lipid laden adipocytes (red). Scale bars: 200 μm
BMSCs treatment improved functional recovery after MCAO
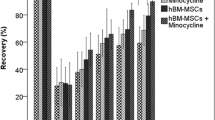
The neurological function was gradually improved with time in all rats evaluated by the mNSS score and removal test score (Fig. 2). There was no significant difference in mNSS score between MCAO+BMSCs group and MCAO group on d1, d3 after MCAO(p > 0.05). However, the MCAO+BMSCs(7.92 ± 0.67) group showed significant improvement in mNSS score compared with the MCAO group(8.67 ± 0.78) as early as 7 days after MCAO(p < 0.05). The therapeutic effects were sustained throughout the study period. The average score of mNSS in MCAO+BMSCs group was 17.4%, 24% and 28.4% less than MCAO group.
Recovery of behavioral deficits. Panel A represents sensorimotor recovery as measured by the mNSS score. Note that, compared with MCAO group, there was significant recovery in the mNSS score in MCAO+BMSCs group. N = 12, *p < 0.05 vs MCAO group. Panel B represents somatosensory recovery as measured by the adhesive-removal test. N = 12, *p < 0.05 vs MCAO group
No significant differences in adhesive-removal test in MCAO+BMSCs group was detected compared with MCAO group on d1, d3, d7 after MCAO(p > 0.05). The MCAO+BMSCs group(82.08 ± 9.73) showed significant improvement in adhesive-removal test compared to the MCAO group(94.42 ± 3.85) as early as 10 days after MCAO(p < 0.05). The average score of adhesive-removal test in MCAO+BMSCs group was 13.1% and 30.6% less than MCAO group on d10 and d14 respectively.
BMSCs treatment increased the protein expression of p-AKT and p-GSK-3β, while decreased the p-CRMP-2
The expressions of p-AKT, p-GSK-3β and p-CRMP-2 were detected by western blot (Fig. 3). Compared with MCAO group, the expressions of p-AKT and p-GSK-3β increased by 60.21% and 31.54% (p < 0.05), and p-CRMP2 decreased by 20.39% (p < 0.05) in MCAO+BMSCs group, respectively. Compared with MCAO+BMSCs group, the expressions of p-AKT and p-GSK-3β decreased by 78.08%, 39.76% (p < 0.05) and p-CRMP-2 increased by 137.36% in MCAO+BMSCs+LY294002 group.
Protein expression in rat cerebral cortex after BMSCs treatment in MCAO. Western blot was probed for p-AKT, p-GSK-3B and p-CRMP-2, respectively. The bar chart shows the ratio of different protein. Values correspond to mean ± SD (n = 6). ▲P < 0.05 vs. Sham group, ★P < 0.05 vs. MCAO group, #P < 0.05 vs. MCAO+BM group
Treatment with BMSCs promoted axon growth in cerebral white matter after MCAO
NF-200 and growth-associated protein-43 were used to detect the axon growth. In the Sham group, NF-200-posivive fibers were red-stained, densely distributed and regularly arranged. In MCAO group, the positive fibers were sparsely distribution and disordered arrangement, and the expression of NF-200 (0.0757 ± 0.00321) was decreased compared with the Sham group (0.1336 ± 0.00495) (p < 0.05). Treatment of BMSCs significantly increased the expression of NF-200 (0.1070 ± 0.00499) and the positive fibers when compared with the MCAO group (0.0757 ± 0.00321) (p < 0.05). These effects can be suppressed by LY294002 (an inhibitor of PI3K/AKT) in MCAO+BMSCs+LY294002 group (0.0718 ± 0.00317), compared with MCAO+BMSCs group (0.1070 ± 0.00499) (p < 0.05) (Fig. 4a). The expression of GAP-43 in cerebral white matter was detected by western blot. Compared with MCAO group, the expression of GAP-43 increased in MCAO+BMSCs group and decreased in MCAO+BMSCs+LY294002 group (p < 0.05)(Fig. 4b).
BMSCs protect axon from MCAO injury. Axon growth was detected by immunofluorescence and Weston blot. The panel A shows neuronal marker NF-200(red) and average fluorescence intensity of NF-200. Scale bar: 20 μm. The panel B represents the expression of GAP-43. ▲P < 0.05 vs. Sham group, ★P < 0.05 vs. MCAO group, #P < 0.05 vs. MCAO+BM group
Discussion
In present study, we show that BMSCs are capable of differentiating into osteocyte of calcium deposits and lipid laden adipocytes. Meanwhile, administration of BMSCs by tail-vein can significantly improve neurological functional outcome and promote axon growth in the MCAO rats, which was associated activation of PI3K/AKT /GSK-3β/CRMP-2 signaling pathway.
Because of easily and safely obtaining and differentiating into many types of cells, BMSCs has been applied in many animal models of neurological disorders in addition to multiple sclerosis, such as stroke and Parkinson’s disease [18,19,20,21,22,23]. BMSCs, including stem and progenitor cells, are identified by the morphology, differentiation and cell-surface antigen. Previous studies showed that a feature of the cells is a CD29+, CD90+, CD106+, CD45-, CD14- cell surface phenotype in BMSCs [24]. In this study, we found that cells derived from rat bone marrow could differentiate into osteoblasts and adipocytes after induction by the morphology.
Intravenous administration of BMSCs can pass through the blood–brain barrier (BBB) and translocate into or “home to” the brain ischemic regions [25]. Transplanted BMSCs concentrated in ischemic boundary zone (IBZ) and subventricular zone [26]. The interaction between stromal-cell-derived factor-1(SDF-1) and CXC chemokine receptor-4(CXCR-4) may lead to the targeted migration of BMSCs [27]. SDF-1 expression was higher in the ischemic boundary zone after stroke, which may facilitate the migration of CXCR4-positive stem cells. CXCR4, the specific receptor of SDF-1, is highly expressed in BMSCs in the bone marrow. The combination of SDF-1 with CXCR4 may contribute to the trafficking of transplanted BMSCs. Matrix metalloproteinases (MMP)-9 also takes part in the migration of BMSCs [28]. MMP-9-positive neutrophil infiltration is associated with BBB breakdown and basal lamina type IV collagen degradation, which sequentially facilitates BMSCs crossing BBB and reaching their destination.
BMSCs can not only survive and migrate to ischemic zone, but they also express neural or glial protein markers and replace dying cells [29]. In the early stage of cerebral infarction, BMSCs have a stimulating effect on the expression of various growth factors in the ischemic zone. During the entire process of recovery, these factors can facilitate functional recovery by inducing angiogenesis, reducing neuronal apoptosis in the IBZ, rebuilding synapses and dendrites, and enhancing axonal regeneration and differentiation of endogenous neural stem and progenitor cells [7,8,9, 30].
Axonal regeneration plays an important role in functional recovery after brain ischemia. Because there are many factors inhibiting the growth of the axon after injury, it is difficult for the axon of central nervous system to regenerate. In a report [31], anterograde tracing with biotinylated dextran amine injected into the right motor cortex was used to assess axonal sprouting in the contralateral motor cortex and ipsilateral rostral forelimb area. It was found that BMSCs further enhanced axonal plasticity and the structural plasticity, which was highly correlated with functional recovery. Some studies have shown MSC could promote axon regeneration by secreting certain neurotrophic factors, such as BDNF (brain-derived neurotrophic factor), and bFGF (basic fibroblast growth factor) [32]. GAP-43 is a highly expressed protein in neuronal growth cones during development that is used as a marker of regenerative neurons with an axon outgrowth [15]. In the cell culture, GAP-43 significantly increased in the BMSCs treatment following oxygen-glucose-deprive injured [33].
Our research indicated that BMSCs implanted intravenously in vivo could increase the GAP-43 protein level remarkably in MCAO rats. Nerve filament-200 is the main component of the cytoskeleton of neuronal cell body and nerve axons, which plays an important role in a series of the pathophysiological changes associated with neurons such as maintenance of the normal neuron morphology and axoplasmic transport. Therefore, NF-200 can be used as the marker of neuronal axons to understand the effect of BMSC treatment on the growth of neurons axon. In our study, we found that administrated with BMSC could increase the density of NF-200 in the cerebral white matter after MCAO. Meanwhile, we found that during the first 2 weeks after administrating with BMSCs, there was a substantial degree of recovery in the mNSS score. The recovery stemmed mainly from improvements in gait, body resistance and spontaneous activity in MCAO+BMSCs rats. Therefore, we concluded that administrated with BMSCs could improve axon growth and benefit neuron function outcome.
Recent studies showed that transplanted MSC after cerebral infarction can activate AKT by phosphorylating [34], which can alleviate ischemic injury and reduce neuronal apoptosis induced by focal cerebral ischemia [35]. AKT is the pivotal effector in the PI3K/AKT pathway, which is a complex signaling pathway involved in crucial cellular functions such as cell proliferation, migration and angiogenesis. Numbers of researchers found that PI3K/AKT pathway also plays an important role in multiple ischemia-reperfusion injury organs, such as brain, heart [36] and kidney [37]. Previously, we have reported that the injured neurons induced by Oxygen–Glucose Deprivation (OGD) can enhance the expression of p-AKT and promote axonal outgrowth through culturing with a conditioned medium of BMSCs [33].Glycogensynthasekinase-3β (GSK-3β) is inactivated by Akt-mediated phosphorylation, so GSK-3β downstream target protein could not undergo phosphorylation including collapsin response mediator protein (CRMP-2) [38]. CRMP-2 is a brain-specific protein involved in neuronal polarity, axonal guidance, and axonal regeneration [39]. Non-phosphorylated CRMP-2 promotes axon growth mainly by interacting with the cytoskeleton. CRMP-2 would lose the activity of regulating neurite growth after phosphorylation [40]. In the adult animal model of cerebral ischemia, CRMP-2 is cleaved or exhibit hypo-phosphorylation state, which is an important cause of neuron death [41]. Our research indicated that the expression of p-AKT and p-GSK-3β increased significantly, but the p-CRMP-2 decreased after administrating BMSCs. The effects were inhibited by LY294002, an AKT phosphorylation inhibitor, injected into the intracerebroventricular before BMSCs intravenously transplanted into MCAO rats. Therefore, it was indicated that BMSCs could stimulate GAP43 protein expression to reinforce neurite outgrowth through activating the AKT/GSK-3β/CRMP-2 signaling pathway.
To sum up, the results from our study demonstrated that BMSCs can promote neurological function recovery of rats following MCAO, and its molecular mechanism is related to the activation of AKT/GSK-3β/CRMP-2. Meanwhile, there are several limitations in our study: firstly, without testing the differentiation of BMSCs implanted into neurons in vivo; secondly, just providing the short-term benefits following ischemia; finally, not determining whether BMSCs directly or indirectly activate the AKT/GSK-3β/CRMP-2 pathway. Nevertheless, our study may be helpful to extend our understanding for transplantation of BMSCs in stroke.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, Sun C, Xu L, Li F, Jiang X (2014) Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation 11:135. https://doi.org/10.1186/1742-2094-11-135
Yang J, Xie QY, Zhang Y, Xiang HX, Guo Z (2007) Pluripotential differentiation of QY1 bone marrow mesenchymal stem cell line. Zhong Nan Da Xue Xue Bao Yi Xue Ban 32(2):268–275
Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts--an in vitro study. Lasers Med Sci 27(2):423–430. https://doi.org/10.1007/s10103-011-0930-1
Chen L, He DM, Zhang Y (2009) The differentiation of human placenta-derived mesenchymal stem cells into dopaminergic cells in vitro. Cell Mol Biol Lett 14(3):528–536. https://doi.org/10.2478/s11658-009-0015-3
Li Y, Chopp M (2009) Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett 456(3):120–123. https://doi.org/10.1016/j.neulet.2008.03.096
Davey GC, Patil SB, O'Loughlin A, O'Brien T (2014) Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol (Lausanne) 5:86. https://doi.org/10.3389/fendo.2014.00086
Song SY, Chung HM, Sung JH (2010) The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther 10(11):1529–1537. https://doi.org/10.1517/14712598.2010.522987
McIver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, Goldberg MP (2010) Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience 169(3):1364–1375. https://doi.org/10.1016/j.neuroscience.2010.04.070
Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B (2012) Bone mesenchymal stromal cells stimulate neurite outgrowth of spinal neurons by secreting neurotrophic factors. Neurol Res 34(2):172–180. https://doi.org/10.1179/1743132811Y.0000000068
Wei L, Fraser JL, Lu ZY, Hu X, Yu SP (2012) Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis 46(3):635–645. https://doi.org/10.1016/j.nbd.2012.03.002
Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, Liu D, Lin F (2011) Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med 9:105. https://doi.org/10.1186/1479-5876-9-105
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147. https://doi.org/10.1126/science.284.5411.143
Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F (2005) HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106(1):59–66. https://doi.org/10.1182/blood-2004-09-3645
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011. https://doi.org/10.1161/01.str.32.4.1005
Schallert T, Hernandez TD, Barth TM (1986) Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain Res 379(1):104–111. https://doi.org/10.1016/0006-8993(86)90261-1
Otero L, Bonilla C, Aguayo C, Zurita M, Vaquero J (2010) Intralesional administration of allogeneic bone marrow stromal cells reduces functional deficits after intracerebral hemorrhage. Histol Histopathol 25(4):453–461. https://doi.org/10.14670/HH-25.453
Kuypers NJ, Hoane MR (2010) Pyridoxine administration improves behavioral and anatomical outcome after unilateral contusion injury in the rat. J Neurotrauma 27(7):1275–1282. https://doi.org/10.1089/neu.2010.1327
Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J (2002) Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke 33(5):1362–1368. https://doi.org/10.1161/01.str.0000014925.09415.c3
Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 20(9):1311–1319. https://doi.org/10.1097/00004647-200009000-00006
Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M (2001) Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett 316(2):67–70. https://doi.org/10.1016/s0304-3940(01)02384-9
Li Y, Chen J, Wang L, Lu M, Chopp M (2001) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56(12):1666–1672. https://doi.org/10.1212/wnl.56.12.1666
Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164(2):247–256. https://doi.org/10.1006/exnr.2000.7389
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59(4):514–523. https://doi.org/10.1212/wnl.59.4.514
Jiao F, Wang J, Dong ZL, Wu MJ, Zhao TB, Li DD, Wang X (2012) Human mesenchymal stem cells derived from limb bud can differentiate into all three embryonic germ layers lineages. Cell Reprogram 14(4):324–333. https://doi.org/10.1089/cell.2012.0004
Li L, Chu L, Fang Y, Yang Y, Qu T, Zhang J, Yin Y, Gu J (2017) Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res Ther 8(1):112. https://doi.org/10.1186/s13287-017-0565-7
Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M (2004) Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res 1030(1):19–27. https://doi.org/10.1016/j.brainres.2004.09.061
Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y (2007) Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res 1183:138–147. https://doi.org/10.1016/j.brainres.2007.08.091
Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39(4):1121–1126. https://doi.org/10.1161/STROKEAHA.107.500868
Shichinohe H, Kuroda S, Lee JB, Nishimura G, Yano S, Seki T, Ikeda J, Tamura M, Iwasaki Y (2004) In vivo tracking of bone marrow stromal cells transplanted into mice cerebral infarct by fluorescence optical imaging. Brain Res Brain Res Protoc 13(3):166–175. https://doi.org/10.1016/j.brainresprot.2004.04.004
Wan H, Li F, Zhu L, Wang J, Yang Z, Pan Y (2014) Update on therapeutic mechanism for bone marrow stromal cells in ischemic stroke. J Mol Neurosci 52(2):177–185. https://doi.org/10.1007/s12031-013-0119-0
Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M (2010) Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab 30(7):1288–1295. https://doi.org/10.1038/jcbfm.2010.8
Yang J, Wu H, Hu N, Gu X, Ding F (2009) Effects of bone marrow stromal cell-conditioned medium on primary cultures of peripheral nerve tissues and cells. Neurochem Res 34(9):1685–1694. https://doi.org/10.1007/s11064-009-9963-2
Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, Chen R, Zheng W, Liu N (2013) Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One 8(11):e78514. https://doi.org/10.1371/journal.pone.0078514
Rowe DD, Leonardo CC, Recio JA, Collier LA, Willing AE, Pennypacker KR (2012) Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction. J Biol Chem 287(6):4177–4187. https://doi.org/10.1074/jbc.M111.296434
Noshita N, Lewen A, Sugawara T, Chan PH (2001) Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 21(12):1442–1450. https://doi.org/10.1097/00004647-200112000-00009
Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T (2012) Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303(1):H75–H85. https://doi.org/10.1152/ajpheart.00241.2012
Jang HS, Kim J, Kim KY, Kim JI, Cho MH, Park KM (2012) Previous ischemia and reperfusion injury results in resistance of the kidney against subsequent ischemia and reperfusion insult in mice; a role for the Akt signal pathway. Nephrol Dial Transplant 27(10):3762–3770. https://doi.org/10.1093/ndt/gfs097
Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K (2005) GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120(1):137–149. https://doi.org/10.1016/j.cell.2004.11.012
Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci 4(8):781–782. https://doi.org/10.1038/90476
Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, Morone N, Usukura J, Kaibuchi K (2005) Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol 25(22):9973–9984. https://doi.org/10.1128/MCB.25.22.9973-9984.2005
Sato Y, Ishida-Nakajima W, Kawamura M, Miura S, Oguma R, Arai H, Takahashi T (2011) Hypoxia-ischemia induces hypo-phosphorylation of collapsin response mediator protein 2 in a neonatal rat model of periventricular leukomalacia. Brain Res 1386:165–174. https://doi.org/10.1016/j.brainres.2011.02.027
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81772452, No. 81802225), Fujian Provincial Special Foundation for Natural Science Innovation Project (No. 2016B014), Joint Funds for the Innovation of Science and Technology, Fujian province (No. 2017Y9030, No. 2019Y9058), Natural Science Foundation of Fujian Province (No. 2020J011017), Teacher Education Research Project of Fujian Education Department (No. JAT170220) and Fujian Provincial Health Technology Project (No. 2019-ZQN-51). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank Dr. Xiaodong Pan, Dr. Lin Fan and Dr. Hongzhi Huang for language revision.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Nan Liu. Performed the experiments: Manli Chen, Xiaohui Lin, Hongbin Chen, Ting Li, Yongxing Lai, Longzai Lin, Peiqiang Lin, Ji Liu, Yixian Zhang, Xinhong Jiang. Analyzed the data: Ronghua Chen. Contributed reagents/materials/analysis tools: Houwei Du. Wrote the paper: Xiaohui Lin, Hongbin Chen.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, X., Chen, H., Chen, M. et al. Bone marrow-derived mesenchymal stem cells improve post-ischemia neurological function in rats via the PI3K/AKT/GSK-3β/CRMP-2 pathway. Mol Cell Biochem 476, 2193–2201 (2021). https://doi.org/10.1007/s11010-021-04073-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04073-w