Abstract
MicroRNAs (miRNAs) are important molecules which implicated in various processes, such as differentiation, development, cell survival, cell apoptosis and also cell metabolism. Investigations over decades have revealed that various genes and signaling pathways are implicated in beginning and development of atherosclerosis, several miRNAs being involved in these dysregulated genes and pathways. miRNAs have provided new molecular vision in the context of atherosclerosis. miRNAs are considered as important regulators of cellular migration, differentiation, proliferation, lipid uptake and efflux, as well as cytokine production. Application of miRNAs as a biomarker in diagnosis, prognosis and even therapy is quiet exciting. Although animal researches showed promising results, still some practical difficulties and technical challenges need to be addressed before translation from researches into clinical practices. In this review, we present important data about three critical cells endothelial cell (EC), vascular smooth muscle cell (VSMC), and monocyte/macrophage and regulation of these cells through miRNAs. Furthermore, we discuss about the potential of miRNAs as a prognostic and diagnostic biomarkers, therapeutic opportunities and challenges, and also future perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disorder characterized through activation of endothelial cell (EC), infiltration of monocytes and differentiation into macrophages and foam cell formation that cause endothelial dysfunction and proliferation of smooth muscle cell (SMC) [1]. Actually, the atherosclerosis pathogenesis consists of several processes with immune and non-immune cells involvement. Investigations through decades have revealed that various genes and signaling pathways are involved in the beginning and development of atherosclerosis [2].
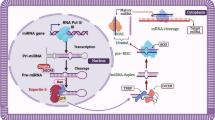
For the first time, microRNAs (miRNAs) were introduced in C. elegans [3]. miRNAs are small (18–22 nucleotides), evolutionarily conserved, and single-stranded non-coding RNAs, which bind to the 3′-untranslated regions (3′-UTRs) of the target mRNA sequences and regulate gene expression. miRNAs through two important mechanisms causes gene suppression; first mechanism is inhibition of the mRNA translation and the next is degradation of the mRNA [4,5,6,7,8,9] (Fig. 1). It has been proposed that more than 60% of protein coding genes are regulated through miRNA [10]. In addition, a specific miRNA can bind and regulate several genes and, on the other hand, a specific gene can be regulated by many miRNAs. miRNAs are implicated in controlling of gene expression in different pathophysiological process, such as atherosclerosis development [2].
Biogenesis of miRNAs. miRNAs are single stranded non-coding RNAs with approximately about 22 nucleotides in length. At first, RNA polymerase II transcribes miRNA genes to generate primary miRNAs (pri-miRNAs). Afterwards, Drosha, which is an endonuclease, catalyze the pri-miRNA to generate precursor miRNAs (pre-miRNAs). Then, the pre-miRNA is transported to cytoplasm through exportin-5 located on the nucleus membrane. In the cytoplasm, Dicer, which is an endoribonuclease, cleaves the pre-miRNA to produce aseymetric duplexes of miRNA containing 19–22 nucleotides. The active strand of miRNA is located in the ribonucleoprotein (RNP) complex, which is named as RNA-induced silencing complexes (RISCs). In order to function, mature miRNAs within the RISC binds to 3′-untranslated region (UTR) of a given mRNA that culminates in to gene suppression. miRNA guide the RISC complex to a particular mRNA then the core component of RISC complex (Argonaute) directly implements the gene silencing [95] (license acquired from RightsLink, License Number: 4818641467208, [96])
Recent studies in the field of microRNA (miRNA) have resulted in a big achievement in recognizing the cell types and diseases [11]. miRNAs are considered as important regulators of cellular migration, differentiation, proliferation, lipid uptake and efflux, and cytokine production. miRNAs have provided new molecular visions about atherosclerosis and been presented as a novel therapeutic approach. miRNAs can be evaluated in the body fluids and this feature confers them the potential as biomarkers for prognosis, diagnosis, and even follow up of patients. Recently, bioinformatic evaluations have provide promising insights on the biogenesis, function, and basic mechanisms of miRNAs in the etiology and pathogenesis of atherosclerosis [2].
This review article intends to review recent findings on the dysregulation of miRNAs in atherosclerosis cells/tissues and incorporate them with the previous knowledge, in order to get an insight on the potential of miRNAs to be used as prognostic marker in atherosclerosis. Ultimately, the implications for miRNA-based therapy will be clarified in the context of the disease.
miRNA abnormalities in different cells/tissues of atherosclerosis
miRNA and endothelial cell
Recent knowledge about miRNAs introduced them as a novel class of inter- and intra-cellular molecules that affect endothelial cells (ECs) and change their profile. Investigations on miRNA signature illustrated that there are associations between miRNAs and pathogenesis of atherosclerosis [11]. Endothelial cells are the first cells that participate in the pathogenesis of atherosclerosis. Under the biochemical stimuli, ECs undergo a series of cellular and molecular changes that initiate the atherosclerotic plaque formation. For instance, expression of adhesion molecules like intracellular adhesion molecule (ICAM)-1, vascular adhesion molecule (VCAM)-1, and E-selectin facilitates the recruitment and migration of leukocyte to the marginal area of vessel that is the first stage of plaque formation [12]. Various miRNAs, including miR-17-3p, miR-31, and miR-126 modulate inflammation through regulation of the adhesion molecules, such as ICAM-1, E-selectin, and VCAM-1 [13]. It has been documented that miR-146a plays an important role in plaque destabilization and also modulates inflammation in atherosclerosis. Part of miR-146a functions originates from its effect on activation of nuclear factor (NF)-κB signal-transduction pathway [14]. In addition, miR-10 influences the NF-κB signaling pathway, hence plays a crucial role in atherosclerosis regulation [15]. On the other hand, miR-146 inhibits the Mitogen-activated protein (MAP) kinase and NF-κB pathways. Furthermore, it has been illustrated that miR-146 target HuR, a RNA binding protein, that leads to activation of ECs through inhibition of endothelial nitric oxide synthase (eNOS or NOS2) [16]. miR-126-5p in endothelial cells plays a protective role in the formation of atherosclerotic lesion through inhibition of Notch1 inhibitor delta-like 1 homolog (Dlk1) [17]. miR-126 has an important role in the angiogenesis through proliferation of ECs by inhibition of suppressors of the phosphatidylinositol kinase (PI3K) pathway [18]. miR-223 is involved in inhibition of cholesterol biosynthesis and controlling of high-density lipoprotein-cholesterol (HDL-C) uptake. In addition, this miRNA plays roles in cholesterol metabolism [19]. miR-26a has a therapeutic role and has been linked to cell death in atherosclerosis [20]. Thus, multiple miRNAs are involved in the modulation of ECs and have been implicated in pathogenesis of atherosclerosis.
miRNAs and monocyte/macrophage
The second stage of atherosclerosis is leukocyte recruitment and migration from blood flow to the artery wall in the areas of lipoprotein retention and endothelial dysfunction. One of important leukocyte which migrates to the artery wall is monocyte [2]. Monocytes are the precursors of myeloid-derived dendritic cells and tissue resident macrophages, which are differentiated into foam cells and cause plaque development [21]. Actually, these cells eventually differentiate into macrophages. These cells play a highlighted role in the pathophysiology of atherosclerosis through two main mechanisms; production of inflammatory mediators and modulation of lipid homeostasis. Lipoprotein uptake by macrophages leads to differentiation of macrophages to foam cell, which is a hallmark of atherosclerosis [22]. These foam cells stay in the location of injury of artery wall and promote the inflammatory immune responses and promote the plaque formation. These cells are the main producers of chemokines and cytokines, which involved in recruitment, migration, differentiation and activation of immune cells, thereby maintain and exacerbate the chronic inflammation [2]. Furthermore, these foam cells are implicated in the destabilization and rupture of plaques, which make these cells the most important cells in atherogenesis. Since doam cells are so important in atherosclerosis, researchers believe that they can modulate the pathological changes in the injury site by manipulating these cells [23]. One of the molecules that affects the infiltration of monocytes is miRNAs. For example, miR-124a, through regulating C-|C motif chemokine ligand 2 (CCL2) expression, promotes the rolling and migration of monocytes into the vessel wall [24]. Different miRNAs, such as miR-106a, miR-20a, and miR-17 control the infiltration of macrophages through direct inhibition of signal-regulatory protein-α (SIRPα) [25]. High expression of miR-145 by VSMCs blocks the macrophage infiltration and it could be a potential target for atherosclerosis management [26]. miR-223 directly inhibits several chemo-attractants, including CCL3 and chemokine C-X-C motif ligand 2 (CXCL2), thereby control infiltration of different myeloid cells [27]. High expression of nitro-oxidative stress through atherosclerotic lesion formation may be one of the functions of miR-342-5p, which is produced by macrophages and results in the atherosclerosis progression. Microarray analysis showed that miR-365, miR-145, miR-143, and miR-155 were downregulated, while miR-352, miR-214, miR-146, and miR-21 were upregulated in the neointimal formation models [28]. miR-342-5p by its effects on Akt pathway facilitates the activation of inflammatory macrophages during atherosclerosis. Since by targeting miR-342-5p in macrophages, the cascade of molecular events which proceed to form an inflammatory macrophage is prevented, it could be a promising therapeutic strategy [29].
The balance among the endogenous synthesis, uptake, efflux, hydrolysis and esterification of cholesterol leads to macrophage cholesterol homeostasis. A number of miRNAs has been identified that are involved in the cholesterol metabolism of macrophages. miR-27a/b through targeting genes implicated in efflux (ABCA1), uptake (CD36, LDL) and cholesterol esterification (ACAT1) may regulate cholesterol homeostasis of macrophages [30]. miR-146a and miR-125a-5p decrease cytokine release and lipid uptake in the oxidized-LDL (ox-LDL)-stimulated macrophages trough targeting toll-like receptor (TLR) 4 and oxysterol binding protein-like 9 (OSBPL9) genes, respectively [31, 32]. miR-155, through targeting HMG box-transcriptional protein 1 (HBP1), controls the development of foam cells. The transcriptional repressor HBP1 negatively regulates macrophage inhibitory factor (MIF), the protein which elevates the uptake of ox-LDL by macrophage [33]. Various miRNAs such as miR-758 [34], miR-302a [35], miR-301b [36], miR-148a [36], miR-130b [36], miR-128-1 [36], miR-26 [37], miR-106 [38], miR-33 [39,40,41], and miR-144 [42, 43] regulate the cholesterol efflux in macrophage through ABCA1 and, thereby, promote formation of macrophage foam cells.
Different environmental factors promote the development of two distinguish class of macrophages; the M1 that is named as classical or proinflammatory macrophage and the M2 that is called as alternative or anti-inflammatory macrophage. Different stages of atherosclerosis are associated with different kinds of macrophage profiles; the M1 phenotype is the predominance kind of macrophage involved in the progression stage and the M2 phenotype is the most frequent type of macrophage playing a role in the plaque regression [22]. There are an increasing list of miRNAs which are involved in regulating the balance between the M1 and M2 macrophages, including miR-223 [44], miR-155 [45], miR-19a [46], miR-33 [47], miR-let7a [48], miR-125a [49], miR-21 [50], miR-214 [51], miR-27a [52], miR-146a [53], and miR-124 [54]. miR-33 plays an important role in controlling efflux of cholesterol, as well as cellular metabolism of macrophages to modify their inflammatory profiles. On the one hand, miR-33 elevates aerobic glycolysis that maintains the M1-like macrophage phenotype, while decreases the oxidation of fatty acid that is critical for M2 macrophages development; however, the ultimate outcome is the predominance of M1 phenotype [47]. miR-33 inhibition metabolically resulted in the M2 predominance that led to tissue repair, resolving inflammation, and finally high level of atheroprotective regulatory T (Treg) cells [47]. miR-155, although with a controversial role in the atherosclerosis, can differentiate M1 from M2 macrophages and results in the elevated number of M1 phenotype [45]. This miRNA is induced by ox-LDL and there is an increased level of miR-155 expression in CD14+ monocytes of coronary artery disease (CAD) patients in comparison to healthy controls [33]. miR-155 inhibits the negative regulators of inflammatory cytokines signaling, including B cell lymphoma 6 (BCL6), Src homology 2 domain-containing inositol-5-phosphatase-1 (SHIP-1), and suppressor of cytokine signaling 1 (SOCS1), thereby promotes the production of proinflammatory cytokines [17, 55,56,57]. On the other hand, miR-223, by targeting Pknox1, can promote the M2 phenotype and also regulates some of lipid metabolism related genes [19, 44]. miR-27a has also been implicated in the formation of foam cell, upregulation of M2 markers like Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN, also known as CD209) and CD206, as well as interleukin (IL)-10 production [52].
miR-21 and miR-147 decrease inflammation by attenuation the TLR-associated signaling of macrophages [58, 59]. Furthermore, miR-146a/b has been implicated in the inflammation resolution through attenuation of cytokine and TLR signaling in the macrophages. miR-146a expression in macrophages is induced by apolipoprotein E (apoE), an anti-atherosclerotic protein, that inhibits the inflammatory response of macrophages in vivo and in vitro [60]. Upregulation of miR-124a and miR-150 by the Krüppel-like factor 2 (KLF2) transcription factor, an anti-atherosclerosis factor, leads to low level of pro-atherosclerotic chemokines, such as CXCL1 and CCL2 [61]. miR-342-5p is one of the most important miRNAs that is induced during early stage of atherosclerosis in the macrophages located in the atherosclerotic lesions [29]. This miRNA, through inhibiting Akt1-mediated suppression of miR-155, increases the secretion of inflammatory mediators, such as IL-6 and inducible NOS (iNOS) from macrophages. Accordingly, miR-342-5p inhibition in Apoe−/− mice alleviates atherosclerotic lesions [29]. The bottom line is that miRNAs can influence on the atherosclerosis development through modulating different pathways in monocyte/macrophages.
miRNAs and vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) are the cellular components of the normal blood vessel wall that maintains structural integrity and also, regulates the diameter of vessels. In the inflammatory condition, these VSMCs change their phenotypes and are converted into a synthetic phenotype that induces signals involved in the proliferation, migration, and finally inflammation [62]. Various miRNAs have been identified to be involved in the regulation of VSMC through different transcription factors, such as SMADs (involved in transforming growth factor (TGF)-β signaling), myocardin (a co-activator), Platelet-derived growth factor (PDGF; involved in the regulation of cytokines and growth factors), and serum-response factor (SRF/KLF4). Some of the important miRNAs implicated in the regulation of VSMCs and atherosclerosis development are presented in the next section [63].
miR-21 plays an important role in proliferation of VSMCs in response to injuries that culminated in the formation of atherosclerotic lesion. On the other side, inhibition of miR-21 in the mechanical balloon injury situation attenuates formation of neointimal lesion. miR-21 by directly targeting Phosphatase and tensin homolog (PTEN) and indirectly elevating the Bcl-2 expression level leads to high proliferation and low apoptosis of VSMCs [28]. Contractile phenotypes of VSMCs is induced by Bone morphogenetic proteins (BMPs) and TGF-β through miR-21. This miRNA inhibits the programmed cell death 4 (PDCD4), which acts as a negative regulator for genes that are involved in contractile form of VSMC. On the other hands, BMP and TGF-β signaling increase the expression of miR-21. This event mediated through Drosha complex by converting the pri-miR-21(primary transcripts of miR-21) into the pre-miR-21 (precursor miR-21) [64]. Further investigations will be required to evaluate the anti-proliferative effects of miR-21 inhibition and also its role in alleviate of atherosclerosis in non-mechanically injury of vessels.
Both miR-221 and miR-222 have an elevated level of expression in neointimal lesions. It has been documented that miR-221 and miR-222 are involved in VSMC proliferation. High expression of miR-221 and miR-222 in VSMCs, resulted in low expression of p27 (Kip1), c-Kit genes and also, some of genes related to SMC contractile [65]. Knockdown of miR-221 and miR-222 showed that proliferation of VSMC and also formation of neointimal lesion were reduced after mechanical injury by targeting p57(Kip2) and p27(Kip1) [66].
miR-143 and miR-145 are downregulated in vessel wall of atherosclerosis patients [67, 68]. Various loss-, and gain-of-function investigations proposed that miR-143 and miR-145 are two important microRNA with regulatory functions in VSMC contractile. Actually, mice with deficiency in miR-143 and miR-145 showed low level of expression in contractile marker and also their function in SMC, impairment in cytoskeletal dynamics and actin stress fibers and decreased medial thickness of vessel wall [67,68,69]. Furthermore, these mice showed a decreased blood pressure in response to vasopressor challenge and this effect attributed to low level of angiotensin converting enzyme (ACE) expression [70]. Conversely, high expression of miR-145 in ApoE−/− mice leads to a decreased size of atherosclerotic plaque and also, reduced macrophage accumulation and necrotic core area [26]. miR-143 and miR-145 play their roles in expression and function of contractile phenotype in VSMCs through low level of K Kruppel-like factor (KLF4) expression and high level of myocardin expression in the ApoE−/− vessel wall of mice [26]. miR-143 and miR-145 have other targets, such as KLF5 and ELK-1 transcriptional regulator which are implicated in differentiation of VSMC [67, 68, 71].
MiRNA as diagnostic tool
Recent researches have illustrated that miRNAs could be applied as diagnostic and prognostic biomarkers for various disorders, such as cardiovascular diseases, diabetes, kidney diseases, rheumatoid arthritis, and cancer. Since the proteins-based biomarkers could not fulfill the diagnostic criteria for diagnosis of cardiac diseases, then diagnostic markers need an improvement. Investigations from clinical samples illustrated that miRNAs appear to be the most important biomarker for a proper diagnosis and even could be applied as a therapeutic agent for various cardiovascular diseases, including hypertension, stroke, atherosclerosis, heart failure, acute myocardial infarction, and even cancer [72, 73]. miR-423-5p showed an elevated level of expression in heart failure patients irrespective of gender and age and could be applied as a sensitive agent for heart disorders [74]. Conversely, miR-126 and miR-145 showed low level of expression in patients with coronary artery disease [75]. Tissue samples from myocardial infarction (MI) patients showed that miR-133a/b and miR-1 were downregulated and miR-208 was upregulated [76]. Therefore, miRNAs could be applied as an important biomarker for diagnosis, prognosis and, even identification of atherosclerosis.
Therapeutic opportunities and challenges
The miRNA ability to target genes opens a door to disease treatment through gene modulations. Since one miRNA could target many genes, there is also potential side effects of other genes targeting. Yet, this approach might be effective in complex disorders such as atherosclerosis which various pathways are implicated in disease pathogenesis [2].
In order to obtain an efficient miRNA-based therapy, it is critical to find important dysregulated genes and pathways which are implicated in atherosclerosis pathogenesis. Investigations on miRNAs illustrated that these molecules have an important role in atherosclerosis development and also its initiation (Table 1). Since miRNA mimics and inhibitors can target genes which are responsible for atherosclerosis pathogenesis, further researches are required to develop a novel miRNA based drug candidates [11].
Anti-sense oligonucleotides provide an opportunity to miRNA silencing to downregulate expression of miRNA or to fine tune special pathways which dysregulated by the disease. To chemically promote the anti-sense oligonucleotide approach, many methods have been used to enhance tissue uptake, stability of miRNAs and target affinity [77]. To minimize unanticipated toxicities and potential side effects, careful assessment of these chemical modifications will be necessary. Single stranded anti-miR oligonucleotides do not need lipid-based delivery systems and can be formulated in saline for intravenous or subcutaneous delivery. After systemic delivery of miRNAs, rapidly taken up by various organs such as liver, kidney, spleen, bone marrow and adipose tissue [78, 79]. After a cell taken up the anti-sense oligonucleotides, the anti-miR forms a high affinity and stable bond with the corresponding miRNA and inhibits the binding of the miRNA to its mRNA target. Preclinical investigations on non-human primates illustrated that using naked anti-miR oligonucleotides could be efficient in targeting of miRNAs (miR-33 and miR-122) especially in the liver [80, 81]. To elevate cellular uptake of anti-miRs, cholesterol analogs have been used and this increased their incorporation into LDL and high-density lipoprotein (HDL) [82,83,84]. miRNA decoy or sponge transcripts is another approach to inhibit miRNA. These transcripts act as competitive inhibitors of the target miRNA [85]. The miRNA sponges have various binding sites which have complementary sequences with the miRNA seed sequence and interfere with miRNA and finally inhibit its function. Viral vectors can be used to deliver miRNA sponges and their expression could be inducible in a specific stage or in a certain cell line through specific promoters. Studies illustrated that after cell transfection with viral vectors containing miRNA sponges, the corresponding miRNAs showed a decreased level of expression [86].
Application of miRNA mimic has two purposes; one is to reconstitute a downregulated miRNA and the second is to decrease expression of genes which are implicated in disease pathogenesis. For instance, downregulation of miR-181b in ECs of CAD patients could be restored with miRNA mimics [87]. Drug delivery vehicles such as lipoprotein-based drug, polymeric micelles, and liposomes carriers have been expanded to deliver these therapeutic oligonucleotides to targets. It is illustrated that to deliver miRNAs between cells, miRNAs are associated with HDL particles and this issue proposes that use of HDL infusion to deliver miRNAs could be efficient [88]. Two of important challenges which the scientists faced with are; specific delivery of miRNAs to a target or a cell, and the second is to achieve an optimal repression multiple doses of miRNA are required.
Since most of oligonucleotide inhibitors and miRNA mimics are mostly taken up by liver, enough amount of these oligonucleotide could not reach to the vessel wall and the success rate is lower than optimal. Studies showed that if miRNAs penetrated specifically to the peripheral blood mononuclear cells and vascular endothelium of the vessel wall the success rate of treatment would be better [87, 89]. In other word, tissue or cell specific delivery of miRNA mimics or inhibitors instead of systemic delivery proposes a novel opportunity to prevent atherosclerosis development and its progression. As a matter of fact, in order to get the highest efficiency with lowest doses and minimal side effects it is critical to find an appropriate translational dosing regimen.
Currently, various miRNA-based therapies are in preclinical stage and two of these therapies are in clinical trials. The first miRNA therapeutic in clinical trial is Miravirsen, a LNA against miR-122, which targets hepatitis C virus (HCV) RNA [90]. Investigations on non-human primates illustrated that inhibition of miR-122 leads to suppression of HCV viremia and considerable side effects and viral resistance has not been reported [91]. The second is the miRNA mimic of miR-34, which stimulating anti-tumor immune responses and inhibit various oncogenic pathways and thereby considered as a tumor suppressor molecule [92]. Investigations on MRX34 [93], a miR-34 mimic encapsulated in a liposomal nanoparticle formulation, in patients with hematological malignancies or advanced solid tumors showed promise results.
Several years of hard working resulted in significant progress in atherosclerosis treatment. Especially, statins (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors) showed promise to treats CAD patients. Although, statins significantly decrease LDL levels and resulted in cardiovascular improvement, but the disease burden even in CAD treated patients remained [2].
To treat disease such as atherosclerosis which complex signaling pathways are involved, novel and complementary therapeutic approaches are required. Since a miRNA may have various targets to suppress gene expression, treatment of patients with miRNAs may have side effects on cell function, biological pathways, and homeostasis in the periphery, liver, and vessel wall. Delivery sets of miRNA mimics or inhibitors could be considered as an attractive and applicable approach in atherosclerosis improvement and also management of its complications [2].
Future perspectives
Over the course of past few years, miRNAs have been evidenced to be involved in the etiology and pathogenesis of atherosclerosis, predominantly through modulating genes playing roles in the process of inflammation. That notwithstanding, it is still critical to further explore and identify the miRNAs with bona fide implication in the atherosclerosis initiation and perpetuation. Furthermore, it is important to clarify miRNA signature in the early and late stage of the disease, thereby facilitating the way toward devising diagnostic biomarkers of the atherosclerosis. On the other hand, miRNA-based therapy or mesenchymal stem cell-derived exosomes could be more efficient in treatment of atherosclerosis [94]. Research in field of miRNA of atherosclerosis is at its infancy, but confers a promising future, as application of miRNAs as a biomarker in diagnosis, prognosis, and even therapy is quiet exciting. Although animal researches showed promising results, still some practical difficulties and technical challenges need to be addressed before translation from researches into clinical practices. As soon as these challenges are resolved, the miRNA-based approaches will be a potential therapy to compete with other therapeutics.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author. All data generated or analyzed during this study are included in this published article.
Abbreviations
- miRNAs:
-
MicroRNAs
- EC:
-
Endothelial cell
- VSMC:
-
Vascular smooth muscle cell
- SMC:
-
Smooth muscle cell
- ICAM:
-
Intracellular adhesion molecule
- VCAM:
-
Vascular adhesion molecule
- eNOS:
-
Endothelial nitric oxide synthase
- Dlk1:
-
Notch1 inhibitor delta-like 1 homolog
- PI3K:
-
Phosphatidylinositol kinase
- HDL-C:
-
High-density lipoprotein-cholesterol
- HBP1:
-
HMG box-transcriptional protein 1
- MIF:
-
Macrophage inhibitory factor
- BCL6:
-
B cell lymphoma 6
- SHIP-1:
-
Src homology 2 domain-containing inositol-5-phosphatase-1
- SOCS1:
-
Suppressor of cytokine signaling 1
- apoE:
-
Apolipoprotein E
- KLF2:
-
Krüppel-like factor 2
- PDCD4:
-
Programmed cell death 4
- ACE:
-
Angiotensin converting enzyme
- HCV:
-
Hepatitis C virus
References
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493
Feinberg MW, Moore KJ (2016) MicroRNA regulation of atherosclerosis. Circ Res 118:703–720
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Ambros V (2004) The functions of animal microRNAs. Nature 431:350
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455:64
Friedman RC, Farh KK-H, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Samanta S, Balasubramanian S, Rajasingh S, Patel U, Dhanasekaran A, Dawn B, Rajasingh J (2016) MicroRNA: a new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med 26:407–419
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473:317
Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, Wybranska I, Chojnacka M, Dembinska-Kiec A (2011) Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn 121:361–366
Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, Zhou W, Xiong B, Zeng Q (2010) miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol 88:555–564
Fang Y, Shi C, Manduchi E, Civelek M, Davies PF (2010) MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci 107:13450–13455
Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE (2013) MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 5:1017–1034
Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20:368
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15:272–284
Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, Palmisano BT, Tabet F, Cui HL, Rye K-A (2014) MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci 111:14518–14523
Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H (2015) MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep 5:9401
Angelovich TA, Hearps AC, Jaworowski A (2015) Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol Cell Biol 93:683
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13:709
Chang R, Ying W, Bazer F, Zhou B (2014) MicroRNAs control macrophage formation and activation: the inflammatory link between obesity and cardiovascular diseases. Cells 3:702–712
Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M, Kumagai S (2009) MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 60:1294–1304
Zhu D, Pan C, Li L, Bian Z, Lv Z, Shi L, Zhang J, Li D, Gu H, Zhang C-Y (2013) MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein α. J Allergy Clin Immunol 132(426–436):e8
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M (2012) MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 126:S81–S90
Dorhoi A, Iannaccone M, Farinacci M, Faé KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf H-J, Oberbeck-Müller D, Jörg S (2013) MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Investig 123:4836–4848
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100:1579–1588
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C, Schober A (2013) The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1-and microRNA-155-dependent pathway during atherosclerosis. Circulation 127:1609–1619
Zhang M, Wu J-F, Chen W-J, Tang S-L, Mo Z-C, Tang Y-Y, Li Y, Wang J-L, Liu X-Y, Peng J (2014) MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 234:54–64
Wang Y-S, Zhou J, Hong K, Cheng X-S, Li Y-G (2015) MicroRNA-223 displays a protective role against cardiomyocyte hypertrophy by targeting cardiac troponin I-interacting kinase. Cell Physiol Biochem 35:1546–1556
Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C (2009) MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83:131–139
Tian F-J, An L-N, Wang G-K, Zhu J-Q, Li Q, Zhang Y-Y, Zeng A, Zou J, Zhu R-F, Han X-S (2014) Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res 103:100–110
Ramirez CM, Dávalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suárez Y, Fernández-Hernando C (2011) MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol 31:2707–2714
Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA (2014) MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol 114:304878
Goedeke L, Rotllan N, Canfrán-Duque A, Aranda JF, Ramírez CM, Araldi E, Lin C-S, Anderson NN, Wagschal A, De Cabo R (2015) MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat Med 21:1280
Sun D, Zhang J, Xie J, Wei W, Chen M, Zhao X (2012) MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett 586:1472–1479
Kim J, Yoon H, Ramírez CM, Lee S-M, Hoe H-S, Fernández-Hernando C, Kim J (2012) MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp Neurol 235:476–483
Marquart TJ, Allen RM, Ory DS, Baldán Á (2010) miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci 107:12228–12232
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328:1566–1569
Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C (2010) MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328:1570–1573
de Aguiar VT, Tarling E, Kim T, Civelek M, Baldan A, Esau C, Edwards P (2013) MicroRNA-144 regulates hepatic ABCA1 and plasma HDL following activation of the nuclear receptor FXR. Circ Res 112:300648
Wanschel A, Zavadil J, Castrillo A, Jungsu K, Suárez Y, Aranda CJF, Cirera-Salinas D, Araldi E, Salerno A, Bonito AC (2013) Control of cholesterol metabolism and plasma HDL levels by miRNA-144
Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S (2012) A novel regulator of macrophage activation: miR-223 in obesity associated adipose tissue inflammation. Circulation 125:2892–2903
Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang C-Y, Zen K (2012) Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol 4:341–343
Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang T, Li N, Gomez-Cabrero A, Reisfeld R, Xiang R (2014) MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 33:3014
Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K (2015) MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Investig 125:4334–4348
Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G (2013) MicroRNA let-7c regulates macrophage polarization. J Immunol 190:6542–6549
Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, Thannickal VJ, Abraham E, Liu G (2013) miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem 288:35428–35436
Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A, Serezani CH (2015) MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS ONE 10:e0115855
Lu S, Gao Y, Huang X, Wang X (2014) Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci 10:415
Saha B, Bruneau JC, Kodys K, Szabo G (2015) Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol 194:3079–3087
Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B (2014) Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol 192:394–406
Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED (2013) IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS ONE 8:e81774
Sun H-X, Zeng D-Y, Li R-T, Pang R-P, Yang H, Hu Y-L, Zhang Q, Jiang Y, Huang L-Y, Tang Y-B (2012) Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 60(6):1407–1414
Yao R, Ma Y-L, Liang W, Li H-H, Ma Z-J, Yu X, Liao Y-H (2012) MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS ONE 7:e46082
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Investig 122:4190–4202
Liu G, Friggeri A, Yang Y, Park Y-J, Tsuruta Y, Abraham E (2009) miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci 106:15819–15824
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’learyRuanJohnsonChenO’neill JJQDSYLA (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11:141
Li K, Ching D, Luk FS, Raffai RL (2015) Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res 114:305844
Manoharan P, Basford JE, Pilcher-Roberts R, Neumann J, Hui DY, Lingrel JB (2014) Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Krüppel-like factor 2 (KLF2)-deficient macrophages. J Biol Chem 289:31638–31646
Doran AC, Meller N, McNamara CA (2008) Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 28:812–819
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A (2009) Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284:3728–3738
Liu X, Cheng Y, Yang J, Xu L, Zhang C (2012) Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol 52:245–255
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee T-H, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460:705
Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN (2009) MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23(18):2166–2178
Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J (2009) The knockout of miR-143 and-145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 16:1590
Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T (2009) Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Investig 119:2634–2647
Cheng Y, Liu X, Yang J, Lin Y, Xu D-Z, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 105:158–166
Faruq O, Vecchione A (2015) microRNA: diagnostic perspective. Front Med 2:51
Sayed ASM, Xia K, Salma U, Yang T, Peng J (2014) Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ 23:503–510
Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM (2010) MiR423-5p as a circulating biomarker for heart failure. Circ Res 106:1035–1039
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M (2010) Circulating microRNAs in patients with coronary artery disease novelty and significance. Circ Res 107:677–684
Boštjančič E, Zidar N, Štajer D, Glavač D (2010) MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 115:163–169
Broderick JA, Zamore PD (2011) MicroRNA therapeutics. Gene Ther 18:1104
Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S (2012) Inhibition of microRNA function by antimiR oligonucleotides. Silence 3:1
Van Rooij E, Olson EN (2012) MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11:860
Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U (2008) LNA-mediated microRNA silencing in non-human primates. Nature 452:896
Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X (2011) Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 478:404
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E (2010) Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28:172
Dowling JE, Wald G (1981) Proceedings of the National Academy of Sciences of the United States of America. Nutr Rev 39:135–138
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432:173
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721
Kluiver J, Slezak-Prochazka I, Smigielska-Czepiel K, Halsema N, Kroesen B-J, van den Berg A (2012) Generation of miRNA sponge constructs. Methods 58:113–117
Sun X, He S, Wara A, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK (2014) Systemic delivery of microRNA-181b inhibits nuclear factor-κb activation, vascular inflammation, and atherosclerosis in apolipoprotein E–deficient mice. Circ Res 114:32–40
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423
Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM (2012) MicroRNA-181b regulates NF-κB–mediated vascular inflammation. J Clin Investig 122:1973–1990
Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309:1577–1581
Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201
Ling H, Fabbri M, Calin GA (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 12:847
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, Smith S, Bader AG, Kim S, Hong DS (2017) Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig New Drugs 35:180–188
Ni J, Sun Y, Liu Z (2019) The potential of stem cells and stem cell-derived exosomes in treating cardiovascular diseases. J Cardiovasc Transl Res 12:51–61
Araujo CL, Quintero IB, Kipar A, Herrala AM, Pulkka AE, Saarinen L, Hautaniemi S, Vihko P (2014) Prostatic acid phosphatase is the main acid phosphatase with 5’-ectonucleotidase activity in the male mouse saliva and regulates salivation. Am J Physiol Cell Physiol 306:C1017–C1027. https://doi.org/10.1152/ajpcell.00062.2014
Wahid F, Shehzad A, Khan T, Kim YY (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta Mol Cell Res 1803:1231–1243
Acknowledgements
The authors are grateful of Deputy of Research from Neyshabur University of Medical Science.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Consent for publication
All authors read the manuscript and consent for its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabaei, S., Tabaee, S.S. Implications for MicroRNA involvement in the prognosis and treatment of atherosclerosis. Mol Cell Biochem 476, 1327–1336 (2021). https://doi.org/10.1007/s11010-020-03992-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03992-4