Abstract
Astrocytes are vitally involved in the development of neurodegenerative diseases and brain cancers. In this work, we investigated the potential ameliorative role of microRNA-194-5p (miR-194-5p) against lipopolysaccharide (LPS)-induced astrocytes activation and the mechanism underneath. Astrocytes were transfected with miR-194-5p mimic or inhibitor and subsequently induced with LPS. Cell proliferation was measured using MTT assay while Transwell assay was used for assessing cell migration. The concentrations of cyclooxygenase 2 (COX2) and cytokines (tumor necrosis factor-α (TNF-α), transforming growth factor β (TGF-β), interleukin (IL)-1β and IL-6) were determined by enzyme-linked immunosorbent assay (ELISA). Gene expression was assessed by quantitative reverse transcription PCR (RT-qPCR) while western blotting was used for quantifying relative protein expression. We found that miR-194-5p, downregulated in LPS-induced astrocytes, significantly inhibited LPS-induced cell proliferation and migration. In addition, miR-194-5p inhibited the release of COX2 and pro-inflammatory cytokines (TNF-α, TGF-β, IL-1β and IL-6). Moreover, the silencing of neurexophilin 1 (NXPH1), an in silico and mechanistically confirmed direct target of miR-194-5p, reverted the anti-inflammatory, anti-proliferative and anti-migratory effects of miR-194-5p. We anticipated that miR-194–5 inhibits the proliferation, invasion, and inflammatory reaction in LPS-induced astrocytes by directly targeting NXPH1. These findings hinted that miR-194-5p/NXPH1 axis exerts vital functions in astrocytes activation and neuroinflammation-associated diseases. This finding will open novel avenues for biomedical and neuroscience research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes are the major cells of the central nervous system and play an important role in maintaining the physiological activities of neurons, including the formation and maintenance of the blood–brain barrier, synaptic development, neurotransmission, metabolic regulation, axonal transmission and inflammation [1,2,3,4]. In case of nervous system injury, astrocytes are activated, proliferate and migrate to the lesion where they form a glial scar, thus preventing from neuronal regeneration [5, 6]. Therefore, exploring the pathways controlling the phenotypes of astrocytes has become a hot spot for research in this area.
In recent years, many microRNAs (miRs) have been reported to be expressed in the brain and nerve cells. These miRs along with other non-coding RNAs regulate cerebral cortical neurons, synapses, and complex neurophysiological network structures, and play a precise regulatory role in the development and function of the nervous system [7,8,9]. Once this regulatory mechanism is disordered, neurological diseases will inevitably occur. Previous studies have shown that miR-194-5p can regulate the growth and metastasis of various cancers, such as endometrial cancer, esophageal adenocarcinoma, gastric cancer, and is considered a candidate biomarker for cancers [10,11,12]. Furthermore, the expression level of miR-194-5p was considerably decreased in glioblastoma patients, suggesting a possible role of miR-194-5p in brain and neurodegenerative diseases [13]. The mechanisms involving miR-194-5p were reported to be related to downstream control of target genes by miR-194-5p or upstream regulations driven by other non-coding RNAs and canonical pathways [14, 15]. However, the role of miR-194-5p in astrocyte activation following seizures is still unclear and needs an in-depth study of its mode of action.
The function of neurexophilins (NXPHs) is poorly understood. However, data suggest that these molecules inhibit neurexins (NRXN)-mediated signaling within the neuronal system [16, 17]. NXPH1-3 are small 29 kDa, secreted, neuropeptide-like proteins found in a variety of tissues [17, 18]. NXPH1 encodes a secreted protein with a variable N-terminal domain and forms a very tight complex with NRXNs. However, whether NXPH1 plays a role in the activation of astrocytes is unknown. Studies are encouraged to elucidate this possible role. Bioinformatics analysis revealed that NXPH1 is a potential target gene for miR-194-5p, therefore, we hypothesized that miR-194-5p exerts its function through targeting NXPH1 expression.
Endotoxin lipopolysaccharide (LPS) is the main component of the cell wall of Gram-negative bacteria [19]. It is often used as a polyclonal immune stimulator in immune response studies to mimic the immune stimulating state of the body, which is a common inflammatory stimulator for studying the information exchange model between the immune system and the nervous system [20, 21]. In the present study, a human astrocytes activation model stimulated by LPS was established. We detected the expression of miR-194-5p and NXPH1, and evaluated the function of miR-194-5p/NXPH1 in LPS-stimulated astrocytes. Notably, we demonstrated that miR-194-5p/NXPH1 axis regulates the proliferation, migration and neuroinflammation in LPS-induced astrocytes.
Materials and methods
Cell culture
Human astrocytes (NHA, Human Astrocytes, Catalog #: CC-2565) were bought from the Lonza (USA). Cells were cultured in a specific astrocyte medium (AGM™ Astrocyte Growth Medium BulletKit™, Lonza, USA). The culture system contained ABM™ Basal Medium (CC-3187) and AGMTMSingleQuots™ Supplements (CC-4123) necessary for growth of Astrocytes. Cultures were performed in a humidified 5% CO2 incubator at 37 °C. Cells were treated with indicated concentrations of LPS (Sigma-Aldrich, St. Louis, USA) for 48 h prior to harvest.
GFP reporter gene construct for miR-194-5p and NXPH1
We constructed a reporting system that correlates miR-194-5p or NXPH1 content in cells with GFP fluorescence. GFP was cloned into the respective EcoRI and NotI sites of pcDNA3.1 with T4 DNA-Ligase (2–3 Weiss-Units). The wild type (WT) NXPH1 3′-UTR was amplified from pRL NXPH1 3′-UTR (plasmid 14,804, Cambridge, MA, USA) and mutant NXPH1 3′-UTR from pRL NXPH1 3′-UTR (plasmid 14,805) using NXPH1 forward primer (5′-ACGAGAAGAAGTGGAAGGCA-3′) and NXPH1 reverse primer (5′-CTCCGATTGCTTGCAGTACC-3′). KpnI and BamHI restriction sites were used to clone the resulting NXPH1 PCR product into GFP downstream into pcDNA3.1-GFP, resulting in GFP reporter vector. Successful cloning was confirmed by using DNA sequencing at GenePharma (Shanghai, China). This GFP reporter assay was used to evaluate the transfection efficiency of different oligonucleotide and the effect of mir-194-5p on NXPH1 in astrocytes.
Cell transfection and quantification of green fluorescent protein fluorescence in astrocytes
The miR-194-5p mimics and miR-194-5p inhibitor were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The miRNA sequences were as follows: miR-194-5p mimic: 5′-UGUAACAGCAACUCCAUGUGGA-3′, mimic control: 5′-UUCUCCGAACGUGUCACGUTT-3′, miR-194-5p inhibitor: 5′-UCCACAUGGAGUUGCUGUUACA-3′, inhibitor control: 5′-CAGUACUUUUGUGUAGUACAA-3′. NXPH1 siRNA interference sequence was synthesized by Genechem co., Ltd. (Shanghai, China). The siRNA sequences were as follows: NXPH1 siRNA: 5′-TACTTGGTCACATGTGCCAATTTAA-3′, control siRNA: 5′-GTTTCATAACTTAGTCGCAGATACT-3′. The miR-194-5p mimic, miR-194-5p inhibitor, NXPH1 siRNA and their respective controls were transfected or co-transfected with the GFP reporter system using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol. Briefly, astrocytes were seeded in 24-well plates (1 × 106 cells/well) and cultured overnight. For each well, 20 μM miR-194-5p mimic, miR-194-5p inhibitor, NXPH1 siRNA or their respective controls in presence (20 μM) or absence of the GFP reporter system was added into 250 μl Opti-MEM medium (Gibco-BRL/Invitrogen, Carlsbad, CA, USA) followed by addition with 5 μl Lipofectamine 2000. Subsequently, the mixture was added to astrocytes and followed by incubation for 6 h. After transfection, cells were cultured in the specific astrocyte medium containing 2 μg/ml LPS (Sigma-Aldrich, St. Louis, USA) for LPS stimulation. For the quantification of green fluorescence, cells were harvested 48 h after co-transfection with the GFP reporter system. The cells were fixed with 4% paraformaldehyde for 15 min, washed with phosphate buffer saline (PBS) three times, then permeabilized with 0.2% Triton X-100 for 5 min, and sealed with 5% BSA for 45 min after rinsing with PBS. The primary antibody diluted in PBS was added and incubated overnight at 4 ℃. On the second day, the cells were washed with PBS and incubated with the secondary antibody at room temperature for 1 h. Then, the nuclei were stained with DAPI (1 μg/ml) for 2 min. Before and after DAPI staining, the cells were washed with PBS three times. Cellular fluorescence intensity was determined by using a microplate reader (Synergy H1, BioTek, Winooski, USA) under excitations at 480 nm, and emissions at 509 nm. Additionally, an epi-fluorescent microscope (Nikon, Tokyo, Japan) was used to capture non-saturating images and the number of cells stained by GFP+ and DAPI+ were calculated using Image J software. Transfection efficiency was determined by the percentage of GFP+ cells in the DAPI+ cells (total cells).
Luciferase reporter assay
Cells at density of 1 × 105/well were plated in 24-well plates 24 h before transfection. Luciferase constructs harboring firefly luciferase with either wild type (WT) NXPH1 3′-UTR or mutant (MUT) NXPH1 3′-UTR and Renilla vector (pRL-TK) were co-transfected with either miR-194-5p mimic or control mimic into the cells using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). Cell lysates were collected 48 h after the transfection, and the luciferase activity measurement was done following the procedures laid out by the manufacturer (Dual-Luciferase system, Promega, E1910). For each sample, firefly luciferase activity was normalized to Renilla luciferase activity.
Transwell assay
Cells were trypsinized and inoculated into Transwell chamber (Costar Corning, NY, US) at a density of 5 × 105 cells/well. 300 μl of serum-free DMEM medium was added to the upper chamber whereas 700 μl of DMEM medium supplement with 20% FBS was added to the lower chamber. After culturing for 8 h, the Transwell chamber was taken out and washed twice with PBS. It was then fixed in a fixing solution (methanol:acetone = 1:1) for 2 h at room temperature, and stained with crystal violet for 30 min. The upper chamber cells were carefully wiped, and 5 fields in each chamber were randomly selected to be photographed under a high-power microscope.
MTT detection of the proliferation of astrocytes
The MTT colorimetric cell proliferation assay was used to determine the proliferation of astrocytes transfected with indicated vectors and treated with LPS according to the instructions provided by the manufacturer. After harvesting of the cells, MTT was added to the cells which were further incubated for 4 h. After that, cell supernatants were removed and cells added with of 100 μl DMSO. Finally, the absorbance (A) value was detected on the microplate reader (ELx808, BioTek Instruments, Winooski, VT, USA) at 490 nm.
Caspase 3/7 activity
The Caspase 3/7 activity was determined by Caspase‐Glo 3/7 Assay (Promega, US) according to the vendor’s instructions. After transfection, cells were seeded into 96-well plates and incubated for 24 h at 37 °C. Subsequently, 100 μl of Caspase‐Glo reagent was added to each well and followed by incubation for 2 h at room temperature. Then, the luminescence was measured by using the Microplate Reader (Bio-Rad, Hercules, US).
RT-PCR detection of miR-194-5p and NXPH1 mRNA expression
According to the Trizol protocol, the total RNA of each group was extracted, and the RNA was reverse transcribed into cDNA. miR-194-5p primers: upstream 5′-CTAGTACCTAGAGGAACCTTTGAAGACTGTTACAGCTCAGCA-3′, downstream 5′-AGCTTGCTGAGCTGTAACAGTCTTCAAAGGTTCCTCTAGGTA-3′; NXPH1 primers: upstream 5′-ACGAGAAGAAGTGGAAGGCA-3′, downstream 5′-CTCCGATTGCTTGCAGTACC-3′; β-actin primers: upstream 5′-CCCATCTATGAGGGTTACGC-3′, downstream 5′-TTTAATGTCACGCACGATTTC-3′. PCR expansion conditions: 94 °C pre-denaturation 5 min; 94 °C denaturation 30 s, 62 °C annealing 45 s, 72 °C extension 1 min, cycle 35 times; 72 °C extension 10 min. Total RNA was used for synthesis of cDNA and the miScript Reverse Transcription kit purchased from Qiagen (Valencia, CA, USA) was used for miR-194-5p qRT-PCR. PCR product were purified on 2% agarose gel electrophoresis imaging, using the Quantity One system for electrophoretic band optical density analysis. Relative mRNA expression was computed using the Ct method [22].
Western blotting
Cells were lysed with 2 × SDS loading buffer and proteins were collected and quantified by Coomassie Brilliant Blue method. After SDS-PAGE electrophoresis, transfer, and 5% skim milk powder were sealed at room temperature. Subsequently, the primary antibodies (NXPH1, Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000; β-actin, Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000) were added overnight at 4 °C, washed 3 times with Tris-buffered saline containing 0.1% Tween-20 (TBST). Next, membranes were incubated with Horseradish Peroxidase (HRP)-labeled secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:5000) for 2 h at room temperature, washed 3 times with TBST, photographed with DAB and analyzed for densitometry using the Image J software (NIH, Bethesda, MD, USA) [23].
ELISA detection
The concentrations of cyclooxygenase 2 (COX2) and cytokines (tumor necrosis factor-α (TNF-α), transforming growth factor β (TGF-β), interleukin (IL)-1β and IL-6) in the supernatant of the cell cultures was determined using the corresponding ELISA kits (Abcam, Cambridge, UK) according to the vendors’ instructions.
Statistical analysis
Statistical analysis was performed using GraphPad prism software (GraphPad Software, Inc., La Jolla, CA, USA). The measurement data were expressed as x ± s. One-way analysis of variance was used for comparison between groups followed by Turkey multiple comparison test. p < 0.05 was considered statistically significant.
Results
miR-194-5p and NXPH1 are dysregulated in LPS-induced astrocytes
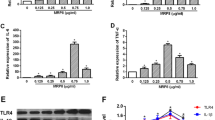
Astrocytes were incubated with LPS for 48 h in order to induce astrocyte activation. MTT assay was used to evaluate the effect of LPS on cell proliferation. As shown in Fig. 1a, LPS induced the proliferation of astrocytes dose-dependently. Decreased caspase3/7 activity indicated that LPS deterred the apoptosis of astrocytes (Fig. 1b). Similarly, transwell assay indicated increased cell migration in the LPS group compared to the control group (Fig. 1c). Furthermore, the determination of cytokines and COX2 by ELISA indicated that TNF-α, TGF-β, COX2, IL-6 and IL-1β were all increased in the LPS group (Fig. 1d), indicating increased inflammation in astrocytes and their activation. Moreover, we found that LPS treatment led to decreased miR-194-5p expression (Fig. 1e) and upregulated the mRNA and protein expression levels of NXPH1 (Fig. 1f, g) in a dose-dependent manner (Fig. 1e), suggesting that miR-194-5p and NXPH1 may play a role in astrocytes activation.
MiR-194-5p and NXPH1 are dysregulated in LPS-induced astrocytes The proliferation (a), apoptosis (b), and migration (c) of astrocytes treated with different concentrations of LPS were detected using the MTT assay (a), Caspase 3/7 activity (b) and transwell assay (c), respectively. d ELISA detected the concentrations of COX2 and cytokines (TNF-α, TGF-β, IL-1β, and IL-6) in LPS-induced astrocytes cell supernatants. e The expression of miR-194-5p in astrocytes treated with different concentrations of LPS was detected using qRT-PCR. The mRNA and protein expression levels of NXPH1 in astrocytes treated with different concentrations of LPS were detected by using qRT-PCR (f) and western blotting (g). The Image J software was used for quantitative analysis of western blots. Data are shown as x ± s. n = 3 independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs the control group
miR-194-5p/NXPH1 axis is a regulatory pathway in astrocytes
To uncover whether the miR-194-5p/NXPH1 axis is a regulatory pathway, we first proceeded to in silico analysis. The online bioinformatics tool Targetscan indicated that miR-194-5p has a binding site in the WT 3′-UTR of NXPH1 (Fig. 2a). Subsequently, this binding site was mutated (NXPH1 3′-UTR MUT) by 7 bp substitution (red letters) (Fig. 2a) and used for luciferase reporter assay. Luciferase reporter assay indicated that miR-194-5p overexpression reduced the luciferase activity of WT-NXPH1, but had no significant impact on MUT-NXPH1, suggesting that NXPH1 is a direct target for miR-194-5p (Fig. 2b). Next, we constructed a GFP-based reporter system to assess both the transfection efficiency of miR-194-5p mimic or inhibitor in astrocytes and the regulatory relationship between miR-194-5p and NXPH1. The NXPH1 3′-UTR was cloned downstream of GFP gene into pcDNA3.1 to construct a reporter system in which GFP fluorescence responded to changes in the level of miR-194-5p in the cells (Fig. 2c). The GFP reporter vector was co-transfected with miR-194-5p mimic or miR-194-5p inhibitor, and the transfection efficiency of astrocytes was about 70%, there was no significant difference between the miR-194-5p mimic and mimic control or between miR-194-5p inhibitor and inhibitor control. The results of cellular fluorescence intensity showed that miR-194-5p mimic effectively inhibited GFP expression and reduced fluorescence by more than 1.5-fold (Fig. 2d). In contrast, miR-194-5p inhibitor significantly reduced miR-194-5p expression in astrocytes, which was reflected by a 1.24-fold increase in GFP fluorescence (Fig. 2d), suggesting that the transfection of miR-194-5p mimic and inhibitor were effective and confirming that NXPH1 is a direct target of miR-194-5p. Moreover, the transfection efficiency of miR-194-5p mimic and inhibitor were also validated by using RT-qPCR. The results showed that miR-194-5p overexpression significantly increased the expression level of miR-194-5p (about 1.9-fold), while inhibition of miR-194-5p markedly declined the miR-194-5p expression (approximately 9.6-fold) (Fig. 2e). Furthermore, western blot analysis indicated that miR-194-5p negatively regulates NXPH1 expression at protein level (Fig. 2f) but had no effect on mRNA expression (Fig. 2g). These results demonstrated that NXPH1 is a direct target of miR-194-5p and that the functional role of miR-194-5p in astrocytes may involve the miR-194-5p/NXPH1 axis as a post-transcriptional regulatory pathway.
miR-194-5p/NXPH1 axis is a regulatory pathway in astrocytes. a Bioinformatics predicts NXPH1 as a direct target of miR-194-5p. b Luciferase reporter assay validated the target gene of miRNA. c Schematic of GFP reporter vector construct. d The transfection efficiency of miR-194-5p overexpression or knockdown was measured using immunostaining, which was analyzed by the percentage of GFP-positive cells in the total DAPI+ cells, and cellular fluorescence intensity was determined by the microplate reader. e The expression of miR-194-5p in LPS-induced astrocytes after transfection with Ctrl mimic, miR-194-5p mimic, Ctrl inhibitor and miR-194-5p inhibitor was detected by using qRT-PCR. The expression of NXPH1 in LPS-induced astrocytes cells after transfection with Ctrl mimic, miR-194-5p mimic, Ctrl inhibitor and miR-194-5p inhibitor was detected by using western blotting (f) and qRT-PCR (g). The Image J software was used for quantitative analysis of western blots. Data are shown as x ± s. n = 3 independent experiments, *p < 0.05, **p < 0.01 and ****p < 0.0001, vs the control group, Ctrl mimic group or Ctrl inhibitor group. (Color figure online)
Overexpression of miR-194-5p suppresses LPS-induced astrocyte activation
To explore the biological roles of miR-194-5p against LPS-induced astrocytes, cells were transfected with miR-194-5p mimic, miR-194-5p inhibitor or their respective controls prior to treatment with LPS (2 µg/ml). As shown in Fig. 3a, cell proliferation was significantly decreased by overexpressing miR-194-5p compared to the mimic control group. On the contrary, inhibition of miR-194-5p noticeably promoted cell proliferation compared to the control inhibitor group. In addition, capase-3/7 activity indicated that miR-194-5p inhibition markedly inhibited capase-3/7 activity while contrary results were obtained with miR-194-5p mimic (Fig. 3b). These results implied that miR-194-5p induces the apoptosis of LPS-induced astrocytes. Similarly, overexpressing miR-194-5p noticeably reversed LPS-induced astrocytes migration while miR-194-5p inhibition promoted this effect (Fig. 3c). Moreover, miR-194-5p overexpression inhibited the release of inflammatory cytokines by LPS-induced astrocytes (Fig. 3d). Taken together, the present findings indicated that miR-194-5p obviously suppressed LPS-induced proliferation, migration and inflammation in astrocytes.
Overexpression of miR-194-5p suppresses LPS-induced proliferation, migration in cytokine release in astrocytes. The proliferation (a), apoptosis (b), and migration (c) of LPS-induced astrocytes after transfection with Ctrl mimic, miR-194-5p mimic, Ctrl inhibitor and miR-194-5p inhibitor were detected using the MTT assay (a), Caspase 3/7 activity (b) and transwell assay (c), respectively. d ELISA detected the concentrations of COX2 and cytokines (TNF-α, TGF-β, IL-1β, and IL-6) in LPS-induced astrocytes cell supernatants after transfection with Ctrl mimic, miR-194-5p mimic, Ctrl inhibitor and miR-194-5p inhibitor. Data are shown as x ± s. n = 3 independent experiments, ****p < 0.0001, vs the control mimic or control inhibitor group
Silencing of NXPH1 inhibits LPS-induced astrocyte activation
Since NXPH1 was confirmed as a direct target of miR-194-5p in astrocytes, specifically silencing NXPH1 should produce phenotypes analogous to those caused by miR-194-5p in astrocytes. Therefore, NXPH1 silencing was achieved to ascertain whether silencing of NXPH1 reproduces the function of miR-194-5p on the proliferation, migration and inflammation of astrocytes. For this purpose, astrocytes were transfected with NXPH1 siRNA. The results showed that NXPH1 silencing significantly downregulated the expression of NXPH1 (about ninefold) compared with the control group, indicating that NXPH1 has satisfactory transfection efficiency (Fig. 4a). Moreover, to confirm the transfection efficiency of NXPH1 siRNA, the GFP report vector was co-transfected with siRNA Ctrl or NXPH1 siRNA, and the results showed that there was no significant difference between the siRNA ctrl and NXPH1 siRNA, but NXPH1 siRNA effectively downregulated the expression of GFP and reduced fluorescence intensity by more than 1.4-fold (Fig. 4b). As expected, after treatment with LPS (2 µg/ml), MTT assay displayed that NXPH1 silencing pointedly repressed the proliferation of astrocytes (Fig. 4c) and increased cell apoptosis (Fig. 4d). In addition, the migration (Fig. 4e) and cytokine release (Fig. 4f) of astrocytes were considerably decreased by NXPH1 silencing. These results suggested that the miR-194-5p inhibits LPS-induced activation of astrocytes by negatively regulating NXPH1.
Silencing of NXPH1 inhibits proliferation and migration and inflammation of astrocytes. a The expression of NXPH1 in LPS-induced astrocytes after transfection with Ctrl siRNA and NXPH1 siRNA was detected by using western blotting. The Image J software was used for quantitative analysis of western blots. b The transfection efficiency of NXPH1 silencing was measured using immunostaining, which was analyzed by the percentage of GFP-positive cells in the total DAPI+ cells, and cellular fluorescence intensity was determined by the microplate reader. The proliferation (c), apoptosis (d), and migration (e) of LPS-induced astrocytes after transfection with Ctrl siRNA and NXPH1 siRNA were detected using the MTT assay (c), Caspase 3/7 activity (d) and transwell assay (e), respectively. f ELISA detected the concentrations of COX2 and cytokines (TNF-α, TGF-β, IL-1β, and IL-6) in LPS-induced astrocytes cell supernatants after transfection with Ctrl siRNA and NXPH1 siRNA. Data are shown as x ± s. n = 3 independent experiments, ****p < 0.0001, vs the control siRNA group
Discussion
Astrocytes, the most abundant cells in the brain, provide a variety of important functions such as nutrition and signal transmission [24,25,26]. In recent years, studies have found that the release of many inflammatory factors leads to inflammatory response and cerebral ischemia or spinal cord injury [27,28,29]. Additionally, after any cellular injury, the local environment undergoes profound biochemical and cellular mutations leading to the release of a large number of cytokines and chemokines that cause cytotoxicity and aggravate cell damage [30,31,32,33,34]. At the same time, the brain lesions can trigger the reaction of astrocytes and trigger the proliferation of these cells, which results in the formation of scars triggered by signaling pathways such as STAT3 and growth factors (TGF-β) and PI3K [35,36,37]. As a result of injury, astrocytes undergo a change in shape and morphology and increase the expression of a number of proteins [38]. In the present study, we established an LPS-induced in vitro injury model for astrocyte culture to investigate the roles and molecular mechanism of miR-194-5p in the regulation of astrocytes activation. In response to LPS stimulation, the contents of TNF-α, TGF-β, IL-1β, IL-6, COX2 and NXPH1 were promoted while miR-194-5p was downregulated. MiR-194-5p overexpression inhibited the proliferation of astrocytes upon LPS stimulation and the resulted inflammatory response. Through binding to the 3′-UTR of NXPH1, miR-194-5p inhibited NXPH1 expression and consequently suppressed astrocyte activation. To the best of our knowledge, this is the first study to convey a regulatory role of miR-194-5p/NXPH1 in astrocyte activation.
In case of spinal cord injury, the alteration of gene expression becomes more and more important. In these changes, miRs play an important role [39,40,41,42]. Many studies have shown that miRs are widely expressed in the brain and keep a number of events running smoothly. More specifically, research has shown that miRs are involved in the regulation of spinal cord injury-related processes, some of which are related to inflammation and apoptosis, while others are related to functional regeneration [43,44,45]. The expression of miRs in astrocytes has also been demonstrated and this stipulates the implication of these molecules in astrocyte biology and internal and external processes related to these cells [24, 46, 47]. In the present study, we demonstrated that miR-194-5p is under-expressed in LPS-induced astrocytes. In addition, the overexpression of miR-194-5p decreased the proliferation of astrocytes while increasing the percentage of apoptosis. Overexpression of miR-194-5p was also followed by decreased cell migration and decreased release of COX2 and cytokines (TNF-α, TGF-β, IL-1β, and IL-6) responsible for inflammation. These results indicate that miR-194-5p regulates the proliferation, migration and inflammation processes in LPS-induced astrocytes. Consequently, miR-194-5p could play a key role in astrocyte protection in particular, and neuroprotection in general. Moreover, given the results obtained, this miRNA could be a therapeutic target in many brain and neurodegenerative diseases. This suggests a complete elucidation of the cellular processes and the molecular mechanisms intrinsically linked to these cells.
Generally, miRs are known to regulate biological processes by post-transcriptionally regulating their target genes. Thus, in order to uncover the mechanism driven by miR-194-5p in astrocytes induced by LPS, bioinformatics was used to predict NXPH1 as a target for miR-194-5p. This was further confirmed by luciferase assay. Further, due to the increased expression of NXPH1 in LPS-induced astrocytes, this gene was knocked down and the results showed that silencing of NXPH1 mimicked the effect of miR-194-5p on the phenotypes of astrocytes. Since we confirmed the regulatory role between miR-194-5p and NXPH1, we inferred that miR-194-5p protects astrocytes from activation by down-regulating NXPH1 protein expression in astrocytes. This is the first study to reveal this regulatory pathway in astrocytes. Our findings will be useful in monitoring the multifunctional role of astrocytes in human brain.
In conclusion, miR-194-5p/NXPH1 axis regulates LPS-induced astrocyte activation and will serve as a promising target in the strategy against neuroinflammation, reactive astrocyte proliferation and migration, which may contribute to the therapy of a multitude of neurodegenerative diseases.
References
Birch AM (2014) The contribution of astrocytes to Alzheimer's disease. Biochem Soc Trans 42(5):1316–1320. https://doi.org/10.1042/bst20140171
Bulc Rozman K, Juric DM, Suput D (2017) Selective cytotoxicity of microcystins LR, LW and LF in rat astrocytes. Toxicol Lett 265:1–8. https://doi.org/10.1016/j.toxlet.2016.11.008
Filous AR, Silver J (2016) Targeting astrocytes in CNS injury and disease: a translational research approach. Prog Neurobiol 144:173–187. https://doi.org/10.1016/j.pneurobio.2016.03.009
Phillips EC, Croft CL, Kurbatskaya K, O'Neill MJ, Hutton ML, Hanger DP, Garwood CJ, Noble W (2014) Astrocytes and neuroinflammation in Alzheimer's disease. Biochem Soc Trans 42(5):1321–1325. https://doi.org/10.1042/bst20140155
Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315. https://doi.org/10.1016/j.expneurol.2015.03.020
Karve IP, Taylor JM, Crack PJ (2016) The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol 173(4):692–702. https://doi.org/10.1111/bph.13125
Danka Mohammed CP, Park JS, Nam HG, Kim K (2017) MicroRNAs in brain aging. Mech Ageing Dev 168:3–9. https://doi.org/10.1016/j.mad.2017.01.007
Quinlan S, Kenny A, Medina M, Engel T, Jimenez-Mateos EM (2017) MicroRNAs in neurodegenerative diseases. Int Rev Cell Mol Biol 334:309–343. https://doi.org/10.1016/bs.ircmb.2017.04.002
Yoon H, Flores LF, Kim J (2016) MicroRNAs in brain cholesterol metabolism and their implications for Alzheimer's disease. Biochem Biophys Acta 1861(12 Pt B):2139–2147. https://doi.org/10.1016/j.bbalip.2016.04.020
Zhai H, Karaayvaz M, Dong P, Sakuragi N, Ju J (2013) Prognostic significance of miR-194 in endometrial cancer. Biomark Res 1(1):12–12
Bus P, Kestens C, Kate FJWT, Peters W, Drenth JPH, Roodhart JML, Siersema PD, Baal JWPMV (2016) Profiling of circulating microRNAs in patients with Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol 51(6):560–570
Bao J, Zou JH, Li CY, Zheng GQ (2016) miR-194 inhibits gastric cancer cell proliferation and tumorigenesis by targeting KDM5B. Eur Rev Med Pharmacol Sci 20(21):4487
Su R, Cao S, Ma J, Liu Y, Liu X, Zheng J, Chen J, Liu L, Cai H, Li Z, Zhao L, He Q, Xue Y (2017) Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122. Mol Cancer 16(1):171. https://doi.org/10.1186/s12943-017-0737-1
Qu F, Cao P (2018) Long noncoding RNA SOX2OT contributes to gastric cancer progression by sponging miR-194-5p from AKT2. Exp Cell Res 369(2):187–196. https://doi.org/10.1016/j.yexcr.2018.05.017
Wei R, Ding C, Rodriguez RA, Del Mar Requena Mullor M (2018) The SOX2OT/miR-194-5p axis regulates cell proliferation and mobility of gastric cancer through suppressing epithelial-mesenchymal transition. Oncol Lett 16(5):6361–6368. https://doi.org/10.3892/ol.2018.9433
Missler M, Hammer RE, Sudhof TC (1998) Neurexophilin binding to alpha-neurexins. A single LNS domain functions as an independently folding ligand-binding unit. J Biol Chem 273(52):34716–34723
Reissner C, Stahn J, Breuer D, Klose M, Pohlentz G, Mormann M, Missler M (2014) Dystroglycan binding to alpha-neurexin competes with neurexophilin-1 and neuroligin in the brain. J Biol Chem 289(40):27585–27603. https://doi.org/10.1074/jbc.M114.595413
Beglopoulos V, Montag-Sallaz M, Rohlmann A, Piechotta K, Ahmad M, Montag D, Missler M (2005) Neurexophilin 3 is highly localized in cortical and cerebellar regions and is functionally important for sensorimotor gating and motor coordination. Mol Cell Biol 25(16):7278–7288. https://doi.org/10.1128/mcb.25.16.7278-7288.2005
Zhu Z, Oh SY, Zheng T, Kim YK (2010) Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin Exp Allergy 40(4):536–546
Espinosa-Oliva AM, Pablos RMD, Herrera AJ (2013) Intracranial injection of LPS in rat as animal model of neuroinflammation. Methods Mol Biol 1041:295
Salvesen Ø, Reiten MR, Heegaard PMH, Tranulis MA, Espenes A, Skovgaard K, Ersdal C (2016) Activation of innate immune genes in caprine blood leukocytes after systemic endotoxin challenge. BMC Vet Res 12(1):241
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Forsyth CB, Banan A, Farhadi A, Fields JZ, Tang Y, Shaikh M, Zhang L, Engen PA, Keshavarzian A (2007) Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J Pharmacol Exp Ther 321(1):84–97
Ouyang YB, Xu L, Liu S, Giffard RG (2014) Role of astrocytes in delayed neuronal death: GLT-1 and its novel regulation by MicroRNAs. Adv Neurobiol 11:171–188. https://doi.org/10.1007/978-3-319-08894-5_9
Ouyang YB, Xu L, Yue S, Liu S, Giffard RG (2014) Neuroprotection by astrocytes in brain ischemia: importance of microRNAs. Neurosci Lett 565:53–58. https://doi.org/10.1016/j.neulet.2013.11.015
Shipman L (2015) Microenvironment: astrocytes silence PTEN to promote brain metastasis. Nat Rev Cancer 15(12):695. https://doi.org/10.1038/nrc4045
Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133(Pt 2):433–447. https://doi.org/10.1093/brain/awp322
Bethea JR (2000) Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res 128:33–42. https://doi.org/10.1016/s0079-6123(00)28005-9
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209(2):378–388. https://doi.org/10.1016/j.expneurol.2007.06.009
Allison DJ, Gabriel DA, Klentrou P, Josse AR, Ditor DS (2017) The influence of chronic inflammation on peripheral motor nerve conduction following spinal cord injury: a randomized clinical trial. Top Spinal Cord Injury Rehabil 23(4):377–385. https://doi.org/10.1310/sci16-00045
DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, Busch SA, Silver J (2015) Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep 5:16795. https://doi.org/10.1038/srep16795
Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R (2015) Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci 68:82–91. https://doi.org/10.1016/j.mcn.2015.04.006
Grau JW, Huie JR, Lee KH, Hoy KC, Huang YJ, Turtle JD, Strain MM, Baumbauer KM, Miranda RM, Hook MA, Ferguson AR, Garraway SM (2014) Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front Neural Circ 8:100. https://doi.org/10.3389/fncir.2014.00100
Hausmann ON (2003) Post-traumatic inflammation following spinal cord injury. Spinal Cord 41(7):369–378. https://doi.org/10.1038/sj.sc.3101483
Dumont CM, Margul DJ, Shea LD (2016) Tissue engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs 202(1–2):52–66. https://doi.org/10.1159/000446646
Kotaka K, Nagai J, Hensley K, Ohshima T (2017) Lanthionine ketimine ester promotes locomotor recovery after spinal cord injury by reducing neuroinflammation and promoting axon growth. Biochem Biophys Res Commun 483(1):759–764. https://doi.org/10.1016/j.bbrc.2016.12.069
Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, Rong LM, Liu S, Ding Y, Shen HY, Long SM, Wu JL, Ling EA, Zeng YS (2016) Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 83:233–248. https://doi.org/10.1016/j.biomaterials.2015.11.059
Brumm AJ, Nunez S, Doroudchi MM, Kawaguchi R, Duan J, Pellegrini M, Lam L, Carmichael ST, Deb A, Hinman JD (2017) Astrocytes can adopt endothelial cell fates in a p53-dependent manner. Mol Neurobiol 54(6):4584–4596. https://doi.org/10.1007/s12035-016-9974-3
Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U, Henzi R (2017) Astrocytes at the hub of the stress response: potential modulation of neurogenesis by miRNAs in astrocyte-derived exosomes. Stem Cells Int 2017:1719050. https://doi.org/10.1155/2017/1719050
Marcuzzo S, Kapetis D, Mantegazza R, Baggi F, Bonanno S, Barzago C, Cavalcante P, Kerlero de Rosbo N, Bernasconi P (2014) Altered miRNA expression is associated with neuronal fate in G93A-SOD1 ependymal stem progenitor cells. Exp Neurol 253:91–101. https://doi.org/10.1016/j.expneurol.2013.12.007
Pilakka-Kanthikeel S, Raymond A, Atluri VS, Sagar V, Saxena SK, Diaz P, Chevelon S, Concepcion M, Nair M (2015) Sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1)-facilitated HIV restriction in astrocytes is regulated by miRNA-181a. J Neuroinflamm 12:66. https://doi.org/10.1186/s12974-015-0285-9
Song YC, Li WJ, Li LZ (2015) Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats. Asian Pac J Trop Med 8(10):807–812. https://doi.org/10.1016/j.apjtm.2015.09.006
Karthikeyan A, Patnala R, Jadhav SP, Eng-Ang L, Dheen ST (2016) MicroRNAs: key players in microglia and astrocyte mediated inflammation in CNS pathologies. Curr Med Chem 23(30):3528–3546
Li XQ, Fang B, Tan WF, Wang ZL, Sun XJ, Zhang ZL, Ma H (2016) miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood–spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC Neurosci 17:10. https://doi.org/10.1186/s12868-016-0243-1
Quinzanos-Fresnedo J, Sahagun-Olmos RC (2015) Micro RNA and its role in the pathophysiology of spinal cord injury - a further step towards neuroregenerative medicine. Cirugia y cirujanos 83(5):442–447. https://doi.org/10.1016/j.circir.2015.05.045
Wang CY, Yang SH, Tzeng SF (2015) MicroRNA-145 as one negative regulator of astrogliosis. Glia 63(2):194–205. https://doi.org/10.1002/glia.22743
Ziu M, Fletcher L, Rana S, Jimenez DF, Digicaylioglu M (2011) Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PLoS ONE 6(2):e14724. https://doi.org/10.1371/journal.pone.0014724
Funding
This study was funded by the Natural Science Foundation of Jiangsu Province (Grant Number BK20161069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Li, Z. & Zuo, Q. miR-194-5p inhibits LPS-induced astrocytes activation by directly targeting neurexophilin 1. Mol Cell Biochem 471, 203–213 (2020). https://doi.org/10.1007/s11010-020-03780-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03780-0