Abstract
Alzheimer’s disease (AD) is the leading cause of dementia, which characterized by toxic senile plaques is composed of amyloid-β (Aβ). β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is the rate-limiting protease in Aβ generation. Therefore, pharmacology BACE1 inhibition is one of the prime targets for potential treatment of AD. Curcumin, a yellow polyphenol derived from the rhizomes of the plant Curcuma longa Linn, has been reported to cross the blood–brain barrier and prevent Aβ aggregation in AD models. However, its neuroprotective mechanism is still unclear. In the present study, we find that curcumin markedly reduces Aβ levels in HEK293-APPswe cells. Our results show that curcumin inhibits BACE1 gene expression in SH-SY5Y cells at transcriptional and translational levels. Furthermore, we reveal that nuclear factor kappa B (NFκB) signaling is involved in the regulation of curcumin on BACE1. Interestingly, the estrogenicity of curcumin is found to partially contribute to its protective action. Our data show that curcumin activates estrogen receptor β (ERβ) selectively and the activation of ERβ directly effects on the upstream factors of the NFκB signaling pathway. The above results indicate that curcumin reduces BACE1 expression through ERβ and NFκB pathway, providing a novel mechanism for curcumin as a candidate for AD therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is characterized by two major histopathological hallmarks: the cerebral accumulation of senile plaques and intracellular neurofibrillary tangles [1]. A fertile area of research supports the amyloid cascade hypothesis that neuronal dysfunction, synaptic loss, neurofibrillary neurodegeneration, and the full manifestation of Alzheimer pathology are initiated by amyloid β (Aβ) [2]. Aβ results from sequential proteolysis of the amyloid-β precursor protein (APP) by β-secretase and γ-secretase, with β-cleavage being the rate-limiting step in this pathway [3]. β-site amyloid precursor protein-cleaving enzyme1 (BACE1) is the major β-secretase and expresses highly in neurons [4]. It has been reported that BACE1−/− mice dramatically abolishes Aβ generation [5]. In addition, BACE1 deficiency mice crossed with APP transgenic mice do not show Aβ-dependent memory loss [6]. Most importantly, the side effects of BACE1 inhibition are relatively moderate to γ-secretase inhibitors [7]. Herein, inhibition of BACE1 is currently one of the viable treatments for AD.
Mounting evidence suggests an early and substantial involvement of inflammation in AD pathogenesis. For instance, elevated concentrations of proinflammatory cytokines can be found in AD brains [8]. Nuclear factor kappa B (NFκB) signaling is the center of immune responses and inflammation [9]. NFκB is a complex including five members: p50, p52, p65 (Rel A), c-Rel, and Rel B. In basic state, inhibitory protein IκBα associates with NFκB in the cytoplasm. Upon activated by stimuli, IκBα undergoes phosphorylation, ubiquitination, and consequently degradation. This enables NFκB dimers (the mainly abundant, p65:p50) transferring to the nucleus to induce target genes. The recent study revealed aberrant activation of NFκB in AD patients [10]. Since the putative binding sites for NFκB in BACE1 promoter have been identified, several groups reported that NFκB signaling regulates BACE1 gene expression at multiple levels [11]. These observations suggest that the regulation of NFκB might be a good target for investigation.
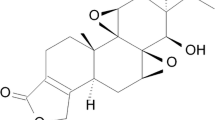
Curcumin (Fig. 1a) is a polyphenolic compound isolated from turmeric which possesses anti-carcinogenic, anti-inflammatory, and anti-oxidant properties [12]. The new study reported that curcumin has received much attention as a fluorochrome in retinal imaging for AD diagnostics [13]. Curcumin was shown to prevent Aβ aggregation from the formation of non-toxic aggregates by Thapa group [14]. Previous research showed that the protective effect of curcumin is associated with its property of CpG demethylation [15]. However, the research about the mechanism of curcumin in AD treatment is not well established, particularly from its anti-inflammatory aspect. In the present study, we aimed to explore the mechanism underlying curcumin’s anti-amyloidogenesis action in AD cell models. Based on its potent modulatory role in the immune response, we reasoned that this process is mediated by NFκB pathway. We further found that, selectively, activation of estrogen receptor β (ERβ) is involved in curcumin’s effects on NFκB. The interaction between ER and NFκB pathway is investigated in our neuroprotective study.
Effects of curcumin on Aβ generation, APP processing, and BACE1 expression in H293-APPswe and SH-SY5Y cells. a The chemical structure of curcumin. b Curcumin reduces Aβ42 levels in a dose manner by ELISA. *p < 0.05, **p < 0.01 versus cont group. c The effects of curcumin on protein mAPP, imAPP, β-CTF(C99), and α-CTF(C83) were detected by western blot, with β-actin as an internal control. Protein levels were quantified by densitometry analysis (right panel). *p < 0.05, **p < 0.01 versus DAPT only group. d Western blot showing that curcumin greatly reduces BACE1 protein levels. *p < 0.05, **p < 0.01 and ***p < 0.001 versus cont group. e QPCR showing a decrease of BACE1 mRNA expression after curcumin treatment. *p < 0.05, **p < 0.01 versus cont group. f Luciferase reporter system shows that curcumin markedly inhibits BACE1 promoter activity. The data were expressed as mean ± SEM. n = 3. *p < 0.05, **p < 0.01 versus cont group
Materials and methods
Cell culture and drug treatment
SH-SY5Y (human neuroblastoma cells) and HEK293 (human kidney cells) were purchased from American Type Culture Collection (USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, BI) and 1% penicillin/streptomycin (Gibco). HEK293 cells stably transfected with a Swedish mutant APP695 (H293-APPswe) were cultured in the medium mentioned above with an additional 200 ng/ml G418 (Sigma, USA). All cells were cultured at 37 °C in a humidified incubator containing 5% CO2. Curcumin (#C1386), DAPT (#D5942), ICI 182,780 (#V900926), DPN (#H5915), PPT (#H6036), and MTT (#M2128) were purchased from sigma. TNFα was from CST. For each experiment, drugs were diluted from its stock solution and added to the growth medium at the indicated concentrations.
Elisa
H293-APPswe cells were incubated with indicated doses of curcumin for 24 h. Or H293-APPswe cells were transfected with pcDNA5-FLAG-P65, and then treated with 10 μM curcumin. Medium was removed and cells were treated with opti-MEM (Gibco, USA) for another 4 h. Medium samples were collected and Aβ levels were determined by ELISA kit (CUSABIO, China) according to the manufacturer’s indication.
Real-time PCR
SH-SY5Y cells were seeded in 6-well culture plates per 106 cells/well, then treated with various concentration of curcumin for 12 h and collected for RNA analysis. Total RNA was isolated with Trizol extraction kits (Cwbio, China). Single-strand cDNA was synthesized from 1 μg of total RNA using reverse transcription system (Promega, USA). The number of transcripts in cDNA samples was measured with SYBR Green I master (TianGen) by quantitative real-time PCR in Bio-rad CFX instrument (USA). The primers used are provided in Supplementary Table 1.
Luciferase report assay and transfection
HEK293 cells were seeded at approximately 105 cells/well in a 24-well plate. Each well was transfected with 400 ng target plasmids and 2 ng pCMV. pCMV was used as an empty vector. After transfection, drugs were added into the medium. Luciferase assays were conducted with the Dual-Luciferase reporter assay system (Promega, USA). Plasmid pBACE1, pBACE1-NFκB, phage-ERα, pEGFP-ERβ, and pGL3-ERE were used in the study. The siRNAs against p65 were synthesized by Genepharma (China). The scramble RNA or si p65 RNA (5′-GGAGUACCCUGAGGCUAUATT-3′) were transfected into SH-SY5Y cells using Lipofectamine 2000 (Invitrogen, USA).
Western blot
After indicated treatments, cells were collected and prepared in lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4, 0.5 μg/ml leupeptin). The protein content of cell lysates was determined by BCA assay (Thermo, USA). Equivalent amounts of protein were separated by 10–15% SDS-PAGE gels and then transferred to PVDF membranes. After incubation with blocking buffer, membranes were incubated with primary antibodies overnight. The following antibodies were used: APP (1:6000, C-terminus, Abcam), BACE1(1:1000, Abcam), Akt (1:1000, CST), phospho-Akt (1:1000, CST), NFκB (1:10,000, Abcam), IκBα (1:1000, CST), phospho-IκBα (1:1000, CST), phospho-NFκB (1:1000, CST), β-actin (1:10,000, ABclonal), ERα (1:1000, CST), and ERβ (1:1000, Sigma). Blots were detected using HRP-conjugated secondary antibodies (Pierce) and the ECL chemiluminescence kit (Millipore, USA). The bands were calculated via quantity one software (Bio-rad, USA).
Nuclear-cytoplasmic fraction
SH-SY5Y cells were pretreated with vehicle or curcumin for 1 h, followed by 10 ng/ml TNFα stimulation for the indicated times, and then harvested and spun down in cold PBS. Approximately 10 volumes of buffer A (10 mM Tris pH 7.4, 10 mM NaCl, 5 mM MgCl2, 1 mM DTT, plus proteinase inhibitors; compared with the volume of the cell pellet) was added to the cells, which were then incubated on ice for 15 min. A certain volume of buffer B (10 mM Tris–HCl pH 7.4, 10 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10% NP40, plus proteinase inhibitors) was then added to ensure the final concentration of NP40 is 0.5%. After agitation and incubation on ice for 1 min, the mixture was centrifuged at 500 g for 10 min. The obtained supernatant was centrifuged at 16,000×g for another 30 min and the new supernatant was taken as the cytoplasmic fraction. The left pellet after the first centrifuge was washed by buffer A for several times before being re-suspended by buffer C (20 mM Hepes pH 7.9, 0.5 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20% glycerol, 1 mM DTT, plus proteinase inhibitors). The mixture after re-suspension was then incubated on ice for 30 min and centrifuged at 16,000×g for 30 min. The resulting supernatant was taken as the nucleus fraction.
Immunocytochemistry staining
SH-SY5Y cells were cultured on a glass coverslip and incubated for 24 h. Cells were treated with 10 ng/ml TNFα or curcumin, or were pretreated with curcumin for 1 h and then co-treated with 10 ng/ml TNFα for 40 min in serum-free medium. Cells were rinsed with PBS before 4% paraformaldehyde fixation for 15 min at ambient temperature. Then cells were permeabilized with 0.1% TritonX-100 for 10 min. Fixed cells were blocked in 2% BSA for 30 min and incubated with primary antibodies at 4 °C overnight. They were gently washed three times with PBS before incubation with fluorescence-conjugated secondary antibody for 1 h at dark situation. The antibody was used: Rabbit anti-NFκB (1:100, CST), Alexa Fluor 594 secondary antibody (1:500, Invitrogen). Counterstaining was performed with DAPI to visualize the cell nuclei. Samples were evaluated by light microscopy (Olympus, Japan) and photomicrographs were digitally captured and stored.
Competitive fluorometric receptor-binding assay
The relative binding affinities of curcumin for estrogen receptors were identified according to the fluorescence polarization method as described previously [16]. Briefly, 40 nM coumestrol and 0.8 μM human GST-ERα or GST-ERβ were added into 384 plates at the indicated doze. Fluorescence polarization was measured after incubating at room temperature for 2 h.
Statistical analysis
All results were repeated at least three duplicates and all values were represented as mean ± SEM. Statistical analysis was performed by ANOVA or student’s t test. Statistical significance is accepted when p < 0.05. All statistical analysis and calculations were done using Origin 8 (Origin Lab, UK).
Results
Curcumin effects on βAPP processing through β-secretase inhibition
To measure cell viability under the treatment of curcumin (0, 1, 2.5, 5, 10 μM), MTT assay was first conducted. We found that curcumin had no detectable cytotoxic effects on cells at the concentration used (Supplementary Fig. S1). We then investigated the effect of curcumin on Aβ accumulation in HEK293-APPswe cells. ELISA analysis was performed which showed that 10 μM curcumin significantly reduced Aβ levels to 52.98 ± 3.11% (p < 0.01) compared to the untreated group (Fig. 1b). Next, we measured the levels of β-C-Terminal fragment (CTF, C99) and α-C-Terminal fragment (CTF, C83), two major APP C-terminal metabolites. To inhibit γ-secretase activity and prevent subsequent proteolysis cleavage, DAPT was added during the measurement thus making CTFs detectable. As shown in Fig. 1c, curcumin treatment attenuated the amount of C99 fragment but not C83 fragment, and yet augmented mature and immature APP (mAPP and imAPP) levels. These results indicated that curcumin affects the amyloidogenic pathway of βAPP and reduces Aβ levels in H293-APPswe cells.
Since BACE1 is the rate-limiting enzyme initiating the production of Aβ in the amyloidogenic pathway, we next checked whether curcumin could decrease its expression. Our results showed that curcumin significantly reduced mature and immature BACE1 (mBACE1 and imBACE1) protein levels in a dose-dependent manner, with a maximum reduction (33.08 ± 2.89% for mBACE1 and 16.87 ± 4.22% for imBACE1) at the concentration of 10 μM (Fig. 1d), respectively. Curcumin treatment was also found to attenuate BACE1 mRNA levels dose-dependently (Fig. 1e). To further test the transcriptional activity of BACE1 promoter under curcumin treatment, we constructed a vector with BACE1 promoter ranging from − 2464 to − 1 bp and found that BACE1 promoter activity was also decreased (Fig. 1f). These findings demonstrated that curcumin might inhibit the amyloidogenic processing of βAPP through inhibiting BACE1 gene expression.
NFκB mediates the suppressive effect of curcumin on BACE1 expression
NFκB is closely correlated with BACE1 levels in AD. In the study, exogenously expressed p65 enhanced BACE1 levels by almost threefold, which was markedly decreased by curcumin exposure (Fig. 2a). To further examine the effect of p65 on APP processing, Aβ generation was measured. Our results showed that p65 expression increased Aβ levels by 171.38 ± 1.18% in H293-APPswe cells, and this increase was reversed after treating with curcumin (Fig. 2b). Meanwhile, disruption of p65 gene by siRNA results in BACE1 levels decreasing to 51.01 ± 1.18% (Fig. 2c), implying that curcumin might affect BACE1 expression through NFκB pathway.
The effect of NFκB on BACE1 expression and Aβ generation, and the effect of curcumin on NFκB transcriptional activity. a Western blot shows that p65 and BACE1 protein levels were both increased in H293 cells transfected with pcDNA-FLAG-p65. *p < 0.05 versus cont group, #p < 0.05 versus Flag-p65 only group. b ELISA showing that the levels of Aβ42 were enhanced by transfecting pcDNA-FLAG-p65. *p < 0.05 versus cont group. c SY5Y cells were transfected with cont si RNA or p65 si RNA. P65 and BACE1 expression were evaluated by western blot and quantified based on densitometry. *p < 0.05 versus cont group. d Luciferase activities of pBACE1-NFκB/pNFκB were performed in H293 cells treated with curcumin and TNFα, respectively. **p < 0.01 versus cont group, ##p < 0.01 and ###p < 0.001 versus TNFα only group. e Luciferase activities of pBACE1-NFκB/pNFκB were performed in H293 cells transfected with pcDNA5-FLAG-P65 and with/without curcumin, respectively. The data were represented as mean ± SEM. n = 3 or 4. ***p < 0.001 versus cont group
To assess the potential role of NFκB in curcumin-regulated BACE1 gene transcription, HEK293 cells were transfected with pBACE1-NFκB or pNFκB followed by TNFα for the activation of NFκB signaling. Dual luciferase revealed that TNFα induced pBACE1-NFκB and pNFκB transcription activity to 181.37 ± 3.79% and 432.78 ± 3.49% relative to control, respectively. Contrarily, curcumin treatment elicits a reduction to 34.23 ± 1.89% and 25.08 ± 1.72% (Fig. 2d). Similar results were obtained by transfecting p65 expression plasmid to HEK293 cells. P65 overexpression dramatically enhanced pBACE1-NFκB activity to 10.73 ± 1.04-fold (p < 0.001) compared to control. This increase was then significantly restored to 2.64 ± 0.25-fold (p < 0.001) by curcumin. Notably, pNFκB activity showed a higher upregulation level (61.64 ± 0.59-fold, p < 0.001) which is also inhibited by curcumin treatment (19.27 ± 1.69-fold, p < 0.001) (Fig. 2e). These aforementioned results unveiled that NFκB mediates the downregulation of curcumin on BACE1 transcriptional levels.
Curcumin inhibits inflammatory NFκB activation
NFκΒ activation comprises numerous steps and integrates with PKB/Akt signaling pathway, which is known to be aberrantly regulated in AD pathology. Accordingly, we examined several key factors to test how NFκB signaling cascade was modulated upon exposure to curcumin.
In our cell line models, we first confirmed that TNFα induced the activation of Akt in time-dependent manner; however, curcumin clearly inhibited the phosphorylation of Akt in co-treating cells (Supplementary Fig. S2). Then, Cell fraction analysis was performed, revealing that TNFα induced rapid phosphorylation and degradation of IκBα in a short time. This response was abolished in the presence of curcumin, accompanied by the inhibition of the ser536 phosphorylation of NFκB p65 subunit in the cytoplasm (Fig. 3a). In addition, immunoblot analysis exhibited the enormous difference of the amount of NFκB p65 in the nucleus between curcumin-treated group and curcumin-untreated group (Fig. 3b), indicating TNFα-induced p65 translocation from the cytoplasm to the nucleus was also blocked by curcumin. And this phenomenon was further proved by immunocytochemical analysis, with no observable stained p65 (red) merging with DAPI-stained nucleus (blue) in curcumin-treated group vs TNFα group (Fig. 3c). Taken together, these results suggest that curcumin inhibits TNFα-induced NFκB activation.
Curcumin inhibits NFκB signaling in SY5Y cells. Cytoplasmic (a) and nuclear (b) extracts were prepared. The expressions of IκBα, p-IκBα, p65, and p-p65 were determined by western blot after curcumin treatment. β-actin and H3 were used as cytoplasmic and nuclear control, respectively. c Immunofluorescence showed that p65 subunit was stained in red, and nuclear was counterstained by DAPI. Scale bar = 20 μm. The results shown are representative of three independent experiments
ERβ mediates curcumin’s inhibition on BACE1 expression
Curcumin is reported to display estrogenecity and antiestrogenecity in various studies. We then investigated whether curcumin regulated BACE1 through estrogen receptor (ER).
The relative binding affinities [17] of curcumin for ER subtypes were first determined by a competitive fluorometric receptor-binding assay. The IC50 values of 17β-estradiol (E2) for ERα and ERβ were 1.09 × 10−8 M and 1.27 × 10−8 M (Fig. 4a), approximating to the theoretical value of 10−8 M, confirming the validity of the assay. The RBA values of curcumin to ERα and ERβ were 0.27% and 1.18% compared to that of E2, and its IC50 values are 4.09E−6 M and 1.02E−6 M (Fig. 4b). These results showed that curcumin has a higher selectivity for ERβ than ERα.
ERβ participates in the regulation of curcumin on BACE1 expression. Competitive binding curves of E2 (a) and curcumin (b) to ERα and ERβ. H293 cells were treated with E2 and curcumin after transfection of pEGFP-ERβ/phage-ERα with pERE luc. c Luciferase assay showing that curcumin was ERα and ERβ agonist like E2. *p < 0.05, **p < 0.01, and ***p < 0.001 versus cont group. d Luciferase assay showed that curcumin was an ERβ antagonist. *p < 0.05 versus cont group. e Representative western blots of ERα and ERβ after curcumin treatments. *p < 0.05, **p < 0.01 versus cont group. f Western blot shows that ICI 182,780 treatment reverses curcumin effect on BACE1 levels. *p < 0.05 versus cont group. g SY5Y cells were treated with DPN, PPT, or vehicle, then with TNFα for additional 15 min. The effect of curcumin on IκBα protein level was detected using western blot. *p < 0.05 versus cont group, #p < 0.05 versus TNFα only group. h SY5Y cells were transfected with pEGFP-ERβ/phage-ERα then with TNFα for additional 15 min. The effect of curcumin on IκBα protein level was detected using western blot. Bar graph shows the densitometric analysis of relative IκBα expression normalized to β-actin levels. Results were mean ± SEM. n = 3 or 4. *p < 0.05 versus cont group,#p < 0.05 versus TNFα only group
Meanwhile, we performed transactivation assay to assess the agonist/antagonist profile of curcumin. Agonist was first tested. As shown in Fig. 4c, E2 induced ERα-mediated transactivation in a dose-dependent manner with significant induction at two concentrations, 10−9 M (2.08 ± 0.20-fold) and 10−8 M (2.83 ± 0.44-fold). A similar result was seen for ERβ (2.69 ± 0.20-fold at 10−9 M and 3.06 ± 0.5-fold at 10−8 M). By contrast, curcumin activates transcription through ERβ (2.54 ± 0.14-fold) at the maximum concentration, but no estrogenic activity was displayed at 1 and 5 μM. While curcumin exhibits no activity through ERα. In parallel, antagonism was conducted in the presence of E2. Curcumin had an additive effect on E2-induced activation in the ERβ group (1.52 ± 0.16-fold) (Fig. 4d), confirming its agonism through ERβ. However, curcumin did not antagonize E2-induced activation via ERα. Therefore, curcumin has been identified as an ERβ agonist.
We then speculated if curcumin could exert its estrogenic effect by influencing ER expression. We found that curcumin treatment decreases ERα levels, whereas it has an upregulation effect on ERβ expression (Fig. 4e). Consequently, regulation of two ER isoforms in opposite directions leads to increase in ERβ/ERα ratio. Regarding the previous results, we speculated that ERβ may be involved in the regulation of BACE1 by curcumin. Truly, we found that curcumin and ER antagonist ICI 182,780 co-treatment restore the inhibitory effect of BACE1 caused by curcumin alone (Fig. 4f). It might occur as a consequence of inhibition of estrogen receptors. Unexpectedly, ERβ overexpression fails to inhibit BACE1 expression, but exhibits a more pronounced inhibition of BACE1 levels when co-acting with curcumin (data not shown).
The activation of ERs is shown to involve in the modulation of multiple signaling pathways. We then tested how ERβ acts on NFκB signaling pathway in our system. Our data showed that both ERβ agonist (DPN) and ERβ overexpression treatment reverse the degradation of IκBα, compared to the TNFα-treated group alone (Fig. 4g, h). In contrast, pretreatment with ERα agonist (PPT) or ERα overexpression did not alter the inhibitory effect of TNFα on IκBα protein levels. These results suggest that ERβ inhibits NFκB signaling by inhibiting IκBα degradation.
Discussion
Though previous researches discovered curcumin’s characteristic for preventing anti-amyloid β in AD, the mechanism underlying this beneficial effect remains to be clarified [18]. In this study, we confirmed curcumin’s capability to reduce Aβ1–42 levels and its upstream intermediate product β-CTF(C99) at the concentration with no obvious negative effects on cell viability in HEK293-APPswe cells. This cell line harbors human APP Swedish mutation (K595N/M596L), enhancing the generation of Aβ, which is a well-established AD cell model [19, 20] (Figs. 1b and S1). β-secretase cleavage is the primary step in the APP processing [21], thus, these data point to BACE1 as the main cause for its anti-amyloidogenesis effect. Given that γ-secretase was blocked by its specific inhibitor DAPT in the intermediate product analysis, we did not inspect curcumin’s role in γ-secretase [22]. Nevertheless, several studies hold that curcumin can act as a dual inhibitor for BACE1 and GSK-3β [23], and GSK-3β is the mediator for controlling γ-secretase activity [24]. Additionally, in SY5Y-APPswe cells, curcumin was shown to inhibit PS1, the major unit responsible for γ-secretase’s cleavage role [25]. Although both BACE1 and γ-secretase are indispensable for Aβ generation, results from Tg2576 mouse model found presenilin-1 could be compensated by other types of γ-secretase, making it a more complex enzyme for such study [26]. Interestingly, also in the intermediate product study, we observed a significant increase of mature and immature APP, a result contrasting with those from Zhang’s group and Ronia’s group, who reported that curcumin would stabilize the immature form of APP by altering its trafficking [27, 28]. The reason for our discovery could be attributed to the APP accumulation triggered by downstream β-secretase inhibition. Based on the above results, we reason that the anti-amyloid effect of curcumin is achieved through regulation of BACE1 expression. In line with this hypothesis, our data show that curcumin is able to suppress BACE1 at the promoter, mRNA, and protein levels. These results were also in agreement with the in vivo study using a 5 × AD mouse model by Zheng’s group [29].
We further discovered that curcumin exerts inhibition effect on BACE1′s transcriptional activity by NFκB signaling. NFκB is reported to link to BACE1 activation [30]. Therefore, we first proved the activity of NFκB and BACE1 is positively correlated. NFκB activation, whether induced by TNFα or p65 transfection, could enhance BACE1 promoter activity and expression, as well as promote Aβ production, while p65 siRNA knockdown would give rise to BACE1 inhibition. On the other hand, we proved that curcumin interferes with NFκB activity in promoter level. According to Chen’s group, human BACE1 promoter region includes four putative NFκB binding sites. Our results confirmed that they are effective, which is also consistent with former results [31]. Here, we provide evidence that targeting NFκB signaling by curcumin confers anti-inflammatory changes, and support the notion that inhibiting the pathway might be therapeutically beneficial in AD.
A broad spectrum of molecular processes has been elucidated as to modulation of NFκB activity [32]. Based on biochemical and cytochemical methods, we discovered that curcumin imposes multiple effects on different steps of this signaling pathway: by reducing its phosphorylation status, it activated and stabilized IκBα. These data can be repeated in other cell lines, as Buharmann found that curcumin inhibits IκBα phosphorylation and degradation in tenocytes [33]. However, other groups found no difference in IκBα levels in chondrocytes under the treatment of curcumin [34]. By inhibiting phosphorylation of p65, it disturbs the shuttle of p65 from the cytoplasm to nuclear. Previous studies show that the phosphorylation of p65 on ser536 determines the amount of p65 remaining in the nucleus by attenuating its association with newly IκΒα [35]. In addition, p65 is phosphorylated at ser536 requiring PI3 K/Akt activity to promote cell proliferation and apoptosis [36]. As our data show, TNFα-induced phosphorylation of Akt was decreased by curcumin, in consistent with the modulatory trend of curcumin on the phosphorylation status of p65. Our results revealed that curcumin might exploit multiple strategies as to suppressing NFκB signaling, which explained its potency in BACE1 inhibition.
Estrogen is restricted in clinical use due to its side effects, such as risks for breast cancer [37]. In spite of its side effects, it is still considered plausible to use estrogen-like substances for AD treatment. Curcumin, generally belonging to plant polyphenols, is deemed to possess the estrogen-like effects [38]. However, Kelly et al. found that curcumin has anti-estrogenic activity in T47D cells [39]. Herein, we provide a thorough analysis of its estrogenic and anti-estrogenic properties. Our results show that curcumin binds to both receptors within the micromolar range and displays a preferential affinity to ERβ over ERα (Fig. 4b). The subsequent reporter assay further demonstrated that curcumin selectively activates ERβ other than ERα. These results suggest that curcumin is an ERβ agonist and could directly associate with the ER. Like most of the phytoestrogens previously reported, curcumin has weaker estrogenic activity than estradiol. Our discovery is supported by Liu’s group whose result using computational molecular docking also noted curcumin as an ER binder [40]. Estrogen deficiency is related to the occurrence of AD [41]. Our lab and Zhao’s group suggest that estrogen relieves Aβ burden by transactivating neprilysin and insulin-degrading enzyme in an ER-dependent manner [42, 43]. Both endogenous and exogenous estrogen were shown to reduce Aβ levels by activating receptors. As our results show, curcumin alters the ratio of two ER subtypes. Both receptors are reported to operate in a complex manner. The disparity of the expression between two receptors might affect their neuronal response. Moreover, ERβ seems to play a more significant role in neuroprotection in the brain than ERα [44]. Previous study showed that estrogen deficiency mice display elevated BACE1 expression and enzyme activity [45], though the detail is unclear. In our data, overexpression and pharmacology inhibition experiments have shown the importance of ERβ in the protective effect of curcumin.
The multiple crosstalks between NFκB and ER signaling pathway have been documented [46]. In vitro, ERα directly binds to Rel A, p50, and c-Rel [47]. ERβ inhibits the DNA binding ability of NFκB p50 dimers [48]. Here, we found that ERβ dampened NFκB activity by heightened IκBα levels, not ERα. This result prompts us that ERβ exerts functions through non-genomic pathway. This is corroborated by Xing’s work that ERβ activation by E2 enhanced IκBα mRNA levels and restored IκBα protein [49]. In addition to this non-genomic signaling, ER and NFκB subunits are reported to form a complex at the gene promoter to facilitate transcription [50]. However, whether this direct genomic activation is implicated in curcumin’s effect needs further study.
In conclusion, we proposed curcumin as a multiple-target reagent for AD in vitro level. However, a clinical study initiated by Baum showed no difference in cognitive impairment among AD patients administered with oral curcumin for 6 months [51]. This may be due to different bioavailability and absorption in human test. Interestingly, one study revealed the benefits of curcumin using solid lipid formulation on cognition and mood in older population [52], suggesting its neuroprotection capacity on clinical level. In our study, we found that the multiple-target effect of curcumin may help increase the impairment of BACE1 transcriptional activity through two separate ways, which is likely to converge at the promoter level. This signaling crosstalk between NFκB and ERβ might also be indispensable for the ultimate BACE1 protein suppression. Therefore, we hold that this multitarget strategy may represent a promising approach to slow AD progression.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid-β
- BACE1:
-
Β-site amyloid precursor protein-cleaving enzyme1
- NFκB:
-
Nuclear factor kappa B
- ER:
-
Estrogen receptor
References
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr, Proceedings of the Meeting of the International Working G, the American Alzheimer’s Association on “The Preclinical State of AD, July and Washington Dc USA (2016) Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 12:292–323. https://doi.org/10.1016/j.jalz.2016.02.002
Du X, Wang X, Geng M (2018) Alzheimer’s disease hypothesis and related therapies. Transl Neurodegener 7:2. https://doi.org/10.1186/s40035-018-0107-y
Das U, Wang L, Ganguly A, Saikia JM, Wagner SL, Koo EH, Roy S (2016) Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat Neurosci 19:55–64. https://doi.org/10.1038/nn.4188
Yan R, Vassar R (2014) Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol 13:319–329. https://doi.org/10.1016/s1474-4422(13)70276-x
Hu X, Das B, Hou H, He W, Yan R (2018) BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. J Exp Med 215:927–940. https://doi.org/10.1084/jem.20171831
Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R (2007) BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis 26:134–145. https://doi.org/10.1016/j.nbd.2006.12.008
Citron M (2004) Strategies for disease modification in Alzheimer’s disease. Nat Rev Neurosci 5:677–685. https://doi.org/10.1038/nrn1495
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Zhang Q, Lenardo MJ, Baltimore D (2017) 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell 168:37–57. https://doi.org/10.1016/j.cell.2016.12.012
Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, Tone Y, Tong Y, Song W (2012) Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int J Neuropsychopharmacol 15:77–90. https://doi.org/10.1017/S1461145711000149
Zheng N, Yuan P, Li C, Wu J, Huang J (2015) Luteolin reduces BACE1 expression through NF-kappaB and through estrogen receptor mediated pathways in HEK293 and SH-SY5Y Cells. J Alzheimers Dis 45:659–671. https://doi.org/10.3233/JAD-142517
Bisht K, Wagner KH, Bulmer AC (2010) Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology 278:88–100. https://doi.org/10.1016/j.tox.2009.11.008
Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, Martins RN (2016) Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 115:449–465. https://doi.org/10.1017/S0007114515004687
Thapa A, Jett SD, Chi EY (2016) Curcumin attenuates amyloid-beta aggregate toxicity and modulates amyloid-beta aggregation pathway. ACS Chem Neurosci 7:56–68. https://doi.org/10.1021/acschemneuro.5b00214
Deng Y, Lu X, Wang L, Li T, Ding Y, Cao H, Zhang Y, Guo X, Yu G (2014) Curcumin inhibits the AKT/NF-kappaB signaling via CpG demethylation of the promoter and restoration of NEP in the N2a cell line. AAPS J 16:649–657. https://doi.org/10.1208/s12248-014-9605-8
Tang C, Li C, Zhang S, Hu Z, Wu J, Dong C, Huang J, Zhou HB (2015) Novel bioactive hybrid compound dual targeting estrogen receptor and histone deacetylase for the treatment of breast cancer. J Med Chem 58:4550–4572. https://doi.org/10.1021/acs.jmedchem.5b00099
Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C (1997) Transcription factor NFk B is activated in primary neurons by amyloid b peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci USA 94(6):2642–2647
Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, Wang R, Pradeepkiran JA, Ogunmokun G, Thamarai K, Quesada K, Boles A, Reddy AP (2018) Protective effects of Indian spice curcumin against amyloid-beta in Alzheimer’s disease. J Alzheimers Dis 61:843–866. https://doi.org/10.3233/JAD-170512
Page RM, Baumann K, Tomioka M, Perez-Revuelta BI, Fukumori A, Jacobsen H, Flohr A, Luebbers T, Ozmen L, Steiner H, Haass C (2008) Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem 283:677–683. https://doi.org/10.1074/jbc.M708754200
Volmar CH, Salah-Uddin H, Janczura KJ, Halley P, Lambert G, Wodrich A, Manoah S, Patel NH, Sartor GC, Mehta N, Miles NTH, Desse S, Dorcius D, Cameron MD, Brothers SP, Wahlestedt C (2017) M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer’s disease genes and improving memory. Proc Natl Acad Sci USA 114:E9135–E9144. https://doi.org/10.1073/pnas.1707544114
Vassar R, Kovacs DM, Yan R, Wong PC (2009) The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci 29:12787–12794. https://doi.org/10.1523/JNEUROSCI.3657-09.2009
Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, Tomita T (2006) C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester). J Biol Chem 281:14670–14676. https://doi.org/10.1074/jbc.M513012200
Di Martino RM, De Simone A, Andrisano V, Bisignano P, Bisi A, Gobbi S, Rampa A, Fato R, Bergamini C, Perez DI, Martinez A, Bottegoni G, Cavalli A, Belluti F (2016) Versatility of the curcumin scaffold: discovery of potent and balanced dual BACE-1 and GSK-3beta inhibitors. J Med Chem 59:531–544. https://doi.org/10.1021/acs.jmedchem.5b00894
Uemura K, Kuzuya A, Shimozono Y, Aoyagi N, Ando K, Shimohama S, Kinoshita A (2007) GSK3beta activity modifies the localization and function of presenilin 1. J Biol Chem 282:15823–15832. https://doi.org/10.1074/jbc.M610708200
Xiong Z, Hongmei Z, Lu S, Yu L (2011) Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacol Rep 63:1101–1108. https://doi.org/10.1016/s1734-1140(11)70629-6
Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang YP (2008) Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol Dis 29:316–326. https://doi.org/10.1016/j.nbd.2007.09.005
Zhang C, Browne A, Child D, Tanzi RE (2010) Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem 285:28472–28480. https://doi.org/10.1074/jbc.M110.133520
Kotani R, Urano Y, Sugimoto H, Noguchi N (2017) Decrease of amyloid-beta levels by curcumin derivative via modulation of amyloid-beta protein precursor trafficking. J Alzheimers Dis 56:529–542. https://doi.org/10.3233/JAD-160794
Zheng K, Dai X, Xiao N, Wu X, Wei Z, Fang W, Zhu Y, Zhang J, Chen X (2017) Curcumin ameliorates memory decline via inhibiting BACE1 expression and beta-amyloid pathology in 5xFAD transgenic mice. Mol Neurobiol 54:1967–1977. https://doi.org/10.1007/s12035-016-9802-9
Buggia-Prevot V, Sevalle J, Rossner S, Checler F (2008) NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem 283:10037–10047. https://doi.org/10.1074/jbc.M706579200
Wang R, Chen S, Liu Y, Diao S, Xue Y, You X, Park EA, Liao FF (2015) All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor kappaB (NFkappaB) signaling. J Biol Chem 290:22532–22542. https://doi.org/10.1074/jbc.M115.662908
Mitchell S, Vargas J, Hoffmann A (2016) Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med 8:227–241. https://doi.org/10.1002/wsbm.1331
Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, Shakibaei M (2011) Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 286:28556–28566. https://doi.org/10.1074/jbc.M111.256180
Wang J, Ma J, Gu JH, Wang FY, Shang XS, Tao HR, Wang X (2017) Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol Med Rep 16:1837–1845. https://doi.org/10.3892/mmr.2017.6771
Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W (1999) IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem 274:30353–30356. https://doi.org/10.1074/jbc.274.43.30353
Liu JY, Jiang L, He T, Liu JJ, Fan JY, Xu XH, Tang B, Shi Y, Zhao YL, Qian F, Wang Y, Cui YH, Yu PW (2019) NETO2 promotes invasion and metastasis of gastric cancer cells via activation of PI3K/Akt/NF-kappaB/Snail axis and predicts outcome of the patients. Cell Death Dis 10:162. https://doi.org/10.1038/s41419-019-1388-5
Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer FE (1995) The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 332:1589–1593. https://doi.org/10.1056/nejm199506153322401
Bachmeier BE, Mirisola V, Romeo F, Generoso L, Esposito A, Dell’Eva R, Blengio F, Killian PH, Albini A, Pfeffer U (2010) Reference profile correlation reveals estrogenlike trancriptional activity of curcumin. Cell Physiol Biochem 26(3):471–482
Hallman K, Aleck K, Dwyer B, Lloyd V, Quigley M, Sitto N, Siebert AE, Dinda S (2017) The effects of turmeric (curcumin) on tumor suppressor protein (p53) and estrogen receptor (ERalpha) in breast cancer cells. Breast Cancer (Dove Med Press) 9:153–161. https://doi.org/10.2147/BCTT.S125783
Liu H, He S, Wang T, Orang-Ojong B, Lu Q, Zhang Z, Pan L, Chai X, Wu H, Fan G, Zhang P, Feng Y, Song YS, Gao X, Karas RH, Zhu Y (2018) Selected phytoestrogens distinguish roles of ERalpha transactivation and ligand binding for anti-inflammatory activity. Endocrinology 159:3351–3364. https://doi.org/10.1210/en.2018-00275
Yun J, Yeo IJ, Hwang CJ, Choi DY, Im HS, Kim JY, Choi WR, Jung MH, Han SB, Hong JT (2018) Estrogen deficiency exacerbates Abeta-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-kB activation in ovariectomized mice. Brain Behav Immun 73:282–293. https://doi.org/10.1016/j.bbi.2018.05.013
Liang K, Yang L, Yin C, Xiao Z, Zhang J, Liu Y, Huang J (2010) Estrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin. J Biol Chem 285:935–942. https://doi.org/10.1074/jbc.M109.051664
Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD (2011) 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging 32:1949–1963. https://doi.org/10.1016/j.neurobiolaging.2009.12.010
Wang L, Andersson S, Warner M, Gustafsson JA (2003) Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA 100:703–708. https://doi.org/10.1073/pnas.242735799
Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y (2013) Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol 47:857–867. https://doi.org/10.1007/s12035-012-8377-3
Sas L, Lardon F, Vermeulen PB, Hauspy J, Van Dam P, Pauwels P, Dirix LY, Van Laere SJJBCR (2012) The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res 14:212. https://doi.org/10.1186/bcr3196
Pradhan M, Bembinster LA, Baumgarten SC, Frasor J (2010) Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J Biol Chem 285:31100–31106. https://doi.org/10.1074/jbc.M110.155309
Galien R, Garcia T (1997) Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res 25:2424–2429. https://doi.org/10.1093/nar/25.12.2424
Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, Chen YF, Nozell S (2012) Estrogen modulates NFkappaB signaling by enhancing IkappaBalpha levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-beta. PLoS ONE 7:e36890. https://doi.org/10.1371/journal.pone.0036890
Stein B, Yang MX (1995) Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol 15:4971–4979. https://doi.org/10.1128/mcb.15.9.4971
Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V (2008) Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28:110–113. https://doi.org/10.1097/jcp.0b013e318160862c
Cox KH, Pipingas A, Scholey AB (2015) Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol 29:642–651. https://doi.org/10.1177/0269881114552744
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31870786, 81573279, and 31371331) and Guidance Project of Science and Technology Research Project of HuBei Provincial Education Department (Category B Project:B2018456). We are grateful to HuZhiYe and NinWenTao (Wuhan university, school of pharmaceutical science, China) for their assistance provided in the receptor-binding experiments.
Author information
Authors and Affiliations
Contributions
PH: Conceived and designed the experiments; PH: performed the data; PH: analyzed the data; PH and NZ: wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, P., Zheng, N., Zhou, Hb. et al. Curcumin inhibits BACE1 expression through the interaction between ERβ and NFκB signaling pathway in SH-SY5Y cells. Mol Cell Biochem 463, 161–173 (2020). https://doi.org/10.1007/s11010-019-03638-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03638-0