Abstract
Thymoquinone (TQ) is a highly perspective chemotherapeutic agent against gliomas and glioblastomas because of its ability to cross the blood–brain barrier and its selective cytotoxicity for glioblastoma cells compared to primary astrocytes. Here, we tested the hypothesis that TQ-induced mild oxidative stress provokes C6 glioma cell apoptosis through redox-dependent alteration of MAPK proteins. We showed that low concentrations of TQ (20–50 μM) promoted cell-cycle arrest and induced hydrogen peroxide generation as a result of NADH-quinone oxidoreductase 1-catalyzed two-electron reduction of this quinone. Similarly, low concentrations of TQ efficiently conjugated intracellular GSH disturbing redox state of glioma cells and provoking mitochondrial dysfunction. We demonstrated that high concentrations of TQ (70–100 μM) induced reactive oxygen species generation due to its one-electron reduction. TQ provoked apoptosis in C6 glioma cells through mitochondrial potential dissipation and permeability transition pore opening. The identified TQ modes of action on C6 glioma cells open up the possibility of considering it as a promising agent to enhance the sensitivity of cancer cells to standard chemotherapeutic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma, the highest grade glioma tumor, is the most common malignant brain tumor [1]. Despite considerable research efforts, glioblastoma remains incurable because of its infiltrating growth, quick-developing chemotherapy resistance and the inability of the majority of anticancer drugs to penetrate blood–brain barrier. Nowadays, only two chemotherapeutic agents, namely carmustine and temozolomide, have been approved by the US FDA for treating malignant gliomas [2, 3]. However, glioma and glioblastoma often show resistance to these drugs due to a variety of cellular mechanisms that include increased efflux of drugs from cancer cells, autophagy, cancer stem cells and miRNAs [4, 5]. Therefore, the development of more efficient therapeutic approaches for the treatment for glioblastoma is required. To date, naturally occurring phytochemicals which have been revealed to induce apoptosis of tumor cells are considered to be one of the most prospective compounds for anticancer therapy [5].
Quinone derivatives are widely used in medicine as antibacterial and antiviral agents, antimicotics and chemotherapeutic compounds [6]. Doxorubicin, one of the high-effective anticancer quinones, is used for treatment for diverse types of tumors such as lymphomas, sarcomas of various etiology and breast cancer. Natural quinones such as menadione and thymoquinone (TQ, 2-isopropyl-5-methyl-1,4-benzoquinone) are considered promising chemotherapeutic drugs [7, 8]. Antitumor activity of TQ has been demonstrated against different tumors in vivo and in vitro. TQ has been shown to cross the blood–brain barrier and induce glioma and glioblastoma cell death [9,10,11] and exhibits antimetastatic and anti-invasive activity. TQ possesses selective cytotoxicity for glioblastoma cells compared to normal human astrocytes [9]. The molecular mechanisms of TQ-induced tumor cell apoptosis involve activation of caspases 8, 9 and 3, Bax up-regulation and Bcl2 down-regulation, and inhibition PI3K/Akt and NF-κB signal pathways [8, 12, 13].
In glioblastoma cells, TQ has been shown to significantly decrease NO production and functions as an autophagy inhibitor, which results in the perturbation of the lysosomal membrane and a leakage of lysosomal protease cathepsin B into the cytosol, finally provoking the caspase-independent cell death [9, 14]. It has also been demonstrated that TQ can induce apoptosis via both p53-dependent and p53-independent pathways [15,16,17,18,19].
Although many cellular effects of TQ have been revealed, the molecular mechanisms of TQ antitumor activity remain uncertain. A growing body of evidence [9, 16, 20, 21] indicates that reactive oxygen species (ROS) generation is a general mechanism underlying the TQ antitumor effect. In lymphoma cells, TQ induces superoxide and hydrogen peroxide production followed by NF-κB inactivation and apoptosis initiation [20]. It is known that ROS can modulate (stimulate or inhibit) redox-dependent Akt and MAPK signal transduction pathways, which also can lead to apoptosis of cancer cells. However, the data of TQ effects on the MAPK pathway are conflicting. To date, the activation or inhibition of the MAPK protein family seems to depend on the cell type and on the TQ concentration used. The aim of this study is to elucidate redox-dependent mechanisms of TQ-induced glioma cell death.
Materials and methods
Chemicals
Carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), dimethyl sulfoxide (DMSO), 3,3′-methylene-bis(4-hydroxycoumarin) (dicumarol), monochlorobimane (MCB), propidium iodide (PI), trypsin–EDTA, TQ, Triton X-100, cyclosporine A, inhibitors PD098059, Sb203580, SP600125, Gӧ6983, LY294002, JSH were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany), and the fluorescent probes 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolocarbocyanine iodide (JC-1) and MitoSOX Red were obtained from Molecular Probes Inc. (USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma Chemical Co. (St. Louis, MO, USA), fetal bovine serum was obtained from HyClone (USA), and gentamicin was purchased from Belmedpreparaty (Minsk, Belarus). Hematoxylin/eosin and HEPES-buffer components were purchased from Analysis X Company (Minsk, Belarus). Apoptosis detection kit was obtained from Trevigen Inc. (USA). All other reagents were purchased from Reakhim (Moscow, Russia). All solutions were made with Milli-Q water. TQ was dissolved in DMSO. Final DMSO concentration in the probe did not exceed 0.1%.
HEPES-buffer containing 131 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, 6 mM glucose and 20 mM HEPES (pH 7.3) was freshly prepared.
Cell culture and treatment
C6 glioma cell line (originally cloned from a cell strain of a rat glial tumor induced by N-nitrosomethylurea) was purchased from the Cell Culture Collection of the Institute of Cytology (St. Petersburg, Russia). The C6 glioma cells were cultured in DMEM supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin at 37 °C in a humidified 5% CO2 and 95% air atmosphere. After cells reached 70% confluency, the cells were harvested, washed with HEPES-buffer, resuspended (1 × 106 cells/ml) and used for experiments.
To study cell proliferation and viability, the cells were plated in Petri dishes at a density of 1 × 105 cells/ml. The culture medium was replaced by fresh one on the 2nd day when the monolayer confluence was about 70–80%. The cells were treated for 24 h with TQ in various doses or DMSO as a control.
Cell viability and proliferation assay
Viability and proliferation of C6 cells exposed to TQ for 24 h were assessed according to [22] with PI using spectrofluorometer SM2203 (Solar, Belarus). It is known that PI penetrates only in damaged (necrotic) cells and intercalates into DNA structure resulting in high-intensive fluorescence. The intensity of PI fluorescence is proportional to the number of damaged cells in a sample. We determined the viability of attached cells after treatment with TQ for 24 h. Attached C6 cells were washed with HEPES-buffer, suspended after trypsin addition and stained with 4 μM PI. Fluorescence intensity F1 (λex = 530 nm, λem = 645 nm) was measured in 5 min. Then, cells were lysed with 0.1% Triton X-100 and fluorescence intensity F2 was registered. Cell viability in each sample was determined as the ratio of viable cells to the total cell number according to the formula (1):
Thereafter, viability in each sample was normalized for viability in control sample.
Proliferation index N relative to the value N0 of the control sample was determined according to the formula (2):
where index “0” denotes the control samples. The background level of PI fluorescence intensity in the cell-free buffer system has been subtracted from each fluorescent intensity values F1 and F2.
Data obtained with PI assay were confirmed by counting of viable cells in Goryaev’s chamber. As seen from Fig. S1 in Supplementary materials, PI-based fluorescent assay and direct counting of cells have showed the similar results.
Mitotic index was determined by direct examination under a light microscope (Nikon eclipse E-100, Japan) of cells fixed with ice-cold ethanol and stained with Böhmer’s hematoxylin, as percentage of mitotic cells from 10,000 cells in a sample.
Assessment of cell apoptosis
Percentage of apoptotic cells was measured by flow cytometry on BD FACScanto II flow cytometer (Becton–Dickinson, Heidelberg, Germany). Cells were treated with TQ (20 μM, 50 μM) for 24 h and then washed in HEPES-buffer and resuspended in binding buffer (total volume was 100 μl per one sample) containing Annexin V-FITC and PI and incubated for 15 min at room temperature in the dark following the manufacturer’s instructions (TACS Annexin V Kits. Apoptosis Detection by Flow Cytometry or In Situ labeling, Trevigen Inc., USA). Minimum of 3 × 105 cells/ml were analyzed for each sample. These experiments were done in triplicate and were independently repeated three times.
Mitochondrial membrane potential quantification
Mitochondrial membrane potential in the C6 glioma cells was evaluated using the mitochondrial membrane potential sensitive carbocyanine dye JC-1 [23]. This lipophilic and cationic dye accumulates within mitochondria driven by the negative membrane potential and forms concentration dependent J-aggregates. When excited at 490 nm, the monomeric form of the dye emits at 530 nm, while the J-aggregates show maximum emission at 590 nm. Briefly, 1 × 106 cells were treated with TQ for 20 min, washed with HEPES-buffer and incubated in the presence of 1 μM JC-1 for 15 min. After washing cells twice again with HEPES-buffer, JC-1-related fluorescence was analyzed using spectrofluorometer. The ratio of fluorescence intensities at 590 and 530 nm (I590/I530) is proportional to mitochondrial membrane potential. Membrane potential in the control sample without quinone was chosen as 100%.
Measurements of ROS generation in mitochondria
For studying mitochondrial superoxide production in C6 glioma cells, MitoSOX Red (fluorogenic dye specifically targeted to mitochondria in live cells [24]) was used. MitoSOX Red was added to cells in 1.25 μM final concentration for 20 min. Cells were washed twice with HEPES-buffer and analyzed. Difference in fluorescence intensity (λex = 510 nm, λem = 580 nm) of MitoSOX Red (\({\text{I}}^{ 10}_{\text{mitoSOX}}\)) between moments 0 min and 10 min after TQ addition to cell suspension was measured using spectrofluorometer. Superoxide production rate in the control sample without quinone was chosen as 100%.
Measurements of cytosolic ROS generation
The ROS generation in cytosol after addition of TQ (10–100 µM) was estimated fluorometrically using a fluorescent probe H2DCF-DA [25, 26]. 1 µM H2DCF-DA was added to cell suspension for 30 min at 37 °C. Then, cells were washed and resuspended in HEPES-buffer (1 × 106 cells/ml). Fluorescence intensity (λex = 488 nm, λem = 530 nm) was recorded by a spectrofluorometer for 10 min after the addition of freshly prepared TQ solution.
Intracellular reduced glutathione level analysis
Concentration of reduced glutathione (GSH) in cells treated with TQ was determined by MCB assay [27]. Cells in suspension 1 × 106 cells/ml were incubated in HEPES-buffer with TQ in different concentrations for 30 min, once washed and incubated with 10 µM MCB for 30 min at 37 °C. Fluorescence intensity of MCB glutathionyl-conjugates (Imcb) was registered (λex = 390 nm, λem = 480 nm). The results are presented as the ratio of concentrations in the tested ([GSH]TQ) and the control ([GSH]0) samples that corresponds to the ratio of MCB fluorescence intensity for both samples (Imcb/Imcb0).
Statistical analysis
The results were expressed as the means of three to five replicates ± SD. The statistical analysis was conducted using the analysis of variance (ANOVA) with post hoc Dunnett’s test for multiple pairwise comparisons with the respective control group for concentration dependences of viability, proliferation, mitochondrial potential, ROS and GSH pool. Bonferroni multiple comparison test was used for all multiple pairwise comparisons to analyze the statistical significance of the inhibitory analysis results.
The number of mitotic cells was expressed as mean ± SD for three independent experiments, each running in triplicate. The unpaired two-tailed Student’s t test was used to determine the significance of differences between means for mitotic index.
p < 0.05 was considered to be statistically significant. Calculations were performed using the statistical software packages StatSoft Statistica 6.0.
Results
TQ inhibits proliferation and reduces viability of C6 glioma cells
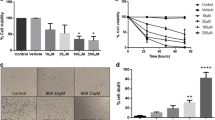
To evaluate the dose-dependent TQ effects on viability and proliferation of C6 glioma cells, PI staining was used. As shown in Fig. 1a, TQ in 10–70 µM concentration induced a dose-dependent proliferation index decrease after treatment of C6 cells for 24 h. The dose of TQ required to induce 50% reduction in the C6 glioma cell number (LD50) was 40 µM. When cells were exposed to 100 µM TQ for 24 h, all cells detached and were lost. This effect indicates cytotoxic and/or cytostatic action of TQ on glioma cells.
Dose-dependent effect of TQ on proliferation and viability (a) and mitotic activity (b) of C6 glioma cells. Cells were exposed to TQ for 24 h. Data were represented as means ± SDs from at least three independent experiments run in triplicate. The statistical significance of the results (a) was analyzed by ANOVA with post hoc Dunnett’s test (*p < 0.05, **p < 0.01). The unpaired two-tailed Student’s t-test was used to determine the significance of differences between means for mitotic index (*p < 0.05)
To differentiate between these two types of action, we determined the viability of attached cells after treatment with TQ for 24 h, as it is known that PI penetrates only in damaged (necrotic) cells. As seen in Fig. 1a, the viability of attached cells decreased at TQ concentrations higher than 30 µM. However, treatment of C6 glioma cells with 20 µM TQ resulted in the proliferation index decrease (26 ± 4)% with unchanged cell viability compared to control (Fig. 1a).
In order to determine whether TQ had effects on cell cycle, we examined the mitotic activity of C6 glioma cells. As shown in Fig. 1b, the mitotic index of cells was twofold decreased in samples treated with 20 μM TQ. Thus, TQ at a concentration of 20 μM could promote cell-cycle arrest.
TQ induces apoptosis in C6 glioma cells
In order to determine whether the observed inhibition of cell proliferation was caused by an increase in apoptosis, flow cytometry analysis using annexin V was performed with C6 glioma cells exposed to TQ. After treatment with increasing concentrations of TQ, a dose-dependent increase in PI and Annexin V double positive cells was observed. As shown in Fig. 2, in non-treated control samples the majority of cells (93%) were viable and non-apoptotic. When cells were treated with 20 µM and 50 μM of TQ for 6 h, a moderate increase (up to 20% and 30%, p < 0.05, respectively) in late apoptotic cells was observed. TQ induced necrosis in glioma cells, as indicated by a slight increase in PI staining compared to non-treated control cells (from 3 to 7%, p < 0.05).
The effect of TQ on apoptosis and necrosis in C6 glioma cells evaluated by annexin V assay. C6 glioma cells were treated for 6 h with TQ. a–c—The representative flow cytometry histograms [Annexin V (FL1A) and propidium iodide (FL2P) labeling] of cell apoptosis for cells treated with solvent control (a) or with 20 µM (b) and 50 μM (c) of TQ. d represents the percentage of late apoptotic and necrotic cells treated with TQ and PI3K inhibitor LY294002. Data are presented as the means ± SDs of three independent experiments. The statistical significance of the results was analyzed by ANOVA with post hoc Dunnett’s test (*p < 0.05, **p < 0.01 vs. the respective control)
Preincubation with phosphoinositol-3-kinase inhibitor (LY294002) significantly attenuated the effect of TQ treatment (Fig. 2d). The number of apoptotic cells significantly decreased from 20 (in the lack of inhibitor) to 14% (p < 0.05), suggestive of participation of PI3K in TQ-induced apoptosis.
TQ disrupts mitochondrial function
Mitochondrial dysfunction is known to participate in the cell death. In order to examine the effect of TQ on mitochondrial functioning in C6 glioma cells, intramitochondrial superoxide level and mitochondrial transmembrane potential were measured.
As shown in Fig. 3a, TQ in a dose-dependent manner rendered both antioxidant and pro-oxidant effects. TQ at 10–20 μM significantly decreased the mitochondrial superoxide production rate (Fig. 3a). A further increase in TQ concentration led to the rise of mitochondrial superoxide yield up to 1.6-fold in cells treated with 100 μM TQ compared to controls. Thus, mitochondrial ROS could be responsible for cell death induction at TQ treatment at high concentration.
TQ effect on mitochondrial functioning in C6 glioma cells. a—The level of mitochondrial superoxide production in cells treated with TQ in different concentrations for 10 min. b—Mitochondrial membrane potential dissipation in C6 glioma cells exposed to TQ for 20 min, FCCP is an ionofore that depolarizes mitochondrial membrane potential. c—Proliferation of C6 glioma cells treated 24 h with 30-μM TQ without inhibitors (TQ) or preincubated for 4 h with 1 μM cyclosporine A (TQ + cycA). Each experiment was done in triplicate. Data were represented as means ± SDs. The statistical significance of the results was analyzed by ANOVA with post hoc Dunnett’s test (*p < 0.05, **p < 0.01)
TQ (30–100 μM) induced mitochondrial depolarization, as shown in Fig. 3b. The total dissipation of mitochondrial transmembrane potential was registered in cells treated with 100 μM TQ for 30 min.
It is known that the mitochondrial permeability transition pore (PTP) opening is a step in apoptosis. Because the observed mitochondrial depolarization can be a sign of PTP opening, we tested cell proliferation under the action of the PTP inhibitor cyclosporine A together with TQ. We observed a decrease in the number of dead cells after cyclosporine A treatment from 37 to 20% (p < 0.05; Fig. 3c). Thus, TQ promotes mitochondrial PTP opening followed by the loss of transmembrane potential and apoptosis. The fact that the number of viable cells after cyclosporine A and TQ treatment did not reach the control level, could suggest that TQ induces both apoptosis and necrosis.
TQ affects the redox state of C6 glioma cells
As intracellular oxidative stress could induce PTP opening and apoptosis, we studied the cytosolic ROS involvement in TQ-induced cell death. To examine whether TQ induces intracellular ROS generation, we quantified the levels of ROS by DCF fluorescence (Fig. 4a). We observed that at a TQ concentration higher than 20 μM cytosolic ROS production in glioma cells was increased (Fig. 4a). Maximal ROS production was detected in cells at 50–70 μM TQ.
TQ effects on the redox state of C6 glioma cells. a—Cytosolic ROS yield in C6 glioma cells exposed to TQ in different concentration for 10 min. Cells were preincubated for 10 min with 1 μM of dicoumarol, and then, TQ was added. b—Intracellular GSH pool changes in C6 glioma cell treated with TQ for 30 min. Before TQ addition, cells were preincubated for 10 min with inhibitors (1 μM dicoumarol, 0.1 μM DPI or 120 U/ml catalase) or the corresponding volume of solvent. Each experiment was done in triplicate. Data were represented as means ± SDs. In a and b, the statistical significance of the results was analyzed by ANOVA with post hoc Dunnett’s test (*p < 0.05, **p < 0.01 for TQ effect in comparison with control sample without quinone). Bonferroni multiple comparison test was used to estimate the statistical significance of the inhibitory analysis results (†p < 0.05 and ††p < 0.01 for inhibitor effect compared to the corresponding sample without inhibitor). c—Proliferation of C6 glioma cells treated for 24 h with 25 μM TQ without inhibitors (TQ) or preincubated for 4 h with 5 mM N-acetylcysteine (TQ + NAC), 100 U/ml catalase (TQ + catalase), 100 U/ml superoxide dismutase (TQ + SOD). Each experiment was done in triplicate. Data were represented as means ± SDs. The statistical significance of the results was analyzed by Bonferroni multiple comparison test (*p < 0.05)
In order to investigate whether one- or two-electron reduction pathways would contribute into the ROS-inducing effects of TQ, dicoumarol, an inhibitor of NADH-quinone oxidoreductase 1 (NQO1, DT-diaphorase), was used. As shown in Fig. 4a, the presence of dicoumarol reduced ROS generation totally in cells exposed to 20–50 μM TQ. We observed that when C6 glioma cells were applied to dicoumarol prior to 70 μM TQ, ROS levels were about 1.4-fold decreased compared to the sample treated with TQ only. At higher TQ concentration (100 μM), dicoumarol had no effect on TQ-induced ROS production (Fig. 4a). Thus, both one-electron reduction and two-electron reduction contribute to the TQ-induced generation of ROS in the cytosol, and likely the two-electron reduction has a major role in TQ action at low concentrations.
GSH is one of the important antioxidants of cellular defense system that also implements thiol-mediated detoxification of anticancer drugs. We evaluated the influence of TQ on GSH levels. GSH levels in C6 cells treated with TQ for 30 min are shown in Fig. 4b. TQ (10–100 μM) decreased the cellular pool of GSH in a dose-dependent manner as indicated by a significant decrease in MCB fluorescence compared to controls. TQ induced more than 50% drop in intracellular GSH level at concentrations of 25 µM and higher. The GSH levels were dramatically decreased in C6 glioma cells exposed to 50–100 μM TQ.
TQ can induce GSH depletion due to generation of ROS or through direct conjuration of GSH with TQ. To evaluate the contributions of each reaction, we used the inhibitors of one- and two-electron quinone reduction enzymes (DPI and dicoumarol) and hydrogen peroxide scavenger (catalase). As shown in Fig. 4b, when C6 glioma cells were applied to catalase prior to 20–100 μM TQ treatment, GSH levels increased compared to the TQ-treated group. In contrast, TQ-induced cellular GSH depletion was more pronounced after DPI and dicoumarol pretreatments of C6 glioma cells. Inhibition of one-electron reduction enzymes by DPI had a significant effect at TQ concentrations of 30 µM and higher, while treatment of cells with dicoumarol results in GSH levels decrease even at 1 µM TQ. Addition of TQ at concentrations of 30 µM and higher caused a total reduction of GSH levels in the presence of dicoumarol (Fig. 4b).
Figure 4c shows the effects of antioxidants [N-acetyl-cysteine (NAC), catalase and superoxide dismutase (SOD)] on TQ-induced C6 glioma cells’ death. Incubation with catalase, but not SOD reduced cell death in samples treated with TQ. Noticeably, a combination of TQ and the ROS scavenger NAC induced significantly less cell death compared to the rates in the individual drug treatments. Thus, production of ROS and depletion of GSH pools may be responsible, at least in part, for cell death induction by TQ.
PI3K and JNK pathways are involved in TQ-induced glioma cell death
To define the molecular mechanisms of redox-mediated apoptosis, we performed an inhibitory analysis of a number of redox-sensitive kinases participating in the redox regulation of cellular functions [6]. Proliferative activity of C6 glioma cells in the presence of inhibitors and exposed to TQ is shown in Table 1.
Inhibition of NF-κB activation with JSH showed a significant suppression in the number of viable cells exposed to TQ. However, proliferation index in PD098059 (ERK1/2 inhibitor), Gӧ6983 (protein kinase C inhibitor) and Sb203580 (p38 inhibitor) pretreatment cell samples exposed to TQ was reduced to the same levels as in the control sample exposed to 25 μM TQ. Preincubation of the cells with the PI3K inhibitor LY294002 or JNK inhibitor SP600125 partially prevented TQ-induced proliferation index decrease.
Discussion
Due to the fact that expression levels of different oncogenes and a variety of gene mutations vary vastly from cell to cell even in the same tumor, it is unlikely to succeed suppressing tumor progression through the inhibition of a single gene or individual signal transduction pathway. Because of this, the quest for common targets, which could provide a general strategy for cancer treatment independent on the type of tumor, has been intense in the research community. Redox alterations in cancer cells associated with mitochondrial dysfunction and elevated ROS generation may be exploited for such therapeutic purpose.
As cancer cells have alterations in redox state and mostly reveal the constitutively elevated rates of ROS production, it has been proposed [28,29,30,31] that these cells would be more vulnerable to agents, which induce the enhanced ROS generation and/or GSH depletion, so-called oxidative therapy. Because of that, pro-oxidant agents could be considered a suitable way to selectively induce cancer cell death while sparing normal cells. To date, several ROS modulating agents have exhibited antitumor activity in preclinical and clinical studies [31].
Diverse natural redox-active polyphenols and quinones, including TQ, demonstrated antitumor action in in vitro and in vivo studies [6,7,8]. These could affect several cellular targets and act as an agents both directly inducing cell death and increasing the sensitivity of cancer cells to standard chemotherapeutic drugs.
When we examined the TQ ability to suppress the growth of glioma cells, we found that proliferation was regulated in a TQ dose-dependent manner and 50% cell death occurred at 40 μM TQ treatment for 24 h (Fig. 1). TQ has been shown to regulate cell-cycle proteins, inducing both G1/S and G2/M cell-cycle arrest in various cancer cell lines [10, 15, 32, 33]. For example, TQ induces G2/M phase cell-cycle arrest and inducing apoptosis of neuroblastoma cells [34]. Our results indicated that TQ significantly reduced mitotic index of C6 cells (Fig. 1b). Taking into account the results described in [10, 15, 32,33,34,35], we hypothesize that TQ could arrest cell-cycle decreasing proliferation of C6 glioma cells.
We revealed pro-apoptotic effects of TQ in C6 glioma cells (Fig. 2), as others have shown in other tumor types [8,9,10, 12, 15, 16, 18, 19, 21, 33,34,35,36,37,38,39,40,41]. Many studies have reported that TQ mediates ROS production as a mechanism to induce apoptosis and growth inhibition [8, 16, 20, 21, 39,40,41,42,43]. Moreover, TQ has been shown to manifest multidirectional redox activity in normal and tumor cells, namely inducing oxidative stress, inhibiting cell proliferation in cultured tumor cells and displaying antioxidant activity or relatively inaction on proliferation in normal cells, as reviewed in [15,16,17, 35, 44].
In the present study, we show that TQ induces oxidative stress in C6 glioma cells accompanied by an increase in intracellular ROS production and GSH reduction (Fig. 4a, b). Quinones are well-known redox-cycling agents which can undergo one- or two-electron reduction by cellular reductases [45, 46]. Potential redox reactions of TQ in C6 glioma cells are shown in Fig. 5. One-electron TQ reduction (Reaction 1), catalyzed by NADPH-cytochrome P450 reductase, NADH-cytochrome b5 reductase, NADH: ubiquinone oxidoreductase, etc., yields semiquinone radicals, which react rapidly with molecular oxygen (Reaction 5) to form superoxide and hydrogen peroxide afterward [45, 46]. Two-electron TQ reduction (Reaction 2), catalyzed by NQO1, leads to formation of quinols (hydroquinones), which have high redox-potential and predominantly possess antioxidant activity [45]. In appropriate conditions, quinols could undergo autooxidation (Reaction 6) or participate in the disproportionation reaction (Reaction 4) with semiquinone radicals formation, resulting in additional ROS generation [47, 48]. We have demonstrated that NQO1 is the essential source of the TQ-induced ROS formation, especially at low doses (20–50 μM) of quinone (Fig. 4a). The higher the TQ concentration, the more considerable the contribution of one-electron reduction of TQ to the ROS generation in C6 glioma cells.
TQ redox reactions in C6 glioma cells. Reaction 1—one-electron TQ reduction, catalyzed by enzymes E1e such as NADPH-cytochrome P450 reductase, NADH-cytochrome b5 reductase, NADH: ubiquinone oxidoreductase, etc. (inhibited by DPI). Reaction 2—two-electron reduction, catalyzed by NADH-quinone oxidoreductase 1 (NQO1, inhibited by Dicoumarol). Reaction 3—Michael-type addition of GSH with formation of TQ-GS conjugate. Reaction 4—disproportionation reaction. Reactions 5 and 6—autooxidation of semiquinone and quinol, respectively. Superoxide dismutation is catalyzed by SOD. Hydrogen peroxide is scavenged in the presence of catalase or GSH
Of particular interest is the fact that many types of tumor cells are characterized by having increased level of NQO1 expression, as compared to normal cells [49]. Therefore, the mechanism of TQ-induced ROS production described in the present study could determine the TQ selectivity for tumor cells. For example, TQ-mediated preferential killing of high-expressing NQO1 glioblastoma cells [50] with low cytotoxicity to primary astrocytes was demonstrated elsewhere [9].
As high levels of intracellular GSH have been associated with tumor drug resistance, depletion of cellular GSH could restore sensitivity to the cytotoxic effect of an anticancer drug [51]. Using catalase, we demonstrated GSH oxidation with hydrogen peroxide in cells treated with TQ. Comparing the dependence of TQ-induced ROS (H2O2) generation (Fig. 4a) and GSH decrease (Fig. 4b), and taking into account the increased GSH depletion by inhibition of the one-electron reduction and two-electron reduction, it is plausible that the decrease in GSH depends on other mechanism.
It is known that quinone-mediated GSH depletion can occur in the nucleophilic addition reaction (Michael-type addition) with formation of quinone conjugates with glutathione (Q-SG), as shown in Fig. 5, Reaction 3 [52,53,54]. Despite that the inhibition of redox-cycling of quinone by dicoumarol and DPI led to the decrease in TQ-induced hydrogen peroxide production in C6 glioma cells, a marked glutathione depletion was observed, probably owing up the enhancement of TQ-SG conjugates formation. As was shown elsewhere [55], TQ forms GSH conjugates even in a non-enzymatic reaction. Thus, TQ can simultaneously display antitumor effects as both redox-cycling and alkylating agents. Inhibition of antioxidant systems, including GSH depletion by modifying thiol groups, constitutes an alternative approach to increase ROS accumulation in tumor cells.
Excessive intracellular ROS production can promote apoptosis via mitochondrial PTP opening with subsequent release of pro-apoptotic factors and caspases activation [56]. As TQ was found to cause PTP opening in C6 glioma cells (Fig. 3c), we have investigated whether ROS level can interfere with TQ-induced growth inhibition. We found that the antiproliferative effect of TQ in C6 glioma cells was partially reversed by NAC and catalase (Fig. 4c). Thus, it could be assumed that oxidative stress leads to a decrease in cell proliferation.
Of note, in our study TQ induced both apoptotic cell death and necrotic cell death (Fig. 2). It has been shown by [9] that TQ inhibited autophagy and induced caspase-independent apoptosis in glioblastoma cells T98G and U87MG with negligible number of necrotic cells. Thus, TQ treatment could be an exciting and emerging strategy in cancer therapy.
Many antitumor compounds (mitocans) have been established to act as pro-oxidant agents, disturbing the mitochondrial respiratory chain and leading to further increase in mitochondrial ROS levels [57]. Indeed, mitochondria are excellent targets for cancer treatment as they determine the aberrant energetic metabolism of malignant cells and regulate intracellular redox homeostasis and cell death by apoptosis and necrosis [57, 58]. Our study indicates that TQ causes mitochondrial potential dissipation and modulates superoxide generation in mitochondria in a dose-dependent manner (Fig. 3). These data may be considered as early events of apoptosis through the mitochondrial pathway. The anticancer effect of TQ, via the induction of apoptosis resulting from mitochondrial dysfunction, was assessed in an acute lymphocyte leukemic cell line [39]. Therefore, TQ could be classified as a mitocan disturbing both cell and mitochondrial redox states.
We have demonstrated that at low doses TQ suppressed mitochondrial superoxide (Fig. 3a). It had been shown that several chemotherapeutic agents such as anthracycline antibiotics can induce ROS production causing toxicity, which in turn limits their use [19]. Therefore, TQ ability of mitochondrial ROS inhibition could reduce the toxicity of these agents. Indeed, TQ was found to suppress doxorubicin-induced cardiotoxicity without influencing the antitumor effects of doxorubicin [44]. It was shown that the ability of TQ to suppress breast tumor growth and the combined treatment with doxorubicin caused significantly higher suppression [37]. On the other hand, mitochondrial ROS production, intensification of mitochondrial interaction with the endoplasmic reticulum and the accumulation of mitochondria in the filopodia of glioma cells are considered to be the factors of invasive growth of these tumors [59]. Thus, the reduction of mitochondrial ROS in glioma cells treated with TQ may cause an anti-invasive effect.
TQ was found to induce apoptosis in tumor cells by regulating NF-κB [35, 60], STAT3 [61], AKT, ERK1/2, JNK [42], p38 [37], p53 [15] signaling pathways, which are redox-sensitive. ROS could directly or indirectly modify signaling proteins [62] activating or suppressing pathways. TQ was shown to induce ROS production and ROS-mediated MAPK phosphorylation in cancer cells [8, 12, 16, 20, 21, 33, 35, 37, 39,40,41,42,43, 63]. However, in our experimental system ERK1/2, PKC and p38 inhibitors had no influence on TQ-induced antiproliferative effect (Table 1). Although TQ is generally considered as a potent suppressor of the direct binding of p65 NF-κB subunit to DNA, down-regulating the expression of different gene products [60, 64], we showed that it acts in combination with NF-κB inhibitor JSH in favor of antiproliferative effect in C6 glioma. In this respect, inhibitory mechanisms of TQ and JSH are proposed to be similar. Such effect of TQ would be useful for treatment for cancers with constitutive NF-κB activity.
PI3K/AKT/mTOR signaling pathway plays an outstanding role in cell proliferation and alterations (amplification) in the proteins involved in this pathway are common in cancer [65]. PI3K inhibiting drugs, including wortmannin, flavonoid derivative LY294002 could suppress proliferation and enhance the sensitivity of tumor cells to apoptosis-inducing agents [65, 66]. Moreover, PI3K/AKT pathway could be involved in tumor drug resistance [67]. GBM patients with an activated PI3K/AKT/mTOR pathway have poor prognosis than patients without oncogenic activation of this pathway [68]. Thus, PI3K/AKT/mTOR (PI3K/AKT/PTEN) pathway is considered to be additional target for glioma treatment [66,67,68]. We have demonstrated here that the reduction of TQ-induced antiproliferative and antiapoptotic effect was registered in C6 glioma cells after PI3K inhibition (Table 1). Therefore, TQ is suggested to suppress PI3K pathway in C6 glioma cells. TQ-induced apoptosis through the suppression of PI3K and AKT activation has also been shown in prostate cancer, human umbilical vein endothelial cells, primary effusion lymphoma cells [33, 40], breast cancer cells (T-47D and MDA-MB-468) [36] and cholangiocarcinoma cells [63].
Of particular interest is the fact that JNK inhibition also resulted in attenuation of TQ antiproliferative effect (Table 1). Although JNK is predominantly activated during apoptosis [69, 70], some studies have demonstrated the involvement of this kinase in tumor cells proliferation and survival [52, 54, 55]. For example, it has been shown that JNK2 activation accelerates KB-3 cells proliferation reducing the transition time from S to G2/M phase of cell cycle [71]. The antiproliferative effect of JNK inhibition in serum-starved C6 glioma cells was reported by us earlier [72].
Conclusions
Our results demonstrate that the antiproliferative and pro-apoptotic effects of TQ in C6 glioma cells are mediated through both cellular redox state modulation via ROS production and GSH depletion, and mitochondrial dysfunction. Therefore, it could be hypothesized that TQ decreases intracellular GSH levels not only through hydrogen peroxide generation, but also by TQ-SG conjugates formation. We demonstrated that TQ induces ROS generation due to two-electron reduction of TQ at low concentrations by NQO1, while one-electron reduction contributes at high TQ concentrations. We proposed that TQ induces apoptosis in C6 glioma cells through the modulation of ROS generation, mitochondrial potential dissipation and permeability transition pore opening.
TQ exhibits significant antitumor effect in C6 glioma cells providing a new possibility to improve cancer therapeutic outcomes. The observed effects of TQ led us to speculate a high selectivity of TQ anticancer activity against tumor cells with over-activated PI3K/AKT pathway as well as a big potential to enhance the sensitivity of glioma cells to standard chemotherapeutic drugs.
References
Delgado-Lopez PD, Corrales-Garcıa EM (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18(11):1062–1071. https://doi.org/10.1007/s12094-016-1497-x
Jungk C, Chatziaslanidou D, Ahmadi R, Capper D, Bermejo JL (2016) Chemotherapy with BCNU in recurrent glioma: analysis of clinical outcome and side effects in chemotherapy-naive patients. BMC Cancer 16:81. https://doi.org/10.1186/s12885-016-2131-6
Khosla D (2016) Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma. Ann Transl Med 4:54. https://doi.org/10.3978/j.issn.2305-5839.2016.01.25
Haar CP, Hebbar P, Wallace GC et al (2012) Drug resistance in glioblastoma: a mini review. Neurochem Res 37:1192–1200. https://doi.org/10.1007/s11064-011-0701-1
Yan Y, Xu Z, Dai S (2016) Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res 35:23. https://doi.org/10.1186/s13046-016-0303-5
Saify ZS, Mushtaq N, Noor F, Takween S, Arif M (1999) Role of quinone moiety as antitumour agents: a review. Pak J Pharm Sci 12(2):21–31
Lamson DW, Plaza SM (2003) The anticancer effects of vitamin K. Altern Med Rev 8(3):303–318
Darakhshan S, Bidmeshki PA, Hosseinzadeh CA, Sisakhtnezhad S (2015) Thymoquinone and its therapeutic potentials. Pharmacol Res 95–96:138–158. https://doi.org/10.1016/j.phrs.2015.03.011
Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA (2013) Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS ONE 8(9):e72882. https://doi.org/10.1371/journal.pone.0072882
Gurung RL, Lim SN, Khaw AK et al (2010) Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS ONE 5:e12124. https://doi.org/10.1371/journal.pone.0012124
Kolli-Bouhafs K, Boukhari A, Abusnina A, Velot E et al (2012) Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Invest New Drugs 30(6):2121–2131. https://doi.org/10.1007/s10637-011-9777-3
Khader M, Eckl PM (2014) Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci 17:950–957
Krex D, Klink B, Hartmann C et al (2007) Longterm survival with glioblastoma multiforme. Brain 130:2596–2606. https://doi.org/10.1093/brain/awm204
Pazhouhi M, Sariri R, Rabzia A, Khazaei M (2016) Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran J Basic Med Sci 19:890–898
Gali-Muhtasib H, DiabAssaf M, Boltze C et al (2004) Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53dependent mechanism. Int J Oncol 25:857–866
Gali-Muhtasib H, Roessner A, SchneiderStock R (2006) Thymoquinone: a promising anticancer drug from natural sources. Int J Biochem Cell Biol 38:1249–1253
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA (2009) Antiinflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 11(5):373–381. https://doi.org/10.1111/j.1477-2574.2009.00059.x
Roepke M, Diestel A, Bajbouj K et al (2007) Lack of p53 augments thymoquinone induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther 6:160–169
Dastjerdi MN, Mehdiabady EM, Iranpour FG, Bahramian H (2016) Effect of thymoquinone on p53 gene expression and consequence apoptosis in breast cancer cell line. Int J Prev Med 7:66. https://doi.org/10.4103/2008-7802.180412
Hussain AR, Uddin S, Ahmed M et al (2013) Phosphorylated IkBa predicts poor prognosis in activated B-cell lymphoma and its inhibition with thymoquinone induces apoptosis via ROS release. PLoS ONE 8(3):e60540. https://doi.org/10.1371/journal.pone.0060540
Rahmani AH, Alzohairy MA, Khan MA, Aly SM (2014) Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid Based Complement Altern Med 2014:724658. https://doi.org/10.1155/2014/724658
Kato F, Tanaka M, Nakamura K (1999) Rapid fluorometric assay for cell viability and cell growth using nucleic acid staining and cell lysis agents. Toxicol In Vitro 13:923–929
Reers M, Smith TW, Chen LB (1991) J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30:4480–4486
Robinson KM, Janes MS, Pehar M et al (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. PNAS 103(41):15038–15043. https://doi.org/10.1073/pnas.0601945103
Gomes A, Fernandes E, Lima JL (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80. https://doi.org/10.1016/j.jbbm.2005.10.003
Wrona M, Wardman P (2006) Properties of the radical intermediate obtained on oxidation of 2′,7′-dichlorodihydrofluorescein, a probe for oxidative stress. Free Rad Biol Med 41:657–667. https://doi.org/10.1016/j.freeradbiomed.2006.05.006
Rice GC, Bump EA, Shrieve DC, Lee W, Kovacs M (1986) Quantitative analysis of cellular glutathione by flow cytometry using monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res 46:6105–6110
Laurent A, Nicco C, Chereau C et al (2005) Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65(3):948–956
Wondrak GT (2009) Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal 11(12):3013–3069. https://doi.org/10.1089/ARS.2009.2541
Rinaldi M, Caffo M, Minutoli L et al (2016) ROS and brain gliomas: an overview of potential and innovative therapeutic strategies. Int J Mol Sci 17(6):E984. https://doi.org/10.3390/ijms17060984
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 8(7):579–591. https://doi.org/10.1038/nrd2803
Alhosin M, Abusnina A, Achour M et al (2010) Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol 79(9):1251–1260. https://doi.org/10.1016/j.bcp.2009.12.015
Arafa E-SA, Zhu Q, Shah ZI et al (2011) Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat Res 706(1–2):28–35. https://doi.org/10.1016/j.mrfmmm.2010.10.007
Paramasivam A, Raghunandhakumar S, Priyadharsini JV, Jayaraman G (2015) In vitro anti-neuroblastoma activity of thymoquinone against neuro-2a cells via cell-cycle arrest. Asian Pac J Cancer Prev 16(18):8313–8319
Banerjee S, Padhye S, Azmi A et al (2010) Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer 62(7):938–946. https://doi.org/10.1080/01635581.2010.509832
Rajput S, Kumar BN, Dey KK, Pal I, Parekh A, Mandal M (2013) Molecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci 93(21):783–790. https://doi.org/10.1016/j.lfs.2013.09.009
Woo CC, Hsu A, Kumar AP, Sethi G, Tan KH (2013) Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS ONE 8(10):e75356. https://doi.org/10.1371/journal.pone.0075356
Yi T, Cho S-G, Yi Z et al (2008) Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and ERK signaling pathways. Mol Cancer Ther 7(7):1789–1796. https://doi.org/10.1158/1535-7163.MCT-08-0124
Salim LZ, Mohan S, Othman R et al (2013) Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 18(9):11219–11240. https://doi.org/10.3390/molecules180911219
Hussain AR, Ahmed M, Ahmed S et al (2011) Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic Biol Med 50:978–987. https://doi.org/10.1016/j.freeradbiomed.2010.12.034
Yu SM, Kim SJ (2013) Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp Biol Med (Maywood) 238(7):811–820. https://doi.org/10.1177/1535370213492685
El-Najjar N, Chatila M, Moukadem H et al (2010) Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 15:183–195. https://doi.org/10.1007/s10495-009-0421-z
Yu SM, Kim SJ (2015) The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int J Mol Med 35(2):325–332. https://doi.org/10.3892/ijmm.2014.2014
Al-Shabanah OA, Badary OA, Nagi MN, Al-Gharably NM, Al-Rikabi AC, Al-Bekairi AM (1998) Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. J Exp Clin Cancer Res 17(2):193–198
Deller S, Macheroux P, Sollner S (2008) Flavin-dependent quinone reductases. Cell Mol Life Sci 65:141–160. https://doi.org/10.1007/s00018-007-7300-y
Siegel D, Reigan P, Ross D (2008) One- and two-electron-mediated reduction of quinones: enzymology and toxicological implications. In: Elfarra A (ed) Advances in bioactivation research. Springer-Verlag, New York, pp 169–199
Buffinton GD, Ollinger K, Brunmark A, Cadenas E (1989) DT-diaphorase-catalyzed reduction of 1,4-naphthoquinone derivatives and glutathionyl-quinone conjugates. Effect of substituents on autoxidation rates. Biochem J 257(2):561–571
Watanabe N, Forman HJ (2003) Autoxidation of extracellular hydroquinones is a causative event for the cytotoxicity of menadione and DMNQ in A549-S cells. Arch Biochem Biophys 411(1):145–157
Belinsky M, Jaiswal AK (1993) NAD(P)H: quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev 12:103–117
Kanamori M, Higa T, Sonoda Y et al (2015) Activation of the NRF2 pathway and its impact on the prognosis of anaplastic glioma patients. Neuro-Oncology 17(4):555–565. https://doi.org/10.1093/neuonc/nou282
Backos DS, Franklin CC, Reigan P (2012) The role of glutathione in brain tumor drug resistance. Biochem Pharmacol 83(8):1005–1012. https://doi.org/10.1016/j.bcp.2011.11.016
Ollinger K, Kägedal K (2002) Induction of apoptosis by redox-cycling quinones. Subcell Biochem 36:151–170
Brunmark A, Cadenas E (1989) Redox and addition chemistry of quinoid compounds and its biological implications. Free Rad Biol Med 7:435–477
Guin PS, Das S, Mandal PC (2011) Electrochemical reduction of quinones in different media: a review. Intern J Electrochem 2011:816202. https://doi.org/10.4061/2011/816202
Khalife KH, Lupidi G (2007) Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic Res 41(2):153–161. https://doi.org/10.1080/10715760600978815
Kowaltowski AJ, Castilho RF, Vercesi AE (2001) Mitochondrial permeability transition and oxidative stress. FEBS Lett 495:12–15. https://doi.org/10.1016/S0014-5793(01)02316-X
Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ (2013) Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 13(3):199–208. https://doi.org/10.1016/j.mito.2012.07.112
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11(1):9–15. https://doi.org/10.1038/nchembio.1712
Katsetos CD, Anni H, Dráber P (2013) Mitochondrial dysfunction in gliomas. Semin Pediatr Neurol 20(3):216–227. https://doi.org/10.1016/j.spen.2013.09.003
Sethi G, Ahn KS, Aggarwal BB (2008) Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res 6(6):1059–1070. https://doi.org/10.1158/1541-7786.MCR-07-2088
Li F, Rajendran P, Sethi G (2010) Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol 161:541–554
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10(8):1343–1374. https://doi.org/10.1089/ars.2007.1957
Connelly L, Barham W, Onishko HM et al (2011) Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene 30:1402–1412. https://doi.org/10.1038/onc.2010.521
Xu D, Ma Y, Zhao B et al (2014) Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol Rep 31(5):2063–2070. https://doi.org/10.3892/or.2014.3059
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30(2):193–204. https://doi.org/10.1016/j.ctrv.2003.07.007
Li X, Wu C, Chen N et al (2016) PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 7(22):33440–33450. https://doi.org/10.18632/oncotarget.7961
Jiang BH, Liu LZ (2008) Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updates 11(3):63–76. https://doi.org/10.1016/j.drup.2008.03.001
Chakravarti A, Zhai G, Suzuki Y et al (2004) The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol 22:1926–1933. https://doi.org/10.1200/JCO.2004.07.193
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103(2):239–252
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23(16):2838–2849. https://doi.org/10.1038/sj.onc.1207556
Du L, Lyle CS, Obey TB et al (2004) Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem 279(12):11957–11966
Krylova NG, Kulahava TA, Koran SV, Semenkova GN (2017) Proliferation of cultured glioma cells mediated by coenzyme Q10 under conditions of serum deprivation. Cell Tissue Biol 11(3):220–226. https://doi.org/10.1134/S1990519X17030063
Acknowledgements
This study was supported by the research grant of the Belarusian Republican Foundation for Fundamental Research (M17M-092) and Belarusian Ministry of Education (the national program of scientific research number 20161385).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krylova, N.G., Drobysh, M.S., Semenkova, G.N. et al. Cytotoxic and antiproliferative effects of thymoquinone on rat C6 glioma cells depend on oxidative stress. Mol Cell Biochem 462, 195–206 (2019). https://doi.org/10.1007/s11010-019-03622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03622-8