Abstract
The protein arginine methyltransferase 5 (PRMT5) and its catalytic partner methylosome protein MEP50 (WDR77) catalyse the mono- and symmetric di-methylation of selective arginines in various histones and non-histone target proteins. It has emerged as a crucial epigenetic regulator in cell proliferation and differentiation; which also reported to be overexpressed in many forms of cancers in humans. In this study, we aimed to assess the modulations in the expression of this enzyme upon exposure to the well-studied natural compound from the spice turmeric, curcumin. We exposed the lung and breast cancer cell lines (A549 and MCF-7) to curcumin (2 and 20 μM) and observed a highly significant inhibitory effect on the expression of both PRMT5 and MEP50. The level of symmetrical dimethylarginine (SDMA) in multiple proteins, and more specifically, the H4R3me2s mark (which predominates in GC-rich motifs in nucleosomal DNA) was also diminished significantly. We also found that curcumin significantly reduced the level and enrichment of the transcription factors Sp1 and NF-YA which shares their binding sites within the GC-rich region of the PRMT5 proximal promoter. Furthermore, the involvement of both PKC-p38-ERK-cFos and AKT-mTOR signalling was observed in reducing the Sp1 and NF-YA expression by curcumin. Therefore, we propose curcumin decreased the expression of PRMT5 in these cells by affecting at least these two transcription factors. Altogether, we report a new molecular target of curcumin and further elucidation of this proposed mechanism through which curcumin affects the PRMT5-MEP50 methyltransferase expression might be explored for its therapeutic application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arginine methylation of various histones and non-histone proteins catalysed by a family of nine protein arginine methyltransferase (PRMT) enzymes has drawn significant scientific interest in the last decade. The protein arginine methyltransferase 5 (PRMT5) was discovered as a JAK2 binding protein (JBP1) [1]. This type-II arginine methyltransferase catalyses the covalent modification ω-nitrogens of arginine residues explicitly into ω-NG-monomethyl and ω-NG, N’G-symmetric dimethylarginine in its target proteins [2]. Multiple nuclear, as well as cytoplasmic protein substrates, have been reported so far which includes histones (H2A, H3 and H4) [1, 3,4,5], small nuclear ribonucleoproteins (snRNPs) [6], Piwi proteins, transcription factors (e.g. HOXA9) [7], signalling proteins including epidermal growth factor receptor (EGFR) [8], C-RAF [9], p53, etc [10]. It also associates with the SWI/SNF (SWItch/Sucrose Non-Fermentable) remodelling complexes [4], spliceosomal snRNP complexes [11], 20S methylosome complex, etc., to accomplish diverse cellular processes including intracellular signalling, protein trafficking and gene transcription, essential for cell growth, division, differentiation and development [2, 5, 12]. Reports suggest it is localised both in the nucleus, cytoplasm and Golgi apparatus, with increased nuclear localisation following global DNA methylation events [7, 9, 13,14,15]. However, in the vertebrates, for its activity and substrate selectivity, PRMT5 partners with other proteins most importantly the 44 kDa WD repeat protein MEP50 (methylosome protein 50) [2, 16, 17]. Together, they could lead to either transcriptional activation through H3R2me1 methylation or transcriptional suppression through H3R8me2s and H4R3me2s methylation ‘marks’ [18]. Therefore, the elevated PRMT5 activity and formation of H3R8me2s and H4R3me2s in the promoters of essential genes could contribute to their transcriptional repression which further promotes cancer cell growth, metastasis as well as epithelial-to-mesenchymal transition (EMT) and is frequently associated with poor prognosis [18,19,20]. This enzyme is overexpressed in multiple forms of cancers including lung cancer [21,22,23], ovarian cancer [24]; prostate cancer [25], liver cancer [26, 27], oral squamous cell carcinoma [20], melanoma [28]; and lymphoma cell lines [29]. Therefore, PRMT5 is an oncogene and has become a promising drug discovery target as seen in the recent years with more reports of various small molecules to selectively bind and inhibit PRMT5 both in vitro and in silico [30,31,32,33,34]. However, promoter level expressional downregulation of PRMT5 could also lead us to a better understanding of the mechanisms that upregulate PRMT5 expression in cancer cells, and if so, then we can target its expression as to augment its therapeutic intervention.
The specificity protein family of transcription factors (including the mostly studied Sp1, Sp3 and Sp4) are overexpressed in various form of cancer and are considered as non-oncogene addiction genes (NOAGs) responsible for regulation of the genes involved in altered cell growth, progression, migration and drug resistance in cancer and thus these factors could be a crucial drug target in chemotherapy [35, 36]. Sp1, which is most extensively studied, binds to the GC-rich motifs and control the expression of many gene promoters. Interestingly, no reports are present whether Sp1 could also bind and control the expression of the PRMT5 (which also overexpressed in many cancers) promoter. The nuclear transcription factor Y α-subunit (NF-YA) forms a heterotrimeric complex with NF-YB (β-subunit) and NF-YC (γ-subunit), that specifically binds to the consensus sequence CCAAT box motifs present in promoters of several target genes. The NF-YA subunit act as a regulatory unit which, depending on its bound cofactor(s), can either activate or silence the respective promoter through its sequence recognition function [37, 38]. NF-Y factor regulate expression of various genes including genes involved in cell cycle and is associated with poor clinical prognosis in multiple cancers [37, 39]. Interestingly, in LNCaP prostate cancer cells, PRMT5 transcription was shown to be positively dependent on NF-YA which is further controlled by PKC-c-Fos signalling [40]. Therefore, inhibiting NF-YA transcription factor or its binding to the promoter of PRMT5 could be one of the mechanisms that might result in downregulation of PRMT5 expression.
Curcumin, the natural polyphenol constituent of the spice turmeric is an anti-cancer chemical, which also modulates cellular epigenetics. Curcumin as a modulator of cellular epigenetics was first reported in 2004 as it was shown to inhibits the p300/CBP-histone acetyltransferase (HAT) explicitly and decreased the level of acetylation of histone H4 as well as of p53 [41]. Contrary to this, several studies have also shown curcumin decreases expression of multiple histone deacetylases (HDACs) in correlation with the decrease in cell proliferation [42]. The histone methyltransferase EZH2 expression, as well as the level of H3K27me3 (a repressive histone mark), was shown to be downregulated in curcumin-treated human cancer cell line [43, 44]. Curcumin was also reported to interact with and inhibit DNMT1 in silico, in vitro and in vivo [45, 46]. Since, increased methylation of specific tumour suppressor gene’s promoter due to increased expression of DNMTs occurs in cancer, resulting in their silencing; curcumin, in contrast, could lead to a reversal of this epigenetic silencing by acting as a DNA hypomethylating agent [47]. However, an alteration of the PRMT5 expression in cancer cells by curcumin exposure is not yet reported. In this study, we aimed to analyse changes in PRMT5 expression after curcumin-treatment and also to investigate the probable underlying mechanism involved in such action of curcumin in two cancer cell lines previously known to overexpress PRMT5.
Materials and methods
Chemicals
Curcumin (#08511), FR180204 (#SML0320), Phorbol 12-myristate 13-acetate (#P8139), Rottlerin (#R5648), SB203580 (#S8307), SC79 (#SML0749), Wortmannin (#W1628) were purchased from Sigma-Aldrich, USA. Gö6976 (#12060) was purchased from Cell Signaling Technology, USA. Stock solutions of curcumin and other chemicals were prepared in DMSO. All other reagents (of analytical grade) required in the study, unless stated otherwise, were obtained from Sigma-Aldrich, USA.
Cell culture
The A549 (lung adenocarcinoma cell line) and MCF-7 (breast cancer cell line) were procured from the National Centre for Cell Science, Pune, India. The cells were grown in the complete medium containing Dulbecco’s Modified Eagle’s Medium–high glucose (DMEM), 10% foetal bovine serum (FBS) and 1x-antibiotic-antimycotic solution in a humidified atmosphere of 5% CO2 at 37 °C. All the above reagents were procured from Himedia laboratories, India. Cells were routinely maintained by sub-culturing them in every 2–3 days with > 75% confluency in the complete medium. For experiments, serum-free and the antibiotics-free medium were used.
Cell viability assays
For Trypan blue viability assay, cells (~ 1 × 105 cells/dish) were allowed to attach overnight, and the spent media was replaced with fresh media (without serum and antibiotics) containing various concentrations of curcumin for 24 h. The cells were harvested, and 10 μL of the cell suspension was mixed with 40 μL of 0.4% Trypan blue solution (#T8154) and the viable (white) or dead (blue) cells were microscopically counted on hemacytometer. For XTT assay, 4000 cells/well in 100 μL culture media in a 96-well plate was allowed to grow overnight and then treated with fresh media (without serum and antibiotics) containing various concentrations of curcumin for 24 h. The freshly prepared XTT (ThermoFisher scientific, #X6493) supplemented with 10 mM Phenazine methosulfate (PMS) was mixed to each well and incubated for 2 h at 37 °C in a CO2 incubator. The absorbance at 450 nm was recorded using a multimode plate reader (PerkinElmer, USA). The absorbance was plotted against each concentration.
RNA extraction, cDNA reverse transcription and real-time quantitative PCR analysis
The cells growing on 60 mm dishes were treated with fresh medium (without serum and antibiotics) containing 0, 2 and 20 μM of curcumin for 12 and 24 h. The cells were lysed directly in culture plates with TRiZol reagent (Invitrogen), and the total RNA was extracted according to the manufacturer’s protocol. One microgram of total RNA was reverse transcribed to synthesize cDNA with the random hexamer primers using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, Germany) as described by the manufacturer. Quantitative real-time PCR (qPCR) analysis was carried out in a LightCycler II 480 instrument (Roche) with LightCycler® 480 SYBR Green I Master, cDNA (100 ng) and gene-specific forward and reverse primers (0.5 μM each) on a 96-well plate. The following PCR parameters were used: 1 cycle of pre-incubation (95 °C for 5 min), 40 cycles of amplification (95 °C for 10 s, 53 °C for 15 s and 72 °C for 15 s per cycle), 1 cycle of melting curve (95 °C for 5 s, 65 °C for 1 min and 97 °C with continuous acquisitions), and finally the plate was cooled at 40 °C for 10 s. The fold differences in mRNA expression were calculated with the 2−∆∆Ct method with GAPDH as the endogenous control, taking into account the efficiency of amplification, determined from a standard curve obtained with the second-derivative maximum method. The primer sequences used in the experiment are provided in Supplementary Table 1.
Western blot analysis
The cells growing on 10 cm dishes were treated with fresh medium (without serum and antibiotics) containing 0, 2 and 20 μM of curcumin for 24 h. For experiments requiring co-treatment with inhibitor/activator, the specified group of cells was first treated with the respective inhibitor/activator for 1–6 h before curcumin addition. The cells were harvested, and the lysate was prepared using the cOmplete™ Lysis-M (Roche) lysis buffer supplemented with cOmplete Mini Protease Inhibitor Cocktail tablet (Roche). After the total protein estimation by Bradford assay, 20 µg protein was separated through SDS–PAGE, electrotransferred onto polyvinylidene fluoride (PVDF) membrane and was incubated overnight with primary antibody of interest at 4 °C under mild shaking conditions. After incubation with horseradish peroxidase-conjugated secondary antibody, the blots were developed using the Clarity™ Western ECL Blotting Substrate (BioRad, USA) for chemiluminescence detection in a Versadoc Instrument (BioRad). For quantifying the band intensity an open source software, ImageJ v1.46 [48] was used. The details of the antibodies used in this study are provided in the Supplementary Table 2.
Nuclear and cytoplasmic extraction and immunoprecipitation
For the cytoplasmic and nuclear protein extract preparation from the confluent cells, the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) were used according to the manufacturer’s instructions. Briefly, the cell pellet was incubated with ice-cold CER I reagent supplemented with protease inhibitor cocktail (Sigma) and 1 mM phenylmethanesulfonyl fluoride (PMSF), followed by incubation with CER II reagent on ice. The contents were centrifuged, and the resultant supernatant which contains cytoplasmic extract was stored at -80 °C until used, the pellet was resuspended with ice-cold NER reagent supplemented with protease inhibitor cocktail and PMSF, and cleaned by centrifugation. The supernatant which contains nuclear extract was stored at -80 °C until used. The extracts obtained were then quantified for protein content by the Bradford assay and subjected to immunoprecipitation and western blotting.
For immunoprecipitation, the cell lysate or nuclear or cytoplasmic extracts were pre-washed with protein A agarose magnetic beads (Cell Signaling, #73778) using a magnetic separation rack (Cell Signaling, #7017). 10% (v/v) of this pre-cleared lysate was preserved for use as input. The protein was incubated overnight with a specific primary antibody or 2 μL of normal rabbit IgG (Cell Signaling, #2729) under shaking conditions, followed by precipitation with pre-washed protein A magnetic beads and after separating the beads in the magnetic separation rack with through washes in the cell lysis buffer on ice. The supernatant with the immunoprecipitated proteins was subjected to western blotting as mentioned above.
Chromatin immunoprecipitation
The chromatin immunoprecipitation (ChIP) assays were performed using the EpiTect ChIP OneDay Kit purchased from Qiagen (#1063182), USA, according to the supplier’s instructions with following modifications. Briefly, post incubation with or without curcumin, the cells were cross-linked with formaldehyde, quenched by stop buffer and fixed with ice-cold PBS. The cells were harvested in PBS containing protease inhibitor cocktail, scrapped, mixed with IP lysis buffer (containing protease inhibitor cocktail) and sonicated, followed by centrifugations to obtain ChIP ready chromatin. Prior to immunoprecipitation, the sheared chromatin was pre-cleared with protein A beads in IP buffer A containing protease inhibitor cocktail. In order to be used as input control, 10 μL of this supernatant (1% of the initial 1 mL IP fraction) was stored at 4 °C until the DNA purification was performed. The target protein-DNA complex was then immunoprecipitated with (1–10 μg) of ChIP-grade anti-Sp1 or anti-NF-YA antibody or control IgG (Cell Signalling, USA) overnight at 4 °C on a rotator. The immunocomplexes were pelleted using protein A beads, and after subsequent washes with cold wash buffers I, II, III and IV, the chromatin was reverse cross-linked by proteinase K and DNA extraction beads, followed by column purification of DNA as instructed. The purified ChIP DNA was quantitatively amplified by SYBR green real-time PCR using a LightCycler II 480 instrument (Roche). The real-time PCR was carried out taking 1 μL of the purified ChIP DNA, 5 μL of SYBR Green master mix (Roche), 0.5 μL of each of the forward and reverse primers (from 5 μM stock) in a 10 μL of final reaction mixture. The following PCR conditions were used: one cycle of initial denaturation for 7 min at 95 °C followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min and finally one cycle of 95 °C for 1 min, 55 °C for 30 s, 95 °C for 30 s. The fold enrichment due to binding of the transcription factors was calculated by 2−ΔΔCt method with modifications of normalisation of the Ct values of DMSO treated and curcumin-treated immunoprecipitation samples was performed with the difference in input DNA. The PCR products were also analysed by electrophoresis on a 2% agarose gel stained with SYBR Green. The data represent mean ± S.E.M from three independent experiments. The primers were designed covering two regions in the proximal promoter of the human PRMT5 gene. These are denominated as region 1 (spanning − 64 to − 272 (relative to the transcription start site) which harbours one Sp1 and two NF-YA predicted binding sites yielding a 209 bp amplicon) and region 2 (spanning − 187 to − 359 (relative to the transcription start site) which harbours a single Sp1 predicted binding site yielding a 173 bp amplicon), respectively. The primers amplifying a non-target region in the PRMT5 distal promoter which does not harbour any Sp1, or NF-YA predicted binding site, was used as negative control and as a positive control, previously published primers amplifying a region containing NF-YA, and Sp1 binding site in the human CCNA2 (Cyclin A2) promoter [40] were used. The details of these primers are provided in the Supplementary Table 3.
siRNA transfection
For experiments involving silencing of PRMT5 and PKCδ expression, 2 × 105 cells/well were grown in a six-well culture plate in DMEM supplemented with 10% FBS but without antibiotics in a CO2 incubator at 37 °C overnight or until they reach 80% confluency. The cells were washed twice with siRNA transfection medium (Santa Cruz, #sc-36868), and then transiently transfected with 1 μg of either control/scrambled siRNA (Santa Cruz, #sc-37007) or PRMT5 siRNA (Santa Cruz, #sc-41073) mixed with siRNA transfection reagent (Santa Cruz, #sc-29528) in siRNA transfection medium for 6 h at 37 °C in CO2 incubator. The transfection mixture was then replaced with standard growth medium containing 10% serum and antibiotics and incubated for another 24 h and subjected to western blotting or real-time PCR protocols as mentioned above. For experiments with curcumin, the cells of each group either transfected with control siRNA or PRMT5 siRNA were treated with either DMSO or 20 μM of curcumin in fresh medium (without serum and antibiotics) for 24 h and subjected for western blotting or real-time PCR protocols as mentioned above.
Assay of protein kinase C activity
The detection of protein kinase C activity after curcumin exposure to the cells was assayed using the PepTag assay for non-radioactive detection of PKC (Promega, #V5330) with the following modifications. The assay utilises a fluorescent-labelled, PKC-specific peptide substrate whose net charge, upon phosphorylation by PKC gets altered and thus migrates separately on an agarose gel which can be quantified. Briefly, the cells were treated with different doses of curcumin (0, 2 and 20 μM) in medium without serum and antibiotics for 24 h; the cells were harvested, and cell lysate was prepared in PKC extraction buffer. Equal amounts of protein from each of the sample were incubated with the fluorescent PepTag C1 peptide (5 μL) in 5 μL PKC reaction buffer (1x), sonicated PKC activator (5 μL) and peptide protection solution (1 μL) for 30 min at 30 °C. Alongside, a negative/blank control (containing 5 μL of only the C1 peptide without sample) was also taken. The reaction was then stopped by heating the tube to 95 °C for 10 min, and the samples (layered with 1 μL of 80% glycerol) were separated on a 0.8% agarose gel at 100 V for 20 min. Phosphorylated peptide migrated towards the cathode while non-phosphorylated peptide migrated towards the anode. The negatively charged phosphorylated bands were excised from the gel and melted by heating at 95 °C. The hot agarose (175 μL) was mixed with 75 μL of warm gel solubilization solution, 100 μL of glacial acetic acid and 150 μL distilled water. The absorbance of the solution was read at 570 nm against the liquified gel (without the peptide) as blank using a spectrophotometer. The activity of PKC was calculated according to the Beer’s law in reference to the negative control. The activity is expressed as units/mL where one unit of the kinase is the number of nanomoles of phosphate transferred to a substrate per minute per mL. The experiment was repeated three times.
Prediction of transcription factor binding site within the PRMT5 proximal promoter
For prediction of the potential binding sites of Sp1 and NF-YA transcription factors within the human PRMT5 proximal promoter DNA spanning − 360 to − 1 bp corresponding to the transcription start site (TSS), the MatInspector tool on the Genomatix software suite [49] was used under a trial account. The tool although displayed many promoters with varying TSS, the most strong promoter with the TSS corresponding to the highest number of CAGE tags were selected for analysis.
Statistical analysis
We used analysis of variance (ANOVA) to indicate the statistical significance at p value < 0.05, which was cut off value to determine the significance of the treated and untreated group of samples.
Results
Increased expression of PRMT5-MEP50 is correlated with the growth of cancer cells
It is well established that PRMT5 is overexpressed in many cancer cells and is essential for their growth, however, to optimise the growth and treatment conditions in our model cell lines we initially traced the correlation of PRMT5 and MEP50 expression and cancer cell growth. We followed the expression of PRMT5 and MEP50 as the A549 (lung), and MCF-7 (breast) cells were grown up to 72 h. The cells after every time interval were subjected to both trypan blue dye exclusion assay and XTT assay for recording their viability. Simultaneously, the level of the transcript (mRNA) and protein were quantified after each time interval by real-time PCR and western blotting, respectively. We found both the cell lines were grown in monolayer cultures divided rapidly after an initial lag of ~ 10 h and reached a peak at 24 h with maximum attainable confluency in the culture plate; then the number of viable cells started to decrease, and by 72 h only 5–20% cells were viable (Supplementary figure S1A and S1B). As expected, the level of both mRNA and protein coincided with the number of viable cells, and at 24 h, the expression of PRMT5, as well as MEP50, were significantly increased (Supplementary figure S1C–F). Moreover, the number of viable cells was decreased significantly when the PRMT5 was knocked down by PRMT5 siRNA (Supplementary S2). Additionally, the immunoprecipitation experiments showed that the protein–protein interaction between PRMT5 and MEP50 was evident in both the cytoplasmic as well as nuclear extracts obtained from the two cell lines (Supplementary figure S3).
Curcumin inhibited cancer cell growth
To determine a suitable dose of curcumin for further studies, the trypan blue dye exclusion assay and XTT assay were performed after exposing both the A549 and MCF-7 cells with curcumin (0.25–100 µM) for 24 h. The results showed that curcumin exerted concentration-dependent cytotoxicity to both cells (Supplementary figure S4). The cells responded differently and the curcumin concentration which inhibited 50% of cell viability (IC-50) after 24 h was found to be 25.3 and 34.6 μM for MCF-7 and A549, respectively. Therefore, for our further studies, two concentrations (2 and 20 μM) were selected.
Curcumin downregulated PRMT5-MEP50 expression and function
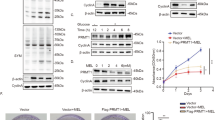
To evaluate the expression and function of PRMT5 and MEP50 in response with curcumin, the A549 and MCF-7 cells were treated without (DMSO) or with curcumin (2 and 20 µM) for 24 h. First, we performed real-time qPCR analysis to monitor the mRNA transcript level of PRMT5 and MEP50 in both cell lines. The results indicated 20 µM curcumin significantly downregulated the PRMT5 as well as MEP50 transcript levels after 24 h of exposure in both cell lines and PRMT5 being most affected by curcumin (Fig. 1a). Next, we performed western blotting to determine the protein level of PRMT5 and MEP50 concerning curcumin exposure. After 24 h of treatment, compared to control, PRMT5 and MEP50 protein levels in both cell lines significantly decreased (Fig. 1b, c). Since curcumin impacted both mRNA and protein levels of both the genes responsible for symmetric dimethylarginine generation in cells; we then analysed the extent of symmetric dimethylarginine formation in histone and non-histone proteins. The western blots after 24 h of treatment indicate curcumin dose-dependently decreased the level of SDMA in at least five different proteins globally (Fig. 1b, c). This effect coincided with the decrease in PRMT5 and MEP50 levels. Interestingly, the SDMA antibody detected different polypeptides/ proteins in A549, and MCF-7 cells and the impact on the SDMA level varied from protein to protein as detected in the blot. Moreover, curcumin also reduced the H4R3me2s repressive mark in both the cell lines as the concentration of curcumin was increased (Fig. 1b, c). The ratio of H4R3me2s to pan H4 suggested the overall level of only the symmetrically dimethylated H4R3 in the cell population. However, the outcomes of curcumin exposure affected the global level of this suppressive mark which also plays a role in further chromatin remodelling. When we compared curcumin-mediated decrease in PRMT5 expression to silencing of PRMT5 by siRNA to PRMT5, curcumin showed an additive effect in reducing the PRMT5 expression when co-treated with 1 µg of PRMT5 siRNA and 20 µM of curcumin for 24 h after transfection (Supplementary figure S5).
Curcumin affects the expression of PRMT5 and MEP50. a The cells were exposed to curcumin with indicated concentration and time periods. Relative mRNA level of PRMT5 and MEP50 was quantified by real-time qPCR. The fold change values are represented as mean ± S.E.M. (n = 9) and the significance was shown as **p < 0.01; and ***p < 0.001 against the DMSO treated control. Similar results were observed in each of three individual experiments. b After 24 h of incubation with or without curcumin, cells were lysed, and an equal amount (20 µg) of protein was subjected to immunoblotting with indicated primary antibodies. The antibody to SDMA detected multiple proteins which were mentioned in Roman numerals. The β-actin was used as loading control. The results represent best observation from three repeated experiments. c Band intensity of PRMT5, MEP50 (left), SDMA (centre) was measured and normalised to that of β-actin. The ratio of band intensities of H4R3me2s and pan-H4 is shown in the right panel. The data are plotted as mean ± S.E.M. (n = 9), and the significance is indicated as *p < 0.05; **p < 0.01; and ***p < 0.001 against the DMSO treated control
Curcumin decreased the transcription factors Sp1 and NF-YA
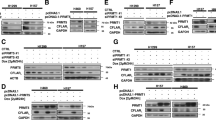
Since curcumin exposure was able to affect the transcription of PRMT5 we wanted to investigate whether the responsible transcription factors which can bind and control transcription of PRMT5 in cancer cells are affected by curcumin too. At first, we examined the promoter of the human PRMT5 gene located on the minus strand of chromosome 14 for prediction of transcription factor binding sites (TFBSs). The TFBS analysis by the MatInspector tool on the Genomatix software suite, indicated the presence of several binding sites of both Sp1 and NF-YA transcription factors represented by V$SP1F and V$CAAT, respectively within the proximal region in the promoter (Fig. 2a). The promoter (GXP_186843, Genomatix) and the TSS (1208) corresponding to the highest number of CAGE tags were selected for analysis as it represents the most potent promoter that encodes the PRMT5 transcript. Since binding of both Sp1 and NF-YA factors were predicted to happen; we doubted whether these two transcription factors get affected by curcumin. When assayed, coincidently mRNA, as well as protein levels of both the transcription factors, was found to be reduced along with the increment in curcumin concentration in both the A549 and MCF-7 cells (Fig. 2b–d). However, the cells exposed to higher concentration of curcumin were affected more significantly in contrast to the untreated (DMSO alone) control cells in a 24-h exposure window. More to our interest, these two transcription factors were found to interact physically in the untreated (control) cells, which was diminished in the 2 µM curcumin-treated cells and were undetectable in 20 µM curcumin-treatment in the immunoprecipitation analysis (Fig. 2e).
Impact of curcumin on the expression of Sp1 and NF-YA transcription factors. a The binding sites of Sp1 and NF-YA transcription factors on the proximal promoter region of the human PRMT5 gene were predicted using the MatInspector tool of the Genomatix software suite. The transcription start site (TSS) is indicated with an arrow, and the binding sites were indicated with boxes. The top panel is entire 1208 bp promoter and the bottom panel represents + 20 to − 300 bp respective to TSS. b Expression of Sp1 and NF-YA transcripts were assayed by real-time qPCR. The cells were exposed to curcumin with indicated concentration and time periods. The Sp1 and NF-YA mRNA expression level were quantified by real-time qPCR. The data were represented as mean ± S.E.M. (n = 9) and the significance was shown as **p < 0.01; and ***p < 0.001 against the DMSO treated control. Similar results were observed in each of three individual experiments. c Post-treatment with the indicated concentration of curcumin for 24 h, the cell lysate was prepared for immunoblotting with the indicated primary antibodies. The β-actin was used as loading control. d Band intensity of Sp1 and NF-YA was measured and normalised to that of β-actin. The data were plotted as mean ± S.E.M. (n = 9), and the significance was shown as *p < 0.05; **p < 0.01; and ***p < 0.001 against the DMSO treated control. e Immunoprecipitation and immunoblotting of Sp1 and NF-YA after curcumin exposure. The cells were exposed to either DMSO or curcumin (2 and 20 µM) for 24 h and then subjected to immunoprecipitation by Sp1 and NF-YA antibodies or IgG (negative control) and followed by immunoblotting with Sp1 and NF-YA antibodies, respectively. For input, 10% of the total protein was used for detection by immunoblotting. The upper and lower panels represent immunoprecipitation with anti-Sp1 antibody and anti-NF-YA antibody, and immunoblotting with anti-Sp1 antibody and anti-NF-YA antibodies, respectively
Curcumin decreased the availability and enrichment of Sp1 and NF-YA to the PRMT5 proximal promoter
As found in the earlier experiment, curcumin decreased the expression of both Sp1 and NF-YA transcription factors having binding sites in the proximal region within the PRMT5 promoter. Together, the 296 bp region spanning from − 359 to − 64 respective to the TSS harbours two binding sites for Sp1 and NF-YA, each. Therefore, we employed chromatin immunoprecipitation to quantify the enrichment of the fold of binding of these two transcription factors on these sites within this promoter region. For better resolution of the outcomes, we divided the entire 296 bp region into two subregions denoted as region 1 (− 272 to − 64) and region 2 (− 359 to − 187) which was amplified using two sets of primers. The region 1 harbours two predicted NF-YA and one Sp1 binding sites while region 2 harbours one Sp1 site only (Fig. 3a). Coincidently, the results suggested both the transcription factors occupied their respective binding sites within the DNA in the control cells, which were seen to overexpress PRMT5 as well as Sp1 and NF-YA in the previous results. On the contrary, as expected, the enrichment of Sp1 in region one, as well as region two, had diminished to almost undetectable level when curcumin was present. Similarly the occupation of NF-YA also significantly reduced in region 1 in response to curcumin. In both cell lines, the effect of curcumin was evident and dose-dependent (Fig. 3b, c).
Curcumin affected the enrichment of both Sp1 and NF-YA in the PRMT5 promoter DNA. a The schematics of the proximal promoter DNA of human PRMT5, flanked by the primers designed for PCR amplification of the immunoprecipitated chromatin DNA. The region 1 (R1) denoted by black arrows spanning − 64 to − 272 respective to the TSS; while red arrows spans indicate region 2 (R2) − 187 to − 359 respective to the TSS. The binding sites of the individual transcription factors are noted by their name with the core DNA element sequence in red colour. b The fold enrichment of the immunoprecipitated chromatin DNA by the Sp1 and NF-YA antibodies as calculated from the real-time PCR using the specific primers for region 1 and region 2. The cells were treated with indicated curcumin concentrations for 24 h, and then chromatin immunoprecipitation was performed. The IgG (mock) was used as a control for antibody specificity determination in the immunoprecipitation. Proper positive and negative control experiments were performed alongside. The data shown here represent fold as pooled mean ± SE (n = 9) from three individual tests and the significance from ANOVA analysis is shown as *p < 0.05, **p < 0.01, and ***p < 0.001 against the DMSO treated control. c The real-time PCR products from the above experiment were electrophoresed on 2% agarose gels, and the image was shown for comparison purpose
Impact of curcumin on the PKC signalling pathway intermediates
Next, we wanted to identify the signalling mechanisms involved in downregulation of PRMT5 through a decrease in Sp1 and NF-YA transcription factors. First, we verified the involvement of PKC activity in response to curcumin and then with the help of PKC activator (TPA) and inhibitors (rottlerin and GÖ6976), we checked the modulations in the PRMT5 level. Secondly, we probed the downstream signalling intermediates including ERK1/2, p38 and c-Fos, and their phosphorylation levels and also the levels of Sp1 and NF-YA in the presence of ERK1/2-selective inhibitor FR180204 and p38MAPK-specific inhibitor SB203580. The results show curcumin dose-dependently activated pan PKC enzymes as the level of the phosphorylated C1 peptide was increased significantly after 24 h of exposure to 2 and 20 μM of curcumin (Fig. 4a, b). The extent of this activation was more prominent in MCF-7 cells than A549 cells. However, when rottlerin (2.5 µM) and GÖ6976 (3 nM) reported inhibiting the activity of different isoforms of PKC was introduced alone, the PRMT5 protein level was found to be increased while co-treatment with 20 μM of curcumin reversed the effect and reduced PRMT5 to basal level in both the cell lines. In contrast, TPA (50 nM) treatment alone affected PRMT5 level differently in two cell lines. In the MCF-7 cells, PKC activation by TPA significantly reduced PRMT5 protein level, while the A549 cells were comparatively less affected. This effect, however, became more prominent when 20 μM curcumin was added after TPA pre-treatment, and the PRMT5 level was significantly reduced to an almost undetectable level (Fig. 4c, d). As our results suggested that increased PKC activity by curcumin might have altered PRMT5 expression, we undertook further assay of activation (phosphorylation) of MAPK pathway signalling molecules like ERK1/2, p38 MAPK, and c-Fos to point out the specific signalling intermediates involved in PRMT5 transcriptional control. The outcome of the assay of phosphoproteins signifies, the involvement of both ERK and p38 MAPK irrespective of the cell line used in this experiment. Curcumin dose-dependently activated both ERK1/2 and p38MAPK which coincided with increased level (3–4 fold) of phospho-c-Fos within 24 h of exposure in both the cell lines (Fig. 4e, f). However, to our surprise, pre-treatment with ERK1/2-specific inhibitor FR180204 (0.5 µM) alone although induced PRMT5 above twofold, did not affect NF-YA or Sp1 expression significantly in any of the cell lines; while co-treatment with 20 µM of curcumin reduced levels of all three proteins (viz. PRMT5, Sp1 and NF-YA). However, pre-treatment with p38MAPK–specific inhibitor, SB203580 (100 nM) did increase the level of PRMT5 and Sp1 (but not NF-YA) only in the A549 cells, and not in MCF-7 cells (Fig. 4g, h); an effect which in presence of curcumin (20 µM) completely reversed in both cell lines.
Impact of curcumin on PKC activity and MAPK signalling intermediates. a The PKC activity in the cells exposed to an indicated concentration of curcumin was assayed by analysing the phosphorylation of the C1 peptide. Phosphorylated peptide with net − 1 charge migrated towards the anode (+) the while non-phosphorylated peptide with a net + 1 charge migrated towards the cathode (-). A proper negative (-Ve) control (containing peptide without sample) was included with samples. b The PKC activity is calculated spectrophotometrically, and the activity is expressed as units/mL. The data shown here represent mean ± SE (n = 3) from three individual experiments and the significance is shown as *p < 0.05, and ***p < 0.001 against the DMSO treated control. c The immunoblotting of the indicated proteins after cells were co-treated with PKC activator (TPA) or inhibitors (rottlerin and Gö6976) alone or together with indicated concentration of curcumin. The β-actin was used as loading control. d The intensity of the bands were quantified using ImageJ and normalised to that of β-actin. The data shown here represent mean ± SE (n = 3) from three individual experiments and the significance is shown as *p < 0.05, **p < 0.01 and ***p < 0.001 against the DMSO treated control. e Western blots of the indicated proteins and their phosphorylated forms after the cells were exposed to the indicated concentration of curcumin for 24 h. The β-actin was used as loading control. f The normalised ratio of intensities of the protein bands was quantified using ImageJ and normalised to that of β-actin. The data shown here represent mean ± SE (n = 3) from three individual experiments and the significance is shown as *p < 0.05, **p < 0.01 and ***p < 0.001 against the DMSO treated control. g The immunoblotting of the indicated proteins after cells were co-treated with ERK1/2-specific inhibitor (FR180204) or p38MAPK-specific inhibitor (SB203580) alone or together in presence or absence of indicated concentration of curcumin. The β-actin was used as loading control. h The intensities of the protein bands were quantified using ImageJ software and normalised to that of β-actin. The data shown here represent mean ± SE (n = 3) from three individual experiments and the significance from ANOVA analysis is shown as **p < 0.01, and ***p < 0.001 against the DMSO treated control
Involvement of the AKT-mTOR pathway in curcumin-mediated alteration of PRMT5 expression
So far, we observed that curcumin-mediated PKC-ERK1/2-p38MAPK-c-Fos signalling is involved in the control of PRMT5 as well as Sp1 expression, but not in NF-YA expression. However, our previous experiments suggested the involvement of NF-YA transcription factor in controlling the PRMT5 expression. Therefore, we further investigated the involvement of AKT-mTOR signalling in modulating the expression of NF-YA, Sp1 and finally PRMT5 under the influence of curcumin. Interestingly, curcumin in order for its increased concentration decreased the activation, but not the basal level of both AKT and mTOR in the A549 and MCF-7 cells with high significance (Fig. 5a, b). The ratio of pAKT (Ser473) to pan AKT and phosphor-mTOR (Ser2448) to mTOR was decreased in the magnitude of ~ 10 fold in both cell lines when treated with 20 µM of curcumin after 24 h. In an attempt to confirm the involvement of AKT-mTOR signalling in the expressional regulation of both NF-YA and Sp1 transcription factors and PRMT5, we pre-treated the cells with phosphoinositide 3-kinase (PI3K)-specific inhibitor (Wortmannin) and AKT activator SC79 alone or with curcumin. The western blots for PRMT5, NF-YA and Sp1 suggest, like curcumin (20 µM) treatment alone, Wortmannin (0.5 µM) also significantly decreased their expression, while co-treatment with curcumin (20 µM) acted synergistically in reducing PRMT5 or NF-YA or Sp1 protein levels (Fig. 5c, d). Now, as Wortmannin mediated inhibition of PI3K would also lead to lack of AKT activation, our results coincided with our previous observation of decreased AKT phosphorylation in the presence of curcumin (Fig. 5a, b) and decreased in PRMT5 (Fig. 1b, c), NF-YA and Sp1 (Fig. 2b, c). However, activation of AKT due to treatment with its strong activator SC79 (10 µM) alone results in a simultaneous increase in PRMT5, Sp1 as well as NF-YA levels, an effect, which in the presence of curcumin was nullified (Figs. 5c, d).
Impact of curcumin on Akt-mTOR signalling affecting PRMT5 expression. a The cells were exposed to the above-mentioned concentrations of curcumin for 24 h, and the cell lysate was subjected to immunoblotting with indicated primary antibodies. The β-actin was used as loading control. Similar results were observed in three repeated experiments. b The ratio of intensities of the bands obtained in the immunoblotting experiment was quantified using ImageJ software and normalised to that of β-actin. The data represent mean ± SE (n = 3) from three individual experiments, and the significance is shown as **p < 0.01, and ***p < 0.001 against the DMSO treated control. c The immunoblotting of the indicated proteins after cells were co-treated with PI3K-specific inhibitor (wortmannin) or Akt-specific activator (SC79) alone or together in the presence or absence of indicated concentration of curcumin. The β-actin was used as loading control. d The intensities of the bands were quantified using ImageJ and normalised to that of β-actin. The data shown here represent mean ± SE (n = 3) from three individual experiments and the significance from ANOVA analysis is shown as **p < 0.01, and ***p < 0.001 against the DMSO treated control
Discussion
Multiple recent pieces of evidence suggest that PRMT5, the dominant type-II protein arginine methyltransferase is involved in tumour initiation, progression, invasion, metastasis as well as poor prognosis in cancer [18,19,20]. It does so either by arginine methylation and functional alteration of a plethora of target proteins involved in regulation of critical signalling pathways essential for cell growth and survival or by epigenetic regulation of gene expression at the chromatin level through arginine methylation of histones. As an enzyme, PRMT5 catalyses the formation of monomethyl arginine (MMA) or symmetrical dimethylated arginine (SDMA) with a preference for RGG/RG (arginine, glycine) rich motifs present on its multiple target proteins that include histones, transcription factors, tumour suppressors, receptors, signalling molecules [12, 19]. However, for the diversity in substrate selection as well as interactions or feedback regulations, PRMT5 almost always requires another binding partner, for example, MEP50 in mammalian cells [16, 17]. On histones, PRMT5 catalyse the formation of either activating H3R2me2s or repressive H3R8me2s and H4R3me2s marks as a part of epigenetic ‘histone code.’ The formation of H4R3me2s by the localised protein arginine methyltransferase PRMT5 is an essential event in DNA methylation, as this mark on histone H4R3 loads DNA methyltransferase DNMT3A onto DNA [50]. As a result, the H4R3me2s might lead to localised silencing of gene expression due to increased DNA methylation. The increasing number of reports suggests that overexpression of PRMT5 promotes invasion and metastasis while blocking the enzyme stops cancer cell growth and prevents migration and invasion [23, 27, 51]. This has led to increased research on finding specific PRMT5 inhibitors in recent years for its high potential regarding prognostic and chemotherapeutic applications. In our initial experiments, we too observed that PRMT5 expression is correlated with the growth and proliferation of two cancer cell lines A549 (lung) and MCF-7 (breast) under in vitro culture conditions. However, knocking down the PRMT5 in these two cells reduced their proliferation and increased cell death. Now, the fact that these cells already express a higher level of PRMT5 (as we observed and also reported in the literature earlier) makes them suitable models to study the underlying mechanisms of PRMT5 upregulation. Moreover, they represent two most common invasive human cancers (viz. non-small cell lung cancer (NSCLC) and breast cancer) worldwide. Keeping all these information about PRMT5 and its catalytic products in mind, in this study, we explored the impact of the much-studied natural inhibitor of cancer cell growth, the critical ingredient of turmeric, curcumin on PRMT5-MEP50 expression and function.
Turmeric is a native to Southeast Asia and is one of the most widely used spices in India for ages and as well as used in an ayurvedic drug for skin ailments, ulcers and wound healing [52]. However, the mechanism of action of curcumin, the major polyphenolic component of turmeric has been deciphered recently, and it has been found to possess potent antioxidant, anti-inflammatory, antimicrobial and antitumor properties [53]. Accumulative literature data suggest that curcumin can induce cytotoxicity of many types of cancer cell lines by both apoptotic and non-apoptotic mechanisms, through induction of Endoplasmic reticulum (ER)-stress and autophagy in some cases, but mostly arresting the cells in either G0/G1 or G2/M phase, with varied sensitivity across different cell lines [54,55,56]. In our study, the presence of curcumin also induced cell death above 25 µM after 24 h in both A549 and MCF-7 cells, with MCF-7 cells being more sensitive. In a very recent study [56], curcumin showed cytotoxicity towards A549 cells above 15 µM but only after longer than 24 h of exposure periods. Although we observed similar effects of curcumin towards A549 cells, however, our serum-free treatment conditions might have played a role in this variation. As it has become evident that curcumin exerts its cytotoxicity through several mechanisms, it is also a well-known epigenetic modulator, whether regarding alteration of micro RNAs or by inhibition of DNMTs, HDACs, HATs and the Polycomb group member, EZH2 [41,42,43,44,45,46,47].
The present study evaluated the ability of curcumin (2 and 20 µM) to reduce expression and formation of the catalytic products of PRMT5 in our cell line models. We analysed the concentration-dependent effect of curcumin on PRMT5 and MEP50 transcript as well as protein expression followed by tracing their catalytic outcomes by real-time qPCR and western blotting. Our result suggested a highly significant decrease of PRMT5 as well as MEP50 both in mRNA and protein levels in two cancer cell lines (which expresses higher levels of these two proteins in the untreated cells) within 24 h of exposure. Since, PRMT5 brings about methylation of many crucial cellular histone or non-histone proteins, in our experiments, the global arginine symmetric di-methylation was checked. Coincidently, the SDMA levels of multiple proteins of molecular weight range 10–200 kDa, was drastically decreased with curcumin in a concentration-dependent way when compared to that of the untreated control cell samples. Since arginine methylation in proteins has been shown to modulate their protein–protein interaction chemistry and biological function as well [57]; it is most likely that these changes induced by curcumin in the global arginine methylation could affect the cellular processes. However, further identification of these global proteins undergoing demethylation at arginine residues due to curcumin would only reveal the extent of its impact. On the other hand, more specifically, decreased the level of H4R3me2s levels by curcumin in both of the cancer cells which coincides with the decrease in PRMT5 levels also confirm that curcumin was able to alter the arginine methylation at histone levels. PRMT5 partners with the coordinator of PRMT5 and differentiation stimulator (COPRS aka COPR5) in the nucleus and preferentially methylate H4R3 over H3R8 [14]. Therefore, tracing the level of H4R3 methylation would ideally reflect the nuclear presence and catalysis event of PRMT5. Curcumin by reducing the symmetric di-methylation at the H4R3 marks the significant action it might play in altering the function of histone H4 (one of the core histones) and the impact it could results on gene expression at the promoter level. Of note, the presence of H4R3me2s histone mark is highly correlated with transcriptionally silenced promoter regions globally [29, 58].
Arginine methylation has explicitly drawn the attention and has emerged as one of the most crucial post-translational modification (PTM) of proteins involved in various critical biological functions including transcriptional control, mRNA splicing, DNA repair and signal transduction [2, 12, 57]. Although the plenty of research has so far focused on the regulation of arginine methylation regarding PRMT5 protein activity as well as its inhibition by small molecules while searching for a PRMT5 inhibitor as chemotherapeutic discovery, much knowledge is lacking about how PRMT expression is regulated. Since, its altered expression and not genetic mutations in multiple forms of cancer has been reported in many studies, understanding the mechanisms responsible for its overexpression would increase the possibilities to find an ideal solution in chemotherapeutic applications. Having found that curcumin attenuated the expression of PRMT5 in cancer cells in this study; we raised the question of how curcumin was able to control PRMT5 transcription if not the protein activity. To illustrate the promoter level control players, we at a first attempt, analysed the promoter on chromosome 14 for the predicted binding sites of two key transcription factors namely Sp1 and NF-YA on the promoter DNA. Previously, NF-YA was highlighted as a critical factor in controlling PRMT5 expression [40], but the role of Sp1 was not yet indicted in this regard. Surprisingly, the proximal region of the human PRMT5 promoter was found to harbour a CpG island (data not shown), and to this GC-rich tract, we observed multiple predicted binding sites of both Sp1 and NF-YA factors. Therefore, we hypothesised curcumin might have affected these two transcription factors in mediating its repressive action on the PRMT5 expression. Our data suggest curcumin significantly decreased the overall expression (in mRNA and protein level) of both of these two transcription factors in a concentration-dependent manner with almost similar extent in both the cell lines after 24 h. These two factors were found to interact with each other in the control cells of both cell lines in the immunoprecitation experiment. Curcumin exposure at 2 µM did not alter the interaction but at 20 µM, the interaction was not detected suggesting either loss of protein level or some other inhibitory mechanism(s) could be responsible for this effect. Therefore, further studies to reveal the importance of this protein–protein interaction upon PRMT5 expression would provide the actual mechanism. The transcription factor Sp1 binds explicitly to the GC-rich regions present in the promoters of several genes. Therefore, have a profound effect on the transcription of genes, and this is the reason Sp1 can regulate critical cellular functions, and its overexpression is reported in cancer [35, 36, 59]. This indicates reducing Sp1 expression could positively affect cancer cell transcriptome and thereby have therapeutic potential. Interestingly, both the transcription factors Sp1 and NF-YA are ubiquitously expressed and interact with each other. Previously, Roder et al. (1999) [60] has shown an interacting domain of NF-YA (located between 55th and 139th amino acid residues) is responsible for its interaction with Sp1 (involving its 139th to 344th residues). Earlier, it was also shown that Sp1/Sp3 interacted with NF-YA but not with NF-YB and NF-YC [61]. NF-YA, on the other hand, cooperates with an Sp1 expression as overexpression of NF-YA showed a synergistic effect on Sp1 overexpression [62, 63]. According to the previous reports on curcumin in controlling Sp1 expression, two different scenarios exist: in one instance curcumin (5 µM) acting as a cytoprotectant against oxidative stress, was found to enhance the Sp1 mRNA and protein levels in human lens epithelial cells (LECs) [64]; while other reports showed Sp1 downregulation by curcumin in cancer cells [65, 66]. Therefore, the action of curcumin on Sp1 is cell line context dependent; and in our study, both cell lines, however, responded similarly.
On the other hand, NF-YA, a very crucial transcription factor for transcription especially for the genes that promote a G2/M transition in cell cycle as well as the cancer-associated genes [38, 67] was also found to be affected by curcumin in our observation. Interestingly, curcumin also arrests the cancer cells at this phase of the cell cycle [54]. Although comparative data on the effect of curcumin on NF-YA could not be found on literature, we observed a significant concentration-dependent downregulation of NF-YA mRNA (which expressed at higher level in both of the untreated cancer cells) and protein expression. Therefore, curcumin exposure to the cancer cells resulted in concurrent inhibition of PRMT5 as well as two transcription factors (Sp1 and NF-YA) which possess their binding sites on the regulatory region of the PRMT5 promoter. Additionally, when the subcellular distribution of Sp1 and NF-YA was analysed, the localisation of Sp1 was double in nuclear fraction than the cytoplasmic counterpart; while NF-YA was only detected in the nuclear fraction of the untreated cells (Supplementary figure S6). However, in the presence of curcumin, the nuclear localisation of Sp1 and NF-YA was diminished as concentration was increased from 2 to 20 µM in both the A549 and MCF-7 cells.
Moreover, the chromatin immunoprecipitation results clearly showed that curcumin (2 and 20 μM) administration reduced the binding of both Sp1 and NF-YA transcription factors to the PRMT5 proximal promoter at both regions for Sp1 and region 1 for NF-YA when probed. Compared to the untreated (DMSO) group, the binding of Sp1 was reduced by about 5.6–6.5 fold at the two regions in both cell lines at 20 μM curcumin-treatment after 24 h; while, that of NF-YA was reduced by 10.2–13.4 fold at the region 1 in both cell lines. The DNA stretch from − 359 to -187 (denoted as region 2) respective to the TSS harbored at least one Sp1-binding site (GGGCGG) and was found to be occupied by Sp1 in the control cells; this region, however, does not harbor any CCAAT or the reverse complementary ATTGG sequence for NF-YA binding. On the contrary, the DNA stretch from − 272 to -64 (denoted as region 1) respective to the TSS harbours at least two NF-YA (ATTGG) and one Sp1 (TCCCCA) binding sites, was found to be occupied by both the transcription factors in the control cells. This signifies that both Sp1 and NF-YA might be involved in PRMT5 promoter activation in these cells which curcumin decreased drastically irrespective of the cell line examined in this study. On the notion that both Sp1 and NF-YA has been found to be associated with the transcription of cell proliferation associated genes [38], our findings that these two factors are involved with PRMT5 promoter binding indicates a possible mechanism of curcumin-mediated downregulation of PRMT5 in the A549 and MCF-7 cells. Structurally, the DNA binding domain of the highly conserved heterotrimeric NF-Y lies within the C-terminus of NF-YA through which it also interacts with NF-YB and NF-YC. The genome-wide analysis using bioinformatic tools mostly suggests the NF-Y enrichment at the CCAAT boxes (which are frequently present on multiple numbers of promoters in human) is mostly associated with the promoters of overexpressed genes in many cancer types [38]. Therefore, curcumin’s ability to alter NF-YA expression and binding to the PRMT5 promoter could be an additional mechanism in the future therapeutic application of curcumin. Although, a curcumin derivative bis-Demethoxycurcumin was previously shown to suppress topoisomerase IIα (TOP2A) expression through an NF-Y dependent mechanism [68]; our findings would technically be the first report on the curcumin-mediated transcriptional repression of PRMT5 through NF-YA.
As of now, we observed a possible coincidence of curcumin-mediated downregulation of Sp1/NF-YA transcription factors resulting in their decreased binding to the PRMT5 proximal promoter. However, the lack of clear perspectives in the literature on how PRMT5 or NF-YA transcription is controlled prompted us to investigate the involvement of the serine/threonine kinases: protein kinase C (PKC) and protein kinase B (PKB/AKT) signalling pathways as curcumin was previously shown to modulate these signalling events [56, 69]. Since the PKC and AKT both are highly studied and central pathways in cellular signalling, we aimed to investigate whether modulation of these pathways by curcumin could, in turn, influence the expression of Sp1/NF-YA and PRMT5.
PKC is a family of ubiquitously expressed 11 isoenzymes categorised into three groups depending on their activating cofactor and structural differences on N-terminal region [70]. These enzymes primarily function as tumour suppressors. Thus, restoration of their activity would serve as an ideal therapeutic measure in cancer [70, 71]. This is also supported by the failures of more than three decades of clinical trials with PKC inhibitors [71]. Several studies have shown the inhibitory action of curcumin on PKC activity above 20 μM; while some reports indicate the effect is Ca2+- and membrane-dependent, as well as cell line context dependent [69]. In our experiments with A549 and MCF-7 cells, however, we observed a very significant increment of PKC phosphorylation activity by 20 μM curcumin even after 24 h as determined in the non-radioactive but highly specific assay. Curcumin exposure increased the pan PKC activity by > 4 and > sixfold in A549 and MCF-7 cells, respectively. Moreover, the analysis of the PRMT5 protein expression levels after the cells was pre-exposed to either TPA (the known activator of PKC) or inhibitors (rottlerin and Gö6976) before 20 μM curcumin exposure signifies the possible modulatory involvement of PKC in PRMT5 expression. The phorbol ester TPA, which is a homolog of diacylglycerol (DAG), activates the DAG-dependent PKC isoforms which include both conventional and novel PKCs [70]. Surprisingly, treatment with TPA alone reduced PRMT5 protein levels, but co-treatment with curcumin further reduced the PRMT5 levels in an additive manner at a greater extent in MCF-7 cells than A549 cells. On the contrary, Gö6976 (which inhibits PKCα and PKCβ) and rottlerin (which inhibits PKCδ) showed opposite effects when the cells were exposed to these PKC inhibitors alone by significantly increasing the PRMT5 level, an effect which curcumin countered when co-treated. Moreover, the A549 and MCF-7 cells showed differential protein expression of AKCα and PKCδ; and thus might have shown a more detrimental effect when inhibited with rottlerin alone, compared to A549 cells (Supplementary figure S7A and S7B). Interestingly, siRNA to PKCδ while inhibiting its expression, increased PRMT5 expression in the MCF-7 cells (Supplementary figure S7C and S7D). Thus, from these observations, it is evident that curcumin-mediated alteration of PRMT5 expression might in part dependent on the PKC activity. To illustrate further, we probed the activation of ERK1/2, p38MAPK and AP1 protein c-Fos as the downstream signalling molecules in an approach to determine the impact of curcumin in the two cell lines. Phosphorylation at the activating residues of these three proteins was increased by curcumin in a concentration-dependent scenario in both of the cell lines investigated. Furthermore, when a specific inhibitor of ERK1/2 (FR180204), as well as selective inhibitor of p38MAPK (SB203580), was introduced to the cells, the response was diversified and inconsistent in the two cell lines. Although PRMT5 expression in the presence of the individual inhibitors alone was doubled in both A549 and MCF-7 cells; the Sp1 expression was only altered in A549 cells but was insignificant in MCF-7 cells whereas, both the cells did not respond to the inhibitors significantly regarding NF-YA protein expression. However, co-treatment with curcumin nullified the effect of both the ERK/p38 inhibitors. This indicates curcumin could be affecting PRMT5 expression through another mechanism(s) as well. In addition to this, a prediction analysis for Sp1 or NF-YA binding site on the human Sp1 and NF-YA promoters reveals that the Sp1 promoter harbours multiple proximal and core promoter binding sites for Sp1 itself and NF-YA, respectively; while the NF-YA promoter is not enriched with NF-YA itself, but only a specific Sp1 binding sites exist (Supplementary figure S8). This indicates that the expression of these two crucial transcription factors could be self-regulatory and further investigations would reveal the underlying mechanisms related to their expressional control by curcumin as observed in this study.
To our interest, very recently, Liu et al. [56] showed that the anti-tumour activity of curcumin in occurs by induction of apoptosis and autophagy through its inhibitory action on PI3K/AKT/mTOR pathway in the human NSCLC cell line, A549. In our experiments, curcumin decreased phosphorylation of AKT as well as mTOR at S473 and S2448 positions, respectively in a concentration-dependent manner. The similar effect we observed in two cancer cell lines of different origin reflects this might be evidence in supporting the curcumin-mediated alteration in the AKT/mTOR signalling. The phosphoinositide 3-kinase (PI3K), acting upon stimulation from upstream tyrosine kinase or G-proteins coupled receptors, activates AKT which in turn, stimulates the mammalian target of rapamycin (mTOR). The resultant PI3K-AKT-mTOR pathway is responsible for induction of signalling events that often leads to tumour development. Multiple genetic mutations and overexpression of isoforms of either PI3K or AKT have been linked with many human cancers [72]. However, it is not clear whether this pathway could modulate Sp1 or NF-YA expression and also directly or indirectly influence PRMT5 expression. Our investigation with a widely used inhibitor for PI3K, Wortmannin reveals PI3K inhibition alone does lead to decrease in both the Sp1 and NF-YA transcription factor protein levels as well as PRMT5 expression while combination with curcumin exacerbated the situation towards more decrease of these proteins. On the other hand, stimulation of AKT by a specific activator, SC79 alone drastically increased PRMT5 expression at par with both Sp1 and NF-YA increased protein expression; however, curcumin combination with SC79 reverted the effect of AKT stimulation in regard of PRMT5 expression. Therefore, the curcumin-mediated inhibitory effect on the PI3K/AKT-mTOR pathway might dramatically interfere with the expression of PRMT5 and the Sp1 and NF-YA which in turn could affect the transcription of PRMT5. In recent times, mono- or combinatorial therapeutic applications of PI3K/AKT/mTOR pathway inhibitors in treating cancer has gained tremendous scientific attention, and several ongoing clinical trials have shown promising outcomes [73, 74]. In this regard, our results show a promising therapeutic application of curcumin in controlling the expression of the oncogene, PRMT5 through a previously known effector mechanism of curcumin by inhibiting AKT/mTOR pathway.
Conclusions
The overexpressed PRMT5 in many cancers positively affect multiple events in cancer initiation and progression. Although we observed a new possible mechanism of curcumin (Fig. 6) in repressing another oncogene, our study needs further identification and characterisation of the intricate mechanisms through which curcumin downregulated the PRMT5 expression. Moreover, the observed inhibition might also be an inclusive mechanism of protein level inhibition. Therefore, we conclude that curcumin-mediated signalling events are responsible for its anti-oncogene action against PRMT5 and this could lead to its therapeutic applications given the fact that the bioavailability and other pitfalls of its success in clinical trials could overcome.
Hypothesis model of curcumin action on downregulating PRMT5 expression. The results from this study indicate curcumin decreased transcription of PRMT5 oncogene through simultaneous modulation of the Akt-mTOR pathway and PKC-ERK1/2-p38-c-Fos kinase pathways. These modulations affect the expression and protein level of two crucial transcription factors (Sp1 and NF-YA) having binding sites on the proximal promoter of PRMT5 in the cancer cell models used in the study. Curcumin-mediated decrease in Sp1, and NF-YA transcription factors result in their inability to bind to the PRMT5 promoter DNA elements, thus decreased transcription of PRMT5 mRNA and subsequently protein level. This mechanism is proposed to explain the inhibitory potential of curcumin in reducing PRMT5 expression in the cells under study
References
Pollack BP, Kotenko SV, He W, Izotova LS, Barnoski BL, Pestka S (1999) The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J Biol Chem 274:31531–31542
Stopa N, Krebs JE, Shechter D (2015) The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci 72:2041–2059
Fabbrizio E, Messaoudi E, Polanowska S, Paul J, Cook C, Lee JR, Negre J-H, Rousset V, Pestka M, Le Cam S, A., et al (2002) Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep 3:641–645
Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S (2004) Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol 24:9630–9645
Morales Y, Cáceres T, May K, Hevel JM (2016) Biochemistry and regulation of the protein arginine methyltransferases (PRMTs). Arch Biochem Biophys 590:138–152
Meister G, Eggert C, Bühler D, Brahms H, Kambach C, Fischer U (2001) Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr Biol 11:1990–1994
Bandyopadhyay S, Harris DP, Adams GN, Lause GE, McHugh A, Tillmaand EG, Money A, Willard B, Fox PL, DiCorleto PE (2012) HOXA9 methylation by PRMT5 Is essential for endothelial cell expression of leukocyte adhesion molecules. Mol Cell Biol 32:1202–1213
Hsu J-M, Chen C-T, Chou C-K, Kuo H-P, Li L-Y, Lin C-Y, Lee H-J, Wang Y-N, Liu M, Liao H-W et al (2011) Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat Cell Biol 13:174–181
Andreu-Perez P, Esteve-Puig R, de Torre-Minguela C, Lopez-Fauqued M, Bech-Serra JJ, Tenbaum S, Garcia-Trevijano ER, Canals F, Merlino G, Avila MA et al (2011) Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci Signal 4:ra58–ra58
Karkhanis V, Hu Y-J, Baiocchi RA, Imbalzano AN, Sif S (2011) Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem Sci 36:633–641
Meister G, Fischer U (2002) Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J 21:5853–5863
Blanc RS, Richard S (2017) Arginine methylation: the coming of age. Mol Cell 65:8–24
Rho J, Choi S, Seong YR, Cho W-K, Kim SH, Im D-S (2001) PRMT5, which forms distinct homo-oligomers, is a member of the protein-arginine methyltransferase family. J Biol Chem 276:11393–11401
Lacroix M, El Messaoudi S, Rodier G, Le Cam A, Sardet C, Fabbrizio E (2008) The histone-binding protein COPR5 is required for nuclear functions of the protein arginine methyltransferase PRMT5. EMBO Rep 9:452–458
Zhou Z, Sun X, Zou Z, Sun L, Zhang T, Guo S, Wen Y, Liu L, Wang Y, Qin J et al (2010) PRMT5 regulates Golgi apparatus structure through methylation of the golgin GM130. Cell Res 20:1023–1033
Burgos ES, Wilczek C, Onikubo T, Bonanno JB, Jansong J, Reimer U, Shechter D (2015) Histone H2A and H4 N-terminal tails are positioned by the MEP50 WD repeat protein for efficient methylation by the PRMT5 arginine methyltransferase. J Biol Chem 290:9674–89
Antonysamy S (2017) The Structure and Function of the PRMT5:MEP50 Complex. Subcell Biochem 83:185–194
Chen H, Lorton B, Gupta V, Shechter D (2017) A TGFβ-PRMT5-MEP50 axis regulates cancer cell invasion through histone H3 and H4 arginine methylation coupled transcriptional activation and repression. Oncogene 36:373–386
Richters A (2017) Targeting protein arginine methyltransferase 5 in disease. Future Med Chem 9:2081–2098
Amano Y, Matsubara D, Yoshimoto T, Tamura T, Nishino H, Mori Y, Niki T (2018) Expression of protein arginine methyltransferase-5 in oral squamous cell carcinoma and its significance in epithelial-to-mesenchymal transition. Pathol Int 68:359–366
Gu Z, Gao S, Zhang F, Wang Z, Ma W, Davis RE, Wang Z (2012) Protein arginine methyltransferase 5 is essential for growth of lung cancer cells. Biochem J 446:235–241
Sheng X, Wang Z (2016) Protein arginine methyltransferase 5 regulates multiple signaling pathways to promote lung cancer cell proliferation. BMC Cancer BioMed Central 16:567
Jing P, Zhao N, Ye M, Zhang Y, Zhang Z, Sun J, Wang Z, Zhang J, Gu Z (2018) Protein arginine methyltransferase 5 promotes lung cancer metastasis via the epigenetic regulation of miR-99 family/FGFR3 signaling. Cancer Lett 427:38–48
Bao X, Zhao S, Liu T, Liu Y, Liu Y, Yang X (2013) Overexpression of PRMT5 promotes tumor cell growth and is associated with poor disease prognosis in epithelial ovarian cancer. J Histochem Cytochem 61:206–217
Deng X, Shao G, Zhang H-T, Li C, Zhang D, Cheng L, Elzey BD, Pili R, Ratliff TL, Huang J et al (2017) Protein arginine methyltransferase 5 functions as an epigenetic activator of the androgen receptor to promote prostate cancer cell growth. Oncogene 36:1223–1231
Shimizu D, Kanda M, Sugimoto H, Shibata M, Tanaka H, Takami H, Iwata N, Hayashi M, Tanaka C, Kobayashi D et al (2017) The protein arginine methyltransferase 5 promotes malignant phenotype of hepatocellular carcinoma cells and is associated with adverse patient outcomes after curative hepatectomy. Int J Oncol 50:381–386
Jiang H, Zhu Y, Zhou Z, Xu J, Jin S, Xu K, Zhang H, Sun Q, Wang J, Xu J (2018) PRMT5 promotes cell proliferation by inhibiting BTG2 expression via the ERK signaling pathway in hepatocellular carcinoma. Cancer Med 7:869–882
Nicholas C, Yang J, Peters SB, Bill MA, Baiocchi RA, Yan F, Sïf S, Tae S, Gaudio E, Wu X et al (2013) PRMT5 Is Upregulated in Malignant and Metastatic Melanoma and Regulates Expression of MITF and p27Kip1. PLoS ONE 8:e74710
Wang L, Pal S, Sif S (2008) Protein arginine methyltransferase 5 suppresses the transcription of the RB family of tumor suppressors in leukemia and lymphoma cells. Mol Cell Biol 28:6262–6277
Ji S, Ma S, Wang W-J, Huang S-Z, Wang T, Xiang R, Hu Y-G, Chen Q, Li L-L, Yang S-Y (2017) Discovery of selective protein arginine methyltransferase 5 inhibitors and biological evaluations. Chem Biol Drug Des 89:585–598
Kong G-M, Yu M, Gu Z, Chen Z, Xu R-M, O’Bryant D, Wang Z (2017) Selective small-chemical inhibitors of protein arginine methyltransferase 5 with anti-lung cancer activity. PLoS ONE 12:e0181601
Mao R, Shao J, Zhu K, Zhang Y, Ding H, Zhang C, Shi Z, Jiang H, Sun D, Duan W et al (2017) Potent, Selective, and cell active protein arginine methyltransferase 5 (PRMT5) inhibitor developed by structure-based virtual screening and hit optimization. J Med Chem 60:6289–6304
Ye F, Zhang W, Ye X, Jin J, Lv Z, Luo C (2018) Identification of Selective, Cell Active Inhibitors of Protein Arginine Methyltransferase 5 through Structure-Based Virtual Screening and Biological Assays. J Chem Inf Model 58:1066–1073
Zhu K, Jiang C, Tao H, Liu J, Zhang H, Luo C (2018) Identification of a novel selective small-molecule inhibitor of protein arginine methyltransferase 5 (PRMT5) by virtual screening, resynthesis and biological evaluations. Bioorg Med Chem Lett 28:1476–1483
Hedrick E, Cheng Y, Jin U-H, Kim K, Safe S (2016) Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget 7:22245–22256
Safe S, Abbruzzese JL, Abdelrahim M, Hedrick E (2018) Specificity protein transcription factors and cancer: opportunities for drug development. Cancer Prev Res 11:371-382
Nardini M, Gnesutta N, Donati G, Gatta R, Forni C, Fossati A, Vonrhein C, Moras D, Romier C, Bolognesi M et al (2013) Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination. Cell 152:132–143
Gurtner A, Manni I, Piaggio G (2017) NF-Y in cancer: Impact on cell transformation of a gene essential for proliferation. Biochim Biophys Acta 1860:604–616
Yamanaka K, Mizuarai S, Eguchi T, Itadani H, Hirai H, Kotani H (2009) Expression levels of NF-Y target genes changed by CDKN1B correlate with clinical prognosis in multiple cancers. Genomics 94:219–227
Zhang H-T, Zhang D, Zha Z-G, Hu C-D (2014) Transcriptional activation of PRMT5 by NF-Y is required for cell growth and negatively regulated by the PKC/c-Fos signaling in prostate cancer cells. Biochim Biophys Acta 1839:1330–1340
Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK (2004) Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 279:51163–51171
Soflaei SS, Momtazi-Borojeni AA, Majeed M, Derosa G, Maffioli P, Sahebkar A (2018) Curcumin: a natural Pan-HDAC inhibitor in cancer. Curr Pharm Des 24:123–129
Hua W-F, Fu Y-S, Liao Y-J, Xia W-J, Chen Y-C, Zeng Y-X, Kung H-F, Xie D (2010) Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur J Pharmacol 637:16–21
Wu G-Q, Chai K-Q, Zhu X-M, Jiang H, Wang X, Xue Q, Zheng A-H, Zhou H-Y, Chen Y, Chen X-C et al (2016) Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget 7:26535–26550
Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li P, Lin J, Fuchs JR, Marcucci G et al (2009) Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett 19:706–709
Yu J, Peng Y, Wu L-C, Xie Z, Deng Y, Hughes T, He S, Mo X, Chiu M, Wang Q-E et al (2013) Curcumin down-regulates dna methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS ONE 8:e55934
Khor TO, Huang Y, Wu T-Y, Shu L, Lee J, Kong A-NT (2011) Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol 82:1073–1078
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942
Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD et al (2009) PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol 16:304–311
Jeon J-Y, Lee J, Park E-R, Shen Y, Kim M-Y, Shin H-J, Joo H-Y, Cho E-H, Moon S, Shin U et al (2018) Protein arginine methyltransferase 5 is implicated in the aggressiveness of human hepatocellular carcinoma and controls the invasive activity of cancer cells. Oncol Rep 40:536–544
Strimpakos AS, Sharma RA (2008) Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–546
Hewlings SJ, Kalman DS (2017) Curcumin: a review of its’ effects on human health. Foods 6:92
O’Sullivan-Coyne G, O’Sullivan GC, O’Donovan TR, Piwocka K, McKenna SL (2009) Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer 101:1585
Rivera M, Ramos Y, Rodríguez-Valentín M, López-Acevedo S, Cubano LA, Zou J, Zhang Q, Wang G, Boukli NM (2017) Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS ONE 12:e0179587
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y, Fan Y et al (2018) Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep 39:1523–1531
Bedford MT, Clarke SG (2009) Protein arginine methylation in mammals: who, what, and why. Mol Cell NIH Public Access 33:1–13
Xu X, Hoang S, Mayo MW, Bekiranov S (2010) Application of machine learning methods to histone methylation ChIP-Seq data reveals H4R3me2 globally represses gene expression. BMC Bioinform 11:396
Beishline K, Azizkhan-Clifford J (2015) Sp1 and the ‘hallmarks of cancer’. FEBS J 10(1111):224–258
Roder K, Wolf SS, Larkin KJ, Schweizer M (1999) Interaction between the two ubiquitously expressed transcription factors NF-Y and Sp1. Gene 234:61–69
Yamada K, Tanaka T, Miyamoto K, Noguchi T (2000) Sp family members and nuclear factor-Y cooperatively stimulate transcription from the rat pyruvate Kinase M gene distal promoter region via their direct interactions. J Biol Chem 275:18129–18137
Nicolás M, Noé V, Ciudad CJ (2003) Transcriptional regulation of the human Sp1 gene promoter by the specificity protein (Sp) family members nuclear factor Y (NF-Y) and E2F. Biochem J 371:265–275
Suske G (2017) NF-Y and SP transcription factors—New insights in a long-standing liaison. Biochim Biophys Acta 1860:590–597
Chhunchha B, Fatma N, Bhargavan B, Kubo E, Kumar A, Singh DP (2011) Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis 2:e234
Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, Li X, Safe S (2008) Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res 68:5345–5354
Jutooru I, Chadalapaka G, Lei P, Safe S (2010) Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem 285:25332–25344
Benatti P, Dolfini D, Viganò A, Ravo M, Weisz A, Imbriano C (2011) Specific inhibition of NF-Y subunits triggers different cell proliferation defects. Nucleic Acids Res 39:5356–5368
Belluti S, Basile V, Benatti P, Ferrari E, Marverti G, Imbriano C (2013) Concurrent inhibition of enzymatic activity and NF-Y-mediated transcription of Topoisomerase-IIα by bis-DemethoxyCurcumin in cancer cells. Cell Death Dis 4:e756–e756
Das J, Ramani R, Suraju MO (2016) Polyphenol compounds and PKC signaling. Biochim Biophys Acta NIH Public Access 1860:2107–2121
Isakov N (2018) Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol 48:36–52
Newton AC (2018) Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol 53:208–230
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E (2014) PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 46:372–383
Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, Fenga C, Spandidos D, Libra M, Tsatsakis AM (2016) Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (Review). Int J Oncol 48:869–885
Pons-Tostivint E, Thibault B, Guillermet-Guibert J (2017) Targeting PI3K signaling in combination cancer therapy. Trends Cancer 3:454–469
Acknowledgements
The authors acknowledge the funding and support of the Science and Engineering Research Board (SERB), New Delhi, India (EEQ-2016/000251).
Author information
Authors and Affiliations
Contributions
BC, and KG designed, carried out the experiments, analysed data and equally contributed to preparation of the manuscript. LS helped to acquire certain experimental data. SRK designed experiments and provided materials. BC, KG, and SRK wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors express no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatterjee, B., Ghosh, K., Suresh, L. et al. Curcumin ameliorates PRMT5-MEP50 arginine methyltransferase expression by decreasing the Sp1 and NF-YA transcription factors in the A549 and MCF-7 cells. Mol Cell Biochem 455, 73–90 (2019). https://doi.org/10.1007/s11010-018-3471-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3471-0