Abstract
Mitochondrial dysfunction plays crucial role in the pathologenesis of myocardial infarction (MI). The present study evaluated the protective effect of α-bisabolol against isoproterenol (ISO)-induced mitochondrial dysfunction and apoptosis in rats. Male albino Wistar rats were pre- and co-treated with intraperitoneal injection of α-bisabolol (25 mg/kg body weight) daily for 10 days. To induce experimental MI, ISO (85 mg/kg body weight) was injected subcutaneously to the rats at an interval of 24 h for 2 days (9th and 10th day). ISO-induced MI was indicated by the decreased activities of heart creatine kinase and lactate dehydrogenase in rats. ISO administration also enhanced the concentrations of heart mitochondrial lipid peroxidation products and decreased the activities/concentrations of mitochondrial antioxidants, Kreb’s cycle dehydrogenases and mitochondrial electron transport chain complexes I, II + III and IV in rats. Furthermore, ISO triggers calcium overload and ATP depletion in the rat’s heart mitochondria followed by the mitochondrial cytochrome-C release and the activation of intrinsic pathway of apoptosis by upregulating the myocardial pro-apoptotic Bax, P53, APAF-1, active caspase-3, active caspase-9 and down regulating the expressions of anti-apoptotic Bcl-2. α-Bisabolol pre and co-treatment showed considerable protective effects on all the biochemical and molecular parameters studied. Transmission electron microscopic study and mitochondrial swelling assay confirmed our biochemical and molecular findings. The in vitro study on hydroxyl radical also revealed the potent free radical scavenging activity of α-bisabolol. Thus, α-bisabolol attenuates mitochondrial dysfunction and intrinsic pathway of apoptosis in ISO-induced myocardial infarcted rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart diseases manifested mainly as acute myocardial infarction (MI) is prevailing worldwide including developed as well as developing nations and often presents a socioeconomic burden with increasing disability. MI is defined as damage of the heart muscles emerging from continuous acute myocardial ischemia. The death of heart tissues in MI involves both necrosis and apoptosis with distinctive morphological changes and alterations in the cardiac function [1,2,3]. To mimic the human MI, there are many experimental animal models with a popular one wherein the subcutaneous administration of isoproterenol (ISO) at larger doses induces myocardial injury in laboratory animals similar to that seen in acute MI in humans. ISO is a synthetic catecholamine and β-adrenergic agonist and provides a rapid, simple and non-invasive method for the evaluation of numerous agents for their cardioprotective effects [2, 3]. ISO triggers the β-adrenergic receptor activation which possesses a pivotal role in modified cardiac contraction and energy metabolism during oxidative stress conditions and it finally regulates the progression of myocardial remodeling and failure [4, 5]. At higher doses, ISO induces severe oxidative stress in the myocardium of rats characterized by gross and microscopic infarct like lesions [6,7,8,9].
Among many mechanisms of oxidative stress, impaired mitochondrial function is one of the most significant sources of reactive oxygen species (ROS) production in the heart and it is usually associated with cardiac dysfunction that further leads MI and heart failure [10]. Synthesis of adenosine triphosphate (ATP) and electron transport chain (ETC) required for cardiac contraction and relaxation are located in the heart mitochondria. The generation of ROS in the failing myocardium triggers mitochondrial DNA damage and consequent cellular injury leading to functional decline. Thus, mitochondrion is serving as a target and source for ROS mediated myocardial injury [11]. Excessive ROS production stimulates cellular dysfunction and causes damage to the electron transport complexes and respiration impairment. The role of energy metabolism in the mitochondria, cell signaling and programmed cell death are the main reasons for focusing the mitochondria as a suitable therapeutic approach to protect the myocardium against ISO-induced MI [12]. During ischemic conditions, the impairment of mitochondrial respiratory chain provides a new therapeutic approach for therapeutic targeting.
Growing evidences have revealed that apoptosis, a highly organized form of cell death participates in ischemic heart diseases including myocardial damage, heart failure and hypertrophy [13]. Oxidative stress has been shown to activate multiple cell signaling pathways including apoptosis [14]. The events of mitochondrial apoptotic pathway are controlled via the proteins of Bcl-2 family which govern the permeability of mitochondrial membrane [15]. The P53 possess a vital role in regulation of the Bcl-2 family proteins and those actions are leading to the activation of caspases and finally cell death [16]. Since apoptosis plays an important role in cell death of cardiomyocytes in MI, thus inhibition of apoptosis could be an important therapeutic target for the treatment of ischemic heart diseases.
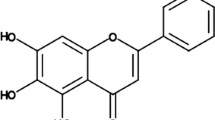
α-Bisabolol is a sesquiterpene alcohol isolated from the essential oil of variety of plants, shrubs and trees. It is abundantly found in the essential oil of Matricaria chamomilla (Asteraceae), popularly known as chamomile, which contains up to 50% of α-bisabolol [17]. Generally, α-bisabolol is extracted by hydrodistillation of the respective essential oils from sage (Salvia runcinata), German chamomile (M. chamomilla), Vanillosmopsis sp. and M. crassifolium [18]. It is a small molecule with favorable physicochemical and pharmaceutical properties in terms of drug discovery and development. It possesses a wide range of pharmacological properties such as anti-inflammatory [19], antibiotic [20], gastroprotective [21], antioxidant [22] and antimutagenic properties [23]. The United States Food and Drug Administration (US FDA) has classified α-bisabolol as ‘generally regarded as safe’ (GRAS) due to its low toxicity that encourage its use as an active ingredient in commercial products [17]. A recent study from Corpas-Lopez et al. [24] has revealed that repeated administration of α-bisabolol did not show any signs of toxicity in hamsters. In spite of the fact that α-bisabolol has been recognized for its multipharmacological properties to treat other human diseases, its conceivable cardioprotective capability has not yet been known. Therefore, our study mainly focuses on evaluation of the α-bisabolol against ISO-induced MI in rats.
Since, mitochondrial dysfunction and apoptosis both are common accompaniment and intimately involved in the pathogenesis of MI, we carried out this study in ISO-induced MI in rats to investigate the effect of α-bisabolol on mitochondrial dysfunction by measuring the enzyme activities and levels of cardiac diagnostic markers, heart mitochondrial lipid peroxidation, antioxidants, tricarboxylic acid (TCA) cycle enzymes and inner mitochondrial respiratory chain complexes as well as calcium (Ca2+) homoeostasis and intrinsic apoptotic pathway. Furthermore, the effect of α-bisabolol on the structure of heart mitochondria was revealed by transmission electron microscopic study and the free radical scavenging activity of α-bisabolol was assessed in vitro.
Materials and methods
Experimental animals
The experiment was performed with male albino Wistar rats weighing 160–180 g acquired from the experimental animal research facility of the College of Medicine & Health Sciences (CMHS), United Arab Emirates University, Al Ain, United Arab Emirates. A maximum of four rats were housed per cage in polypropylene cages (47 × 34 × 20 cm) lined with husk (replaced every 24 h). The animals were housed under standard laboratory conditions of a 12 h light/dark cycle at around 22◦C. Rats were fed on standard rodent chow diet (National Feed and Flour Production and Marketing Company LLC., Abu Dhabi, UAE) and water ad libitum. The experimental protocol (A31/14) for animal experimentation was approved by the Animal Ethics Committee of United Arab Emirates University.
Drugs and chemicals
α-Bisabolol, ISO hydrochloride, nitroblue tetrazolium, phenazine methosulphate, 1-chloro-2,4-dinitro benzene, potassium dichromate, 2,4-dinitro phenyl hydrazine, xylenol orange, glutathione, α-ketoglutarate, thiamine pyrophosphate, sodium succinate, bovine serum albumin, oxaloacetate, cytochrome-C and decylubiquinone were purchased from Sigma Chemical Company, St. Louis, MO, USA. All other chemicals used were of analytical grade.
Induction of experimental MI
ISO (85 mg/kg body weight) dissolved in saline was subcutaneously injected into rats at an interval of 24 h for 2 days [25,26,27,28]. ISO-induced MI was confirmed by decreased activities of heart creatine kinase (CK) and lactate dehydrogenase (LDH) in rats.
Experimental design
The animals were divided into four experimental groups, each containing 15 rats. Group I: normal control rats; Group II: rats were intraperitoneally treated with α-bisabolol (25 mg/kg body weight) daily for a period of 10 days; Group III: rats were subcutaneously injected with ISO (85 mg/kg body weight) at an interval of 24 h for 2 days (on 9th and 10th day); Group IV: rats were pre- and co-treated intraperitoneally with α-bisabolol (25 mg/kg body weight) daily for a period of 10 days and were subcutaneously injected with ISO at an interval of 24 h for 2 days (9th and 10th day). The dose and the mode of administration of α-bisabolol was selected based on our previous pilot study wherein we have screened many doses of α-bisabolol with different treatment protocol using different routes of administration [29]. Twelve hours after the second dose of ISO injection (i.e. on 11th day), the rats were anesthetized by pentobarbital sodium (60 mg/kg body weight) and then sacrificed by cervical decapitation. For serum, blood was collected in dry tubes without anticoagulant. The heart was excised immediately and rinsed in ice chilled saline. The heart tissue with determined weight were homogenized in Tris–HCl buffer (0.1 M; pH 7.4) and used for biochemical estimations. For western blotting, the heart tissues were homogenized in RIPA buffer. The heart tissues were also used for TEM and mitochondrial swelling studies.
Biochemical estimations
Assay of cardiac marker enzymes
The activity of CK and LDH enzymes were assayed using Vet Test 8008 Chemistry Analyzer, UK.
Isolation of heart mitochondria
The mitochondrial fraction of the heart tissue was isolated by the mitochondrial isolation kit purchased from Abcam, MA, USA.
Estimation of mitochondrial lipid peroxidation products
The concentration of thiobarbituric acid reactive substances (TBARS) in the heart mitochondrial fraction was estimated by the method of Fraga et al. [30]. 2 mL of thiobarbituric acid–trichloro aectic acid–hydrochloric acid reagent was mixed with 1 mL of mitochondrial fraction and kept in a boiling water bath for 15 min. After cooling, the tubes were kept for brief centrifugation and the fluorescence on the butanol layer was measured at 535 nm against the reagent blank.
The levels of lipid hydroperoxide (LOOH) in the heart mitochondrial fraction were estimated by the method of Jiang et al. [31]. For this assay, 0.2 mL of the heart mitochondrial fraction was mixed with 1.8 mL of Fox reagent and incubated at room temperature for 30 min. The developed chromophore was read at 560 nm.
Estimation of mitochondrial antioxidant enzymes
Superoxide dismutase (SOD) activity in the heart mitochondrial fraction was assayed by the method of Kakkar et al. [32]. For this assay, 0.5 mL of heart mitochondrial fraction was diluted to 1.0 mL with distilled water. Then, 1.5 mL of chloroform and 2.5 mL of ethanol (all chilled) were mixed and centrifuged. The activity of enzyme in the supernatant was measured. To the assay mixture contained 1.2 mL of sodium pyrophosphate buffer, 0.3 mL of nitroblue tetrazolium and 0.1 mL of phenazine methosulfate and 0.2 mL of NADH, appropriately diluted enzyme preparation was added and make up to total volume of 3.0 mL with distilled water. Following incubation for 90 s at 30 °C, 1.0 mL of glacial acetic acid was added to arrest the reaction. The mixture was vigorously stirred and shaken with 4.0 mL of n-butanol. The chromogen intensity was measured at 560 nm against butanol blank.
The activity of catalase in the heart mitochondrial fraction was assayed by the method of Sinha [33]. Briefly described, 0.1 mL of heart mitochondrial fraction, 0.9 mL of phosphate buffer, 0.4 mL of hydrogen peroxide was added. After incubation for 60 s at room temperature, 2.0 mL of dichromate-acetic acid mixture was added. The tubes were kept in a boiling water bath for 10 min and the colour developed was read at 620 nm.
The levels of glutathione (GSH) in the heart mitochondrial fraction were performed by the method of Ellman [34]. For this assay, 0.5 mL of mitochondrial fraction was allowed to precipitate with 2.0 mL of 5% TCA. After centrifugation, 1.0 mL of the supernatant was taken and added 0.5 mL of Ellman’s reagent and 3.0 mL of phosphate buffer. The yellow colour developed was read at 412 nm.
Assay of Kreb’s cycle dehydrogenases
The activity of isocitrate dehydrogenase (ICDH) in the heart mitochondrial fraction was assayed in the heart mitochondria by the method of King [35]. To the assay mixture (0.4 mL of Tris–HCl buffer, 0.2 mL of manganese chloride, 0.2 mL of substrate and 0.2 mL of NADP), 0.2 mL of mitochondrial fraction was added and mixed well. 0.2 mL of saline was used instead of NADP for control. The tubes were incubated in room temperature for 1 h. After incubation period, 1.0 mL of coloring reagent (0.001 M; 2,4-dinitro phenyl hydrazine) and 0.5 mL of ethylene diamine tetraacetic acid were added and mixed well. The tubes were incubated at 37 °C for 20 min and 10 mL of sodium hydroxide (0.4 N) was added. The colour intensity was measured at 420 nm.
The activity of malate dehydrogenase (MDH) in the heart mitochondrial fraction was assayed by the method of Mehler et al. [36]. To the assay mixture (0.75 mL of phosphate buffer, 0.75 mL of oxaloacetate and 0.15 mL of NADH), 0.2 mL of mitochondrial fraction was added and the reaction was done at 25 °C. The control tubes contained all reagents except NADH and the change in absorbance was measured for 2 min at 340 nm at an interval of 15 s.
The succinate dehydrogenase (SDH) activity in the heart mitochondrial fraction was assayed by the method of Slater and Borner [37]. The reaction mixture contains 1.0 mL of phosphate buffer, 0.1 mL of sodium cyanide, 0.1 mL of ethylene diamine tetra-acetic acid, 0.3 mL of sodium succinate, 0.2 mL of potassium ferricyanide, 0.1 mL of bovine serum albumin was made up to 2.8 mL with distilled water. Then, 0.2 mL of mitochondrial fraction was added to initiate the reaction. The change in optical density was measured at 420 nm for 5 min at 15 s intervals.
The activity of α-ketoglutarate dehydrogenase (α-KGDH) in the heart mitochondrial fraction was assayed by the method of Reed and Mukherjee [38]. The incubation mixture contains 0.1 mL of thiamine pyrophosphate, 0.1 mL potassium α-ketoglutarate, 0.1 mL of phosphate buffer, 0.1 mL of magnesium sulfate and 0.1 mL of potassium ferricyanide was made up to 1.4 mL with distilled water followed by the addition of mitochondrial fraction. The control tube contains all the reagents except mitochondrial fraction. The mixture was allowed for incubation at 30 °C for 30 min. after incubation period, 1.0 mL of 10% TCA was added to terminate the reaction. After the addition of 10% TCA, the reaction mixture was mixed well and centrifuged. To 1.0 mL of supernatant, 1.5 mL of distilled water, 1.0 mL of 10% TCA and 1.0 mL of 4% duponol and 0.5 mL of ferric ammonium sulfate-duponol reagent were added. The tubes were kept for 30 min at room temperature and the absorbance was measured at 540 nm.
Assay of mitochondrial respiratory chain complexes
The activities of inner mitochondrial ETC complexs-I, II + III were determined by the method of Kramer et al. [39].
The complex-I (rotenone sensitive NAD-coenzyme Q reductase) activity was determined with two different 2-point rate assays (4 min) by measuring the total and rotenone-insensitive complex I activities. To 19 µL of mitochondrial fraction, 350 µL of substrate solution (pH-7.2) (27 mmol of potassium phosphate buffer, 2.66 g/L bovine serum albumin, 5 mmol/L magnesium chloride, 0.213 mmol/L NADH, 2 mmol/L potassium cyanide, 0.1065 mmol/L decylubiquinone, 0.002 g/L antimycin A) was added and the decreased absorbance was monitored. The activity of rotenone-insensitive complex-I was measured by adding 39.9 µmol/L rotenone with 350 µL of substrate solution and the decreased absorbance was monitored. The difference between the activity of total and rotenone insensitive complex was used to calculate complex-I enzyme activity.
The activity of complex-II + III: Succinate cytochrome-C reductase was measured with 2-point rate assay (5 min). Mitochondrial fraction (50 µL) was added to 300 µL of substrate solution (pH-7.8) (37.5 mmol/L potassium phosphate, 25 mmol/L succinic acid, 1.87 mmol/L potassium cyanide, 0.005 g/L rotenone and 0.5 mmol/L ethylene diamine tetraacetic acid). Then 37 µL of 0.5 mmol/L cytochrome-C was added after 1.5 min. The increased absorbance was read at 546 nm to calculate the complex II + III enzyme activity.
The activity of cytochrome-C-oxidase in the heart mitochondrial fraction was assayed by the method of Pearl et al. [40]. The assay was done by adding the reaction mixture containing 1.0 mL of phosphate buffer, 0.1 mL of cytochrome-C (0.01%), 0.2 mL of N-phenyl-p-phenylene diamine (0.2%) and 0.5 mL of distilled water. Then, 0.2 mL of mitochondrial fraction was added and the absorbance was recorded for 5 min (interval of 15 s each) at 550 nm. The tube contains all the reagents except cytochrome-C was used as control.
Estimation of Ca2+ and ATP
The heart mitochondrial Ca2+ levels were measured by the calcium colorimetric assay kit obtained from Sigma Chemical Company, St. Louis, MO, USA. The heart mitochondrial ATP concentration was measured by the method of Williams and Coorkey [41]. To the incubation mixture contains 2 mL of phosphate buffer (pH 7.4), 5 mM ethylene diamine tetraacetic acid, 10 mM magnesium chloride, 10 µL of adenosine diphosphate, 5 µL of glucose-6-phosphate dehydrogenase; 0.2 mL of mitochondrial fraction was added. Mixed thoroughly and the fluorescence was recorded. Then, 10 µL of ATP and 5 µL of hexokinase were added and the increased absorbance was recorded at 340 nm.
Mitochondrial swelling assay
The mitochondrial permeability transition pore activation in isolated cardiac mitochondria was measured by Ca2+-induced swelling by the method of Maloyan et al. [42]. Opening of the pore results in swelling of the mitochondria and the decrease in absorbance was measured at 540 nm. Mitochondrial fraction was resuspended in the swelling buffer contains ten Tris–HCl (pH 7.4), 120 mmol/L potassium chloride and five potassium dihydrogen phosphate to a final protein concentration of 250 µg/mL. The pore opening was induced by 250 µmol/L of calcium chloride and measured at 540 nm.
Western blot analysis
Western blotting analysis was performed to analyze the expression pattern of pro-apoptotic B-cell lymphoma-2 associated-x (Bax), anti-apoptotic B-cell lymphoma-2 (Bcl-2), P53, apoptotic protease activating factor 1 (APAF-1), active caspase-3, active caspase-9, cytochrome-C and β-actin. The heart tissue samples were homogenized in an ice-cold RIPA buffer and the homogenate was centrifuged at 2800×g/min for 30 min at 4 °C to remove debris. The protein contents in the sample were estimated using the Pierce™ BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). The protein samples were loaded and separated by SDS-polyacrylamide (12%). The membranes were incubated with the blocking buffer containing 5% bovine serum albumin for 1 h to reduce non-specific binding sites. The proteins were transferred onto PVDF membrane and incubated with specific primary antibodies; 1:500 dilution for Bax (Santa Cruz Biotechnology, Dallas, TX, USA), Bcl2, P53, APAF-1, active caspase-3 (1:1000; Abcam, MA, USA), active caspase-9 (1:1000; Cell signalling technology, Danvers, MA, USA), voltage dependent anion channel (VDAC) (1:5000; Sigma St. Louis, USA) and β-actin (1:5000; MERCK Millipore, USA) with gentle shaking overnight at 4 °C. Then, membranes were incubated with their corresponding secondary antibodies (anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase) for 1 h at room temperature and the protein bands were visualized using an enhanced chemiluminescence pico kit (Thermo Fisher Scientific, Rockford, IL, USA). The intensity of bands was quantified using Image J software public domain Java image processing software, Wayne Rasband, NIH, Bethesda, MD, USA, which of control was set to 1.
Estimation of protein in the heart mitochondrial fraction
Protein content in the mitochondrial fraction was estimated using Pierce™ BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA).
Transmission electron microscopic study
The ultrastructure of the heart mitochondrial specimen was examined by transmission electron microscopy (TEM) (Fei, Teenai BioTwin G2, Holland) according to the method of Lang [43]. Small pieces of heart tissues were taken and rinsed in 0.1 M phosphate buffer (pH 7.2). Approximately, 1-mm heart pieces were trimmed and immediately fixed into 3% ice-cold glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) and kept at 4 °C for 12 h. Then, tissue processing for TEM study was carried out. The grids containing sections were stained with 2% uranyl acetate and 0.2% lead acetate. Then, the sections were examined under a transmission electron microscope.
The in vitro study
The free radical scavenging effect of α-bisabolol in vitro on hydroxyl radical (OH•) was determined by the method of Halliwell et al. [44]. Varying volumes of α-bisabolol (10, 20, 30, 40 and 50 µM) was mixed with 0.1 mL of 100 mM potassium phosphate buffer, 0.2 mL of 500 mM ferric chloride, 0.1 mL of 10 mM hydrogen peroxide, 0.1 mL of 1 mM ethylene diamine tetraacetic acid, 0.1 mL of 1 mM ascorbic acid, and 0.2 mL of 2-deoxyribose. The contents were mixed well and incubated for 60 min at room temperature. Then, 1 mL of 1% thiobarbituric acid in 0.05 N sodium hydroxide and 1 mL of 28% trichloroacetic acid was added and kept in a boiling water bath for 30 min. The absorbance was measured at 532 nm with distilled water as blank. Decreased absorbance of the mixture indicates increased OH• scavenging activity.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance followed by Duncans multiple range test (DMRT) using Statistical Package for the Social Science (SPSS) software package version 12.00. Results were expressed as mean ± standard deviation (SD) for eight rats in each group. The criterion of statistical significance was set on P values < 0.05.
Results
α-Bisabolol protects the myocardium by preventing the leakage of CK and LDH
ISO-induced MI was confirmed by decreased activities of heart CK and LDH in rats compared to normal control rats. Pre and co-treatment with α-bisabolol daily for a period of 10 days significantly (P < 0.05) increased the activities of heart CK and LDH in ISO-induced rats compared to ISO control rats (Fig. 1a, b).
α-Bisabolol attenuates lipid peroxidation and maintained the antioxidant status
ISO-induced rats revealed significant (P < 0.05) increased levels of mitochondrial TBARS and LOOH with significant (P < 0.05) decreased activities/levels of SOD, catalase and GSH in the heart mitochondria compared to normal control rats. Pre and co-treatment with α-bisabolol revealed significant (P < 0.05) decreased levels of mitochondrial TBARS and LOOH and significant (P < 0.05) increased activities/levels of SOD, catalase and GSH in the heart mitochondria of ISO-induced rats compared to ISO control rats (Fig. 2a, b).
Measurement of oxidative stress in heart mitochondria. a Levels of TBARS and LOOH in the heart mitochondria. b Activities/levels of SOD, catalase and GSH in the heart mitochondria. Each column is mean ± SD for eight rats in each group; *P < 0.05 as compared to normal control (Group-I), **P < 0.05 as compared to ISO control (Group-III) (DMRT). *Units: nM/mg protein for TBARS; nM/100 mg protein for LOOH; units/100 mg protein for SOD; nM of H2O2 consumed/min/mg protein for catalase; nM /100 mg protein for GSH
α-Bisabolol defends the myocardium by maintaining the status of Krebs cycle dehydrogenases and inner mitochondrial ETC complexes
The activities of Kreb’s cycle dehydrogenases such as ICDH, SDH, MDH, α-KGDH and inner mitochondrial ETC complexes I, II + III and IV in the heart mitochondria were significantly (P < 0.05) decreased in ISO-induced rats compared to normal control rats. Pre and co-treatment with α-bisabolol showed significant (P < 0.05) increased activities of these enzymes in ISO-induced rats compared to ISO control rats (Fig. 3a–d).
Activities of TCA cycle and mitochondrial ETC enzymes. a Activities of TCA cycle enzymes in the heart mitochondria. *Units: nM of NADH oxidized/h/mg protein for ICDH; nM of ferro cyanide formed/h/mg protein for α-KGDH; nM of NADH oxidized/min/mg protein for MDH and nM of succinate oxidized/min/mg protein for SDH. b Activity of complex-I in the heart mitochondria. c Activity of complex-II + III in the heart mitochondria. d Activity of complex-IV in the heart mitochondria. Each column is mean ± SD for eight rats in each group; *P < 0.05 as compared to normal control (Group-I), **P < 0.05 as compared to ISO control (Group-III) (DMRT)
α-Bisabolol reduced Ca2+ overload, mitochondrial swelling and increased ATP production in the mitochondria
The levels of Ca2+ and the swelling of mitochondria were considerably increased and the levels of ATP were considerably (P < 0.05) decreased in the heart mitochondria of ISO-induced rats. α-Bisabolol pre and co-treatment revealed significant (P < 0.05) decreased levels of Ca2+ and mitochondrial swelling with significant (P < 0.05) increased levels of ATP in the heart mitochondria of ISO-induced rats compared to ISO control rats (Fig. 4a, b).
Measurement of Ca2+ homeostasis and energy production in the heart mitochondria. a Levels of Ca2+ and ATP in the heart mitochondria. b Mitochondrial swelling assay. Each column is mean ± SD for eight rats in each group; *P < 0.05 as compared to normal control (Group-I), **P < 0.05 as compared to ISO control (Group-III) (DMRT)
α-Bisabolol abrogates intrinsic pathway of apoptosis by preventing the mitochondrial cytochrome-C release
ISO administration showed upregulated expressions of Bax, P53, APAF-1, active caspase-3, active caspase-9 and downregulated expression of Bcl-2 in the myocardium. Furthermore, ISO triggered the release of cytochrome-C from mitochondria to the cytosol. α-Bisabolol pre and co-treatment downregulated the expressions of pro-apoptotic proteins, such as Bax, P53, APAF-1, active caspase-3, active caspase-9, and downregulated the expression of anti-apoptotic Bcl-2 protein (Fig. 5a, b). Also, α-bisabolol prevents the release of cytochrome-C from mitochondria to the cytosol (Fig. 6a, b). The results have clearly revealed that α-bisabolol abrogates the mitochondrial pathway of apoptosis by efficiently modulating the pro-apoptotic and anti-apoptotic markers in ISO-induced MI in rats.
TEM study on heart mitochondria
The TEM image of α-bisabolol alone treated rats showed normal mitochondrial architecture with intact myofibrils (Fig. 7a). ISO-induced mitochondria showed swelling, loss of cristae, irregular size and shape, vacuolation and loss of myofibrils heart of rats (Fig. 7b). α-Bisabolol pre and co-treated ISO-induced rats showed near normal architecture of mitochondria with intact myofibrils (Fig. 7c).
TEM study on heart mitochondria. a Rats treated with α-bisabolol showing normal mitochondria with intact myofibrils (Group-II) (×5000). b ISO-induced rats showing loss of cristae (↑), swelling of mitochondria, irrugular shape and size (→), vacuolation (←) with damaged myofibrils (↓) (Group-III) (×5000). c α-Bisabolol pre and co-treated ISO-induced rats showed near normal architecture of mitochondria with intact myofibrils (Group-IV) (×5000)
α-Bisabolol showed potent OH• scavenging activity
Figure 8 shows the percentage in vitro radical scavenging effects of α-bisabolol on OH•. α-Bisabolol scavenges OH• in vitro in a concentration-dependent manner. The percentage scavenging effects of α-bisabolol on OH• at various concentrations (10, 20, 30, 40 and 50 µM) were found to be 17.3, 29.2, 55.3, 71.2 and 79.96, respectively. Thus, α-bisabolol at the concentration of 50 µM showed highest percentage scavenging effect of OH• around 79.96%. Thus, α-bisabolol is a potent free radical quencher (Fig. 8).
Discussion
Subcutaneous injection of ISO-induced MI in rats make a more suitable experimental model for the evaluation of cardioprotective agents due to low mortality, high reproducibility and validity compared with other animal models [2, 3]. ISO by pharmacological stimulation induces MI in rats conveniently due to the smaller size of coronary arteries and activation of β-adrenergic G-protein coupled receptor after ISO-induced cardiac damage is responsible for the changes in the integrity and/or permeability of the plasma membrane results in the leakage of cardiac diagnostic marker enzymes [8]. It could be due to the sarcolemmal damage induced by β-receptor agonist and rendering it leaky [1]. During hypoxic conditions, myocardial cells are vulnerable for damage which results in the leakage cardiac diagnostic markers such as CK and LDH into the serum which reflects the lowered CK and LDH activity in the myocardium [45]. α-Bisabolol appear to protects ISO challenged myocardium by preventing the leakage of myocardial CK and LDH into the circulation.
ISO-induced oxidative stress alters the function of myocardium and its ultrastructure in rats [46]. The generation of free radicals after myocardial injury plays a vital role in the progression of the damage [47]. The mitochondrial damage mediated by lipid peroxidation has been observed in ISO-induced MI in rats. Increased index markers of lipid peroxidation such as TBARS and LOOH may decrease the mitochondrial membrane fluidity and alters the proton permeability which uncouples the oxidative phosphorylation in the myocardium [46]. Thus, enhanced lipid peroxidation damages both the structure and function of mitochondria in ISO-induced rats. α-Bisabolol counters free radical mediated lipid peroxidation in ISO-induced MI in rats by decreasing the levels of mitochondrial lipid peroxidation products and reinstates normal mitochondrial architecture as well as function. The maintenance of mitochondrial lipid peroxide levels reasonably ascribed to the free radical scavenging activity of α-bisabolol.
Impairment in the efficiency of antioxidants leads to enhanced deleterious effects of ROS exerted through the quinine metabolites of ISO and interferes with antioxidant systems (SOD, catalase and GSH) results in the decreased activities and levels of these antioxidants. Glutathione protects the mitochondrial membrane by attenuating lipid peroxidation and its decreased concentrations seem to be a crucial mechanism for imbalanced mitochondrial function [48]. Increased intracellular GSH content may prevent cellular and mitochondrial damage. α-Bisabolol treatment protects the mitochondria by increasing the mitochondrial antioxidant status and counters ROS-mediated oxidative stress in ISO-induced myocardial infarcted rats by virtue of its potent free radical scavenging property.
ICDH is the predominant enzyme involved in glutathione regeneration by producing reduced nicotinamide adenine dinucleotide phosphate (NADPH) in the heart mitochondria [49]. SDH regulates mitochondrial ATP production whereas MDH present in outer mitochondrial membrane and both are vulnerable to free radical attack [50, 51]. Conversion of α-ketoglutarate to succinyl-CoA and reduced nicotinamide adenine dinucleotide (NADH) in the heart mitochondria is catalyzed by α-KGDH. The aerobic oxidation of pyruvate might be the reason for the reduced activities of TCA cycle enzymes which results in declined ATP production in ISO-induced rats. During free radical triggered hypoxic conditions, the activities of TCA enzymes were declined in ISO-induced rat’s heart mitochondria [50]. Previous reports have showed that the inner mitochondrial respiratory chain was inhibited during cardiac damage [52]. Decreased activities of inner mitochondrial respiratory chain enzymes play a crucial role in the progression of left ventricular remodeling and heart failure during MI [53]. It might be due to the unavailability of cardiolipin which is required for their functioning results in the decreased production of ATP in ISO-induced rats [53, 54]. The oxidative damage during MI could have created blocks in the flow of electrons along the respiratory chain. The impairment of mitochondrial complexs might increase the leakage of electrons from the ETC and producing damage to the mitochondrial inner membrane constituents [54, 55]. In our study, the complexes I–IV activities were significantly decreased in the ISO challenged rats compared to normal control rats. α-Bisabolol pre and co-treatment significantly enhanced the activities of these mitochondrial ETC complexes by virtue of its potent free radical scavenging property.
The Ca2+ homeostasis in the mitochondria is mandatory for the physiological functions and cell survival. Also, Ca2+ is a regulator and the key second messenger for the function of the mitochondria. Excessive ROS may cause extensive oxidative damage which results in impaired cardiomyocyte functions including ion transport, contractility and Ca2+ cycling [56]. During ischemia, the depleted ATP levels caused loss of ionic gradients, swelling and alterations in the structure and function of the mitochondria [57]. Swelling of mitochondria is found in line with mitochondrial dysfunction and damage [58]. ISO triggers the mitochondrial permeability transition pore opening which disrupts permeability barrier of the inner mitochondria, which results in uncoupling of oxidative phosphorylation, osmotic swelling, and rupture of the outer membrane and ultimately cell death [51]. α-Bisabolol treatment decreased the mitochondrial Ca2+ overload, swelling and increased the ATP levels thereby maintaining the structure and function of the mitochondria.
Apoptosis is one of the inevitable contributors of the cardiac damage [59]. It has been implicated as a possible mechanism in the progress of heart failure due to its pathophysiological consequences contributing to the loss and functional abnormalities of the cardiac muscle [60]. The β1-adrenergic receptor activation in cardiomyocytes triggers excessive apoptosis by increasing the myocardial contractility which consequently produces hypoxia followed by ischaemia [12]. ISO, a potent β-adrenergic receptor agonist administration has been reported to induce cardiomyocyte death by activating apoptosis in the myocardium [60, 61]. The members of the Bcl-2 family possess crucial role in the progress of apoptosis as evidenced by various experimental studies. The proteins of Bcl-2 family and caspases are checkpoints of the apoptotic pathways [61]. Bax, a proapoptotic gene of the family usually resides in the cytosol is expressed abundantly during apoptosis and promotes the programmed cell death [62]. Increased level of ROS activates Bax which ensures cell death by creating mitochondrial pore formation giving rise to the release of mitochondrial cytochrome-C to the cytosol. On the other side, this process can be countered by anti-apoptotic protein Bcl-2 [63]. Released cytochrome c binds to APAF-1, a key regulator of mitochondrial intrinsic pathway of apoptosis and activates executioner caspases by the formation of apoptosome [61]. The apoptosome then activates the initiator caspase 9, which subsequently leads to the activation of downstream executioner caspases, such as caspase 3 [64]. P53 is a regulator of cell cycle progression and apoptosis have been revealed to regulate APAF-1 in response to severe stress conditions and it also possesses the ability to activate pro apoptotic Bax protein [65]. ISO is an abundant producer of ROS and it treatment showed upregulated expressions of Bax, P53, APAF-1, active caspase-3 and active caspase-9 followed by downregulated expression of Bcl-2. Futhermore, ISO treatment showed decreased expression of cytochrome-C in the mitochondrial fraction and increased expression in the cytosolic fraction. These results have clearly revealed the activation of intrinsic apoptotic pathway in the myocardium. α-Bisabolol administration prevented ISO-induced overexpression of Bax, P53, APAF-1, active caspase-3 and active caspase-9. Also, it decreased the release of mitochondrial cytochrome-C from the myocardium. Therefore, α-bisabolol suppresses the activation of mitochondrial pathway of apoptosis in the myocardium.
Ultrastructural alterations of mitochondria are the most prominent finding to reveal cardiac damage in ISO-induced MI. ISO-induced heart mitochondria showed irregular size and shape, vacuolation, swelling and distruption of cristae. α-Bisabolol pre and cotreatment revealed normal mitochondrial architecture without distruption of cristae, swelling and vacuolation. Thus, α-bisabolol protected the structure of heart mitochondria against ISO-induced damage. The radical scavenging activities are crucial in biological systems due to the role of free radicals in triggering lipid peroxidation [66]. ISO is an abundant producer of OH• which possess the ability to alter the normal function of cell membranes via lipid peroxidation. In our study, α-bisabolol scavenges OH• in a dose-dependent manner. The highest percentage scavenging of OH• was observed at the concentration of α-bisabolol (50 µM). Thus, α-bisabolol scavenges excess OH• produced by the metabolism of ISO and attenuates cardiac mitochondrial lipid peroxidation by virtue of its free radical scavenging capacity.
The overall protective effects of α-bisabolol seems to be closely involved with the restriction of cardiac marker leakage, decreasing mitochondrial oxidative stress, maintaining TCA cycle enzymes, ETC complexes, Ca2+ homeostasis and structure of heart mitochondria and standing against the mitochondrial pathway of apoptosis.
Conclusion
Our findings revealed that α-bisabolol has maintained cardiac mitochondrial structure and function in ISO-induced MI in rats. Taken together the biochemical parameters, transmission electron microscopic, molecular and in vitro studies clearly suggest the therapeutic potential of α-bisabolol in attenuating myocardial injury. However, the translation of these therapeutic benefits in humans require many further studies. The possible mechanisms for the observed protective effects of α-bisabolol are ascribed to the prevention of oxidative stress triggered mitochondrial dysfunction and intrinsic apoptotic pathway in IS-induced MI in rats.
Abbreviations
- ISO:
-
Isoproterenol
- MI:
-
Myocardial infarction
- ATP:
-
Adenosine triphosphate
- Bax:
-
B-cell lymphoma-2 associated-x
- APAF-1:
-
Apoptotic protease activating factor 1
- Bcl-2:
-
B-cell lymphoma-2
- ETC:
-
Electron transport chain
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid
- Ca2+ :
-
Calcium
- CK:
-
Creatine kinase
- LDH:
-
Lactate dehydrogenase
- TBARS:
-
Thiobarbituric acid reactive substances
- LOOH:
-
Lipid hydroperoxide
- SOD:
-
Superoxide dismutase
- GSH:
-
Reduced glutathione
- ICDH:
-
Isocitrate dehydrogenase
- MDH:
-
Malate dehydrogenase
- SDH:
-
Succinate dehydrogenase
- α-KGDH:
-
α-Ketoglutarate dehydrogenase
- TEM:
-
Transmission electron microscopy
- OH• :
-
Hydroxyl radical
- DMRT:
-
Duncan’s multiple range test
- SPSS:
-
Statistical Package for the Social Science
- SD:
-
Standard deviation
- NAD:
-
Nicotinamide adenine dinucleotide
- NADH:
-
Reduced nicotinamide adenine dinucleotide
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
References
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2015) Catecholamine toxicity triggers myocardial membrane destabilization in rats: thymol and its counter action. RSC Adv 5:43338–43344. https://doi.org/10.1039/C5RA00903K
Hansi Priscilla D, Stanely Mainzen Prince P (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179:118–124. https://doi.org/10.1016/j.cbi.2008.12.012
Sivakumar R, Anandh Babu PV, Shyamaladevi CS (2008) Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem Biol Interact 176:227–233. https://doi.org/10.1016/j.cbi.2008.08.008
Xiang YK (2011) Compartmentalization of β-adrenergic signals in cardiomyocytes. Circ Res 109:231–244. https://doi.org/10.1161/CIRCRESAHA.110.231340
Xu Q, Dalic A, Fang L et al (2011) Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol 252:1012–1028. https://doi.org/10.1111/j.1476-5381.2010.01043.x
Hemalatha KL, Stanely Mainzen P, Prince (2016) Preventive effects of zingerone on cardiac mitochondrial oxidative stress, calcium ion overload and adenosine triphosphate depletion in isoproterenol induced myocardial infarcted rats. RSC Adv 6:12332. https://doi.org/10.1039/C6RA23330A
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2015) Thymol attenuates altered lipid metabolism in β-adrenergic agonist induced myocardial infarcted rats by inhibiting tachycardia, altered electrocardiogram, apoptosis and cardiac hypertrophy. J Funct Foods 14:51–62. https://doi.org/10.1016/j.jff.2015.01.013
Nagoor Meeran MF, Stanely Mainzen Prince P, Hidhayath Basha R (2012) Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study. Eur J Pharmacol 677:116–122. https://doi.org/10.1016/j.ejphar.2011.11.043
Nagoor Meeran MF, Stanely Mainzen Prince P (2012) Protective effects of N-acetyl cysteine on membrane-bound adenosine triphosphatases and minerals in isoproterenol-induced myocardial-infarcted rats: an in vivo and in vitro study. J Biochem Mol Toxicol 26:276–281. https://doi.org/10.1002/jbt.21419
Rimbaud S, Garnier A, Ventura-Clapier R (2009) Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep 61:131–138. https://doi.org/10.1016/S1734-1140(09)70015-5
Murray AJ, Edwards LM, Clarke K (2007) Mitochondria and heart failure. Curr Opin Clin Nutr Metab Care 10:704–711. https://doi.org/10.1097/MCO.0b013e3282f0ecbe
Mari Kannan M, Darlin Quine S (2012) Ellagic acid protects mitochondria from β-adrenergic agonist induced myocardial damage in rats; evidence from in vivo, in vitro and ultra structural study. Food Res Int 45:1–8. https://doi.org/10.1016/j.foodres.2011.09.018
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Nat Rev Cardiol 9:620–633. https://doi.org/10.1161/CIR.0b013e31826e1058
Hare JM (2001) Oxidative stress and apoptosis in heart failure progression. Circ Res 89:198–200
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
El-Missiry MA, Amer MA, Hemieda FA et al (2015) Cardioameliorative effect of punicalagin against streptozotocin-induced apoptosis, redox imbalance, metabolic changes and inflammation. Egypt J Basic Appl Sci 2:247–260. https://doi.org/10.1016/j.ejbas.2015.09.004
Kamatou GPP, Viljoen AM (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87:1–7. https://doi.org/10.1007/s11746-009-1483-3
Buitrago A, Rojas J, Rojas L et al (2015) Essential oil composition and antimicrobial activity of Vismia macrophylla leaves and fruits collected in Tachira-Venezuela. Nat Prod Commun 10:375–377
Zargaran A, Borhani-Haghighi A, Faridi P et al (2014) Potential effect and mechanism of action of topical chamomile (Matricaria chammomila L.) oil on migraine headache: a medical hypothesis. Med Hypotheses 83:566–569. https://doi.org/10.1016/j.mehy.2014.08.023
Brehm-Stecher BF, Johnson EA (2003) Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol and apritone. Antimicrob. Agents Chemother 47:3357–3360. https://doi.org/10.1128/AAC.47.10.3357-3360.2003
Moura Rocha NF, Venancio ET, Moura BA (2010) Gastroprotection of (-) alpha-bisabolol on acute gastric mucosal lesions in mice: the possible involved pharmacological mechanisms. Fundam Clin Pharmacol 24:63–71
Braga PC, Dal Sasso M, Fonti E et al (2009) Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell free systems. Pharmacology 83:110–115. https://doi.org/10.1159/000186049
Gomes-Carneiro MR, Dias DM, De-Oliveira AC (2005) Evaluation of mutagenic and antimutagenic activities of alpha-bisabolol in the Salmonella⁄microsome assay. Mutat Res 585:105–112
Corpas-Lopez V, Merino-Espinosa G, Lopez-Viota M et al (2016) Topical treatment of Leishmania tropica infection using (-)-α-bisabolol ointment in a Hamster model: effectiveness and safety assessment. J Nat Prod 79:2403–2407. https://doi.org/10.1021/acs.jnatprod.6b00740
Ojha S, Goyal S, Kumari S et al (2012) Pyruvate attenuates cardiac dysfunction and oxidative stress in isoproterenol induced cardiotoxicity. Exp Toxicol Pathol 64:393–399. https://doi.org/10.1016/j.etp.2010.10.004
Ojha S, Bhatia J, Arora S (2011) Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity:a biochemical and histopathological evaluation. J Environ Biol 32:731–738
Ojha S, Goyal S, Sharma C, Arora S, Kumari S, Arya DS (2013) Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats. Human Exp Toxicol 32:492–503
Malik S, Goyal S, Ojha SK et al (2011) Seabuckthorn attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity in rats. Int J Toxicol 30:671–680. https://doi.org/10.1177/1091581811417898
Ojha S, Azimullah S, Al Taee H, Meeran MFN (2017) Cardioprotective effect of (-)-α-Bisabolol in animal model of myocardial infarction. Planta Med Int 4:83. https://doi.org/10.1055/s-0037-1608133
Fraga CG, Leibovitz BE, Toppel AL (1988) Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–251. https://doi.org/10.1016/0891-5849(88)90023-8
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389. https://doi.org/10.1016/0003-2697(92)90122-N
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Sinha K (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
King J (1965) Isocitrate dehydrogenase. In: King JC, Van D (eds) Practical clinical enzymol. Nostrand Co, London, p 363
Mehler H, Kornberg A, Grisolia S et al (1948) The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J Biol Chem 174:961–977
Slater EC, Borner WD Jr (1952) The effect of fluoride on the succinic oxidase system. Biochem J 52:185–196
Reed LJ, Mukherjee RB (1969) α-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Lowenstein JM (ed) Methods in enzymology. Academic Press, London, pp 53–61
Kramer KA, Oglesbee D, Hartman SJ (2005) Automated spectrophotometric analysis of mitochondrial respiratory chain complex enzyme activities in cultured skin fibroblasts. Clin Chem 51:2110–2116. https://doi.org/10.1373/clinchem.2005.050146
Pearl W, Cascarano J, Zweifach BW (1963) Microdetermination of cytochrome oxidase in rat tissues by the oxidation on N-phenyl-p-phenylenediamine or ascorbic acid. J Histochem Cytochem 11:102–104
Williams JR, Coorkey BE (1967) Assay of intermediates of the citric acid cycle and related compounds by flourimetric enzymatic methods. In: Lowenstein JM (ed) Methods in enzymol. Academic Press, New York, pp 488–492
Maloyan A, Sanbe A, Osinska H et al (2005) Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 112:3451–3461. https://doi.org/10.1161/CIRCULATIONAHA.105.572552
Lang RAD (1987). In: Darely-Usmar VM, Reckwood D, Wison MT (eds) Mitochondria, a practical approach. IRL Press Ltd, Washington, pp 17–20
Halliwell B, Gutteridge M, Aruoma OI, The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 255:215–219
Moovendhan M, Seedevi P, Shanmugam A (2016) Exploration of the preventive effect of S. lessoniana liver oil on cardiac markers, hematological patterns and lysosomal hydrolases in isoproterenol-induced myocardial infarction in Wistar rats: a novel report. RSC Adv 6:64147–64154. https://doi.org/10.1039/C6RA11369A
Senthil Kumaran K, Stanely Mainzen Prince P (2010) Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones 15:791–806. https://doi.org/10.1007/s12192-010-0187-9
Zheng XH, Liu CP, Hao ZG et al (2017) Protective effect and mechanistic evaluation of linalool against acute myocardial ischemia and reperfusion injury in rats. RSC Adv 7:34473–34481. https://doi.org/10.1039/C7RA00743D
Basha RH, Priscilla DH (2011) An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Exp Toxicol Pathol 65:7–14. https://doi.org/10.1016/j.etp.2011.05.002
Plaut WE, Cook M, Aogaichi T (1983) The subcellular location of isozymes of NADP-isocitrate dehydrogenase in tissues from pig, ox and rat. Biochim Biophys Acta 760:300–308. https://doi.org/10.1016/0304-4165(83)90177-0
Yogeeta SK, Raghavendran HR, Gnanapragasam A et al (2006) Ferulic acid with ascorbic acid synergistically extenuates the mitochondrial dysfunction during beta-adrenergic catecholamine induced cardio toxicity in rats. Chem Biol Interact 253:250–259. https://doi.org/10.1016/j.cbi.2006.04.018
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2016) Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in β-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chem Biol Interact 244:159–168. https://doi.org/10.1016/j.cbi.2015.12.006
Ambrosio G, Zweier J, Duilio C (1993) Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268:18532–18541
Sudheesh NP, Ajith TA, Janardhanan KK (2013) Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. Int J Cardiol 165:117–125. https://doi.org/10.1016/j.ijcard.2011.07.103
Suchalatha S, Srinivasan P, Shyamala Devi C (2007) Effect of T. chebula on mitochondrial alterations in experimental myocardial injury. Chem Biol Interact 259:145–153. https://doi.org/10.1016/j.cbi.2007.06.034
Ide T, Tsutsui H, Hayashidani S et al (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88:529–535. https://doi.org/10.1161/01.RES.88.5.529
Riba A, Deres L, Sumegi B et al (2017) Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid Med Cell Longev 2017:10. https://doi.org/10.1155/2017/6819281
Starkov AA, Polster BM, Fiskum G (2002) Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem 83:220–228. https://doi.org/10.1046/j.1471-4159.2002.01153.x
Abell ED, Doenst T (2011) Mitochondrial adaptations to physiological vs pathological cardiac hypertrophy. Cardiovasc Res 90:234–242. https://doi.org/10.1093/cvr/cvr015
Wang M, Si JY, Yu Y (2014) Red clover flavonoids protect against oxidative stress-induced cardiotoxicity in vivo and in vitro. RSC Adv 4:54668–54676. https://doi.org/10.1039/C4RA08407A
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2016) Synthetic catecholamine triggers β1-adrenergic receptor activation and stimulates cardiotoxicity via oxidative stress mediated apoptotic cell death in rats: abrogating action of thymol. Chem Biol Interact 251:17–25. https://doi.org/10.1016/j.cbi.2016.03.017
Radhiga T, Rajamanickam C, Sundaresan A (2012) Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie 94:1135–1142. https://doi.org/10.1016/j.biochi.2012.01.015
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619. https://doi.org/10.1016/0092-8674(93)90509-O
Sahu BD, Anubolu H, Koneru M (2014) Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: possible involvement of mitochondrial dysfunction and apoptosis. Life Sci 107:59–67. https://doi.org/10.1016/j.lfs.2014.04.035
Joo JH, Ueda E, Bortner CD et al (2015) Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T-lymphoblastic leukemia Molt4 cells. Biochem Pharmacol 97:256–268. https://doi.org/10.1016/j.bcp.2015.08.086
Zhou B, Wu LJ, Tashiro S et al (2006) Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation of cell cycle. Biol Pharm Bull 29:1900–1905. https://doi.org/10.1248/bpb.29.1900
Wickens P (2011) Ageing and the free radical theory. Respir Physiol 128:379–391. https://doi.org/10.1016/S0034-5687(01)00313-9
Acknowledgements
The authors wish to thank United Arab Emirates University for the funding through University Program for Advanced Research (UPAR) (Grant No. 31M195).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagoor Meeran, M.F., Laham, F., Azimullah, S. et al. α-Bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol Cell Biochem 453, 89–102 (2019). https://doi.org/10.1007/s11010-018-3434-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3434-5