Abstract
Oxidative stress is implicated in the pathogenesis of a plethora of cardiovascular diseases including interstitial fibrosis, contractile dysfunction, ischemia-reperfusion injury, and cardiac remodeling. However, antioxidant therapies targeting oxidative stress in the progression of those diseases have largely been unsuccessful. The current study evaluated the effects of a NADPH oxidase inhibitor, apocynin (Apo), on the production of reactive oxygen species and the development of pathological cardiac hypertrophy under sustained β-adrenergic stimulation in male Wistar rats. As evident from the HW/BW ratio, HW/TL ratio, echocardiography, and histopathology, hypertrophic responses induced by isoproterenol (Iso; 5 mg/Kg body weight, subcutaneous) were blocked by Apo (10 mg/Kg body weight, intraperitoneal). Iso treatment increased the transcript levels of cybb and p22-phox, the two subunits of Nox. Iso treatment also caused a decrease in reduced glutathione level that was restored by Apo. Increase in mRNA levels of a number of markers of hypertrophy, viz., ANP, BNP, β-MHC, and ACTA-1 by Iso was either partially or completely prevented by Apo. Activation of key signaling kinases such as PKA, Erk, and Akt by Iso was also prevented by Apo treatment. Our study thus provided hemodynamic, biochemical, and molecular evidences supporting the therapeutic value of Apo in ameliorating adrenergic stress-induced cardiac hypertrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac hypertrophy is a pathophysiological response to increased cardiac workload, resulting in increase in heart size [1]. While physiological hypertrophy is associated with normal cardiac function and occurs during development, pregnancy, and sustained exercise; pathological hypertrophy is promoted by hypertension, myocardial injury, excessive neurohumoral activation, etc, eventually leading to heart failure [2]. High and sustained β-adrenergic activity with elevated level of blood catecholamines is a hallmark of pathological hypertrophy in heart failure patients [3, 4]. Pathological hypertrophy is characterized by increase in myocyte size, increased rate of protein synthesis, and reinduction of fetal genes through multiple signaling pathways, viz., MAP-, PI3-, Akt kinases, and Ca++-calcineurin system [5,6,7]. Transcription factors NFκB, NFAT, MEF2, GATA-4, etc, have also been associated with the reprogramming of gene expression in the hypertrophied heart [8]. Besides these canonical signal transduction pathways, reactive oxygen species (ROS) has also been attributed to the hypertrophic responses, but mechanisms are largely obscure [9, 10].
In recent years, NADPH oxidases (Noxes) have emerged as the major source of intracellular ROS generation under various pathophysiological setups [10,11,12]. Out of seven isoforms of mammalian Noxes, Nox1/2/4 are expressed in heart [13]. While tightly regulated ROS generation is essential for the maintenance of various cardiac functions, over production of ROS leads to conditions like interstitial fibrosis, contractile dysfunction, ischemia-reperfusion injury, and cardiac remodeling [14]. Therapeutic targeting of Nox-mediated generation of ROS is currently an area of intense research [15]. We have recently demonstrated that the stimulation of β-adrenergic receptor co-stimulates Nox2 and the crosstalk between the kinase and the redox (Nox) signaling determines the downstream responses [16]. In the present study, we demonstrate that Apo, a widely used inhibitor of Nox, attenuates the hypertrophic response elicited by the β adrenergic receptor agonist isoproterenol (Iso). Apo prevents the decrease in reduced glutathione level and the upregulation of a number of marker genes of hypertrophy by Iso.
Materials and methods
Reagents
All chemicals were purchased from Sigma Aldrich, USA, unless otherwise specified. Rabbit monoclonal antibodies against Erk1/2, phospho-Erk1/2 (Thr202/Tyr204), Akt, phospho-Akt (Ser473), and GAPDH were purchased from Cell Signaling Technologies, USA. Rabbit monoclonal antibodies against CREB and phospho-CREB (Ser133) was purchased from Abcam, UK. Rabbit polyclonal antibodies against PKA and phospho-PKA (Ser96) were procured from Santa Cruz Biotechnology, USA. Horseradish peroxidase-conjugated anti-rabbit IgG antibody was obtained from Santa Cruz Biotechnology, USA. All the oligonucleotides used for the transcript analyses were synthesized from Sigma Aldrich (Table 1).
Animals
Animal studies were carried out using male Wistar rats of 6–8 weeks. Animal were kept under standard laboratory conditions (temperature; 25 ± 2 °C, relative humidity; 50 ± 15% and 12 h-dark/12-h light period) and provided feed and water ad libitum. All animal procedures were reviewed and approved by Institutional Animal Ethics Committee (IAEC code 09/2014) of the Jawaharlal Nehru University, New Delhi (Registration No. 19/GO/ReBi/S/99/CPCSEA Dated: 10.03.1999). All animal care and experimental protocols were performed in compliance with the National Institutes of Health (NIH) guidelines for the care and use of the Laboratory Animals (NIH Publication no. 85-23, revised 1996).
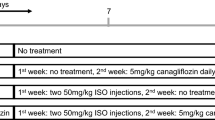
Rats were randomly divided into five groups (n = 6 in each group); (i) Control groups were administered subcutaneously with saline, (ii) Iso groups were subcutaneously injected with isoproterenol (5 mg/kg body weight; in saline), (iii) Iso + Apo groups were administered intraperitoneally with Apo (10 mg/kg body weight; in 10% DMSO) followed by subcutaneous injection of isoproterenol (5 mg/kg body weight; in saline), (iv) Apo groups were administered intraperitoneally with Apo (10 mg/kg body weight; in 10% DMSO), and (v) DMSO groups were administered intraperitoneally with DMSO (10%; in saline). All the drugs were freshly prepared and given once daily for 14 days.
After 14 days, body weight and tail length were measured and rats were sacrificed by overdose of pentobarbitone. The hearts were carefully excised, washed in ice cold PBS, blotted dry with tissue paper, and weighed. The ratio of heart weight to body weight and the ratio of heart weight to tail length were taken as parameters of cardiac hypertrophy. Tissues were snap frozen in liquid nitrogen, and stored at − 80 °C for later analysis.
Echocardiography
Rats were anesthetized with ketamine (50 mg/kg body weight) and xylazine (10 mg/kg body weight) and the chest hair was shaved. Transthoracic echocardiography was performed using a fully digitized Philips, USA system (Vivid 7 dimension) with a 10–11.5 MHz neonatal cardiac probe transducer. The transducer was placed on the left hemithorax. Two-dimensional M-mode images of left ventricle (LV) at the papillary muscle level were obtained from the parasternal short-axis view. LV posterior wall thickness (LVPWd), interventricular septal thicknesses (IVSd), and LV internal dimensions at the end of diastole (LVIDd) and systole (LVIDs) were measured from M-mode images. Based on these measurements, various other parameters were also calculated using the following formula:
Hematoxylin and eosin (H&E) staining
Left ventricle sections were cut from the hearts and were fixed in 10% formalin overnight at room temperature followed by embedding in paraffin. Four to five micrometer sections were cut and subjected to H&E staining by following standard protocols. Slides were viewed with Nikon Eclipse TiS microscope and images were captured at 10X magnification.
Glutathione assay
Reduced glutathione (GSH) concentration was determined essentially as described by Rahman et al. [17]. Briefly, the LV section of heart was gently homogenized in 0.6% sulfosalicylic acid and 0.1% Triton X-100 solution in 0.1 M KPE (potassium phosphate buffer containing 5 mM EDTA disodium salt, pH 7.5). The homogenate was centrifuged at 8000 g for 10 min at 4 °C and the supernatant obtained was used for the assay.
Real-time PCR
Total RNA was isolated from LV section of heart tissues using TRI reagent according to the manufacturer’s instructions. One microgram of RNA was reverse transcribed using Applied Biosystems reverse transcription kit as per the manufacturer’s protocol. Gene-specific primers were used to analyze the transcript level using SYBR green master mix (Applied Biosystems) in an Applied Biosystems 7500 real-time PCR system. Data were normalized using the GAPDH as an internal control, and fold change over control was calculated.
Western blotting
The LV sections of heart were homogenized in RIPA buffer (50 mM Tris; pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA) with phosphatase inhibitor cocktail, 1 mM phenylmethylsulphonyl fluoride (PMSF), and protease inhibitor cocktail followed by sonication. The homogenates were centrifuged and supernatant was assessed for protein concentration by the Bradford method using BSA as standard. Proteins were separated (80 μg per well) on 10% SDS-polyacrylamide gel and transferred onto PVDF membrane (Millipore, USA). Membranes were blocked with 3% BSA in 0.1% TBST for 2 h at room temperature followed by incubation with specific primary antibodies at 4 °C overnight. Then the membranes were incubated with secondary antibody conjugated to peroxidase (HRP) for 40 min at room temperature. Membranes were exposed to enhanced chemiluminescence reagent and visualized on X-ray film (Kodak, USA). Quantification of band intensities was performed with the ImageJ software system. The signal intensity of each protein was normalized with the corresponding total protein signal and fold change over control was calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism, PC version 5 (GraphPad software). Data are expressed as mean ± SEM. Experimental groups were compared with the use of one-way ANOVA if there was one independent variable (Tukey’s test) or two-way ANOVA if there were two independent variables. Values of P < 0.05 were considered statistically significant.
Results
Isoproterenol-induced cardiac hypertrophy parameters are prevented by Apo
Sustained activation of β-adrenergic receptors is an important hallmark of pathological cardiac hypertrophy and ROS is attributed to it [18]. Isoproterenol (Iso), a non-selective β-adrenergic receptor agonist, is widely used for inducing cardiac hypertrophy in experimental rats while Apo, a non-toxic inhibitor of Nox is also used for inhibiting Nox2 activity in vivo [19, 20]. To explore the role of Nox, if any, in β-agonist-induced cardiac hypertrophy, we injected male Wistar rats with Iso (5 mg/kg body weight, subcutaneous) for 14 days. Apo was also co-administered (10 mg/kg body weight, intraperitoneal), 30 min prior to the injection of Iso [21]. Iso-induced cardiac hypertrophy, as evident from increase in heart weight-to-body weight ratio (HW/BW), heart weight-to-tail length ratio (HW/TL), and the echocardiogram, was prevented by Apo (Fig. 1a, b). As summarized in Table 2 and shown in Fig. 1b, Iso increased the IVSDd, LVPWd by ~ 2- and ~ 1.5-fold, respectively, indicating concentric hypertrophy. There was no significant change in LVIDd, LVIDs, FS, EF, and SV (stroke volume) in Iso-treated heart, suggesting absence of any cardiac dysfunction (Table 2). Also, there were no significant changes in Apo groups. Further, microscopic examinations of H&E-stained LV sections demonstrated enlarged cardiomyocytes under Iso treatment while concurrent treatment with Apo partially prevented the increase in the cardiomyocytes size (Fig. 1c). Taken together, these data suggest the involvement of Noxes in inducing cardiac hypertrophy under sustained adrenergic stimulation.
Isoproterenol-induced cardiac hypertrophy in rat is prevented by the Nox inhibitor apocynin. Wistar rats were administered with Iso (5 mg/kg body weight, subcutaneous) and Apo (10 mg/kg body weight, intraperitoneal) for 14 days. Upon completion of treatments, a Rats were sacrificed and the heart were excised. Upper panel: representative images of whole heart excised from treated rats. Lower panel: ratio of HW/BW (heart weight to body weight) and HW/TL (heart weight to tail length). b M-mode echocardiography was done on treated rats. Upper panel: representative echocardiogram of different groups on day 14. Lower panel: Quantitative representation of LVIDd and LVPWd on day 1 (baseline) and day 14. (c) Representative histology of H&E-stained LV sections of hearts captured at 10X magnification using Nikon TiS microscope. LVIDd left ventricular internal dimensions at diastole, LVIDs left ventricular internal dimensions at systole, IVSd interventricular septal thickness at diastole, LVPWd left ventricular posterior wall thickness at diastole. **P ≤ 0.01 vs. Control; ***P ≤ 0.001 vs. Control; # P ≤ 0.05 vs. Iso; ## P ≤ 0.01 vs. Iso; $$ P ≤ 0.01 vs. Day 1; $$$ P ≤ 0.001 vs. Day 1
Isoproterenol upregulates the expression of cybb and p22-phox subunits of Nox
Activation of Nox2 at mRNA and protein levels by diverse stimuli has been demonstrated in different cell types [22]. To further establish the involvement of Nox2 in isoproterenol-induced cardiac hypertrophy, we measured the transcript levels of two of its subunits, i.e., cybb and p22-phox. The total RNA was extracted from the LV section of hearts and the expression of those subunits were assayed by Real-time PCR. As shown in Fig. 2a, in Iso-treated hearts, the levels of cybb and p22-phox mRNAs were increased by ~ 7.0- and ~ 3.0-fold, respectively. Concurrent treatment with Apo further increased the mRNA levels though it was not significant as compared to the Iso group. Since Apo is a pharmacological inhibitor of Nox activities [23], the levels of cybb and p22-phox mRNAs were unaffected with Apo treatment as expected and similar results have also been reported by others [24].
Apocynin pretreatment prevents isoproterenol-induced activation of NADPH oxidase and subsequent oxidative stress. Wistar rats were administered with 5 mg/kg body weight (subcutaneous) of Iso and 10 mg/kg body weight of Apo (intraperitoneal). After 14 days, rats were sacrificed and heart was excised. a Total RNA was isolated from the left ventricle and assayed for cybb and p22-phox transcripts using gene-specific primers by Real-time PCR. Normalization of input RNA was done by using GAPDH level as an internal control. Fold differences of mRNA levels over control were calculated. b The concentration of GSH was measured in the LV homogenate. *P ≤ 0.05 vs. Control; ***P ≤ 0.001 vs. Control; ##P ≤ 0.01 vs. Iso
Iso treatment increases oxidative stress in heart and Apo reduces it
Reduced glutathione is a key constituent of cellular antioxidant defense and a well-established marker of oxidative stress. In many experimental setups, following the generation of ROS, the level of GSH is reduced [25, 26]. We thus measured the level of reduced glutathione in Iso-treated heart and the effect of Apo on it. As shown in Fig. 2b, the level of GSH was reduced by ~ 30% in Iso-treated heart and Apo restored it by ~ 15%. Apo alone or the vehicle (DMSO) affected the GSH level only marginally. The data thus suggest that the induction of cardiac hypertrophy by Iso involves ROS generation and its inhibition by Apo reverses its effects.
Apocynin pretreatment prevents isoproterenol-induced expression of the markers of hypertrophy
To further establish the role of Nox-derived ROS in inducing cardiac hypertrophy by Iso, we assayed the mRNA levels of a number of well-established markers of hypertrophy. Total RNA were extracted from the LV section of hearts and the expression of markers genes were assayed by Real-time PCR [27]. As shown in Fig. 3, in Iso-treated hearts, the levels of transcripts for β-MHC, ANP, BNP, and ACTA-1 were increased by ~ 5.2-, ~ 2.5-, ~ 5.3-, and ~ 2.2-fold, respectively, that were prevented either partially (ANP, and BNP) or completely (β-MHC and ACTA-1) by the Apo treatment.
Apocynin pretreatment prevents isoproterenol-induced alteration in transcripts level. Wistar rats were administered with 5 mg/kg body weight (subcutaneous) of Iso and 10 mg/kg body weight of Apo (intraperitoneal). After 14 days, rats were sacrificed and heart was excised. Total RNA was isolated from the left ventricle and assayed for β-MHC, ANP, BNP, and ACTA-1 transcripts using gene-specific primers by Real-time PCR. Normalization of input RNA was done by using GAPDH level as an internal control. Fold differences of mRNA levels over control were calculated. ***P ≤ 0.001 vs. control; ## P ≤ 0.01 vs. Iso; ### P ≤ 0.001 vs. Iso
Apocynin prevents isoproterenol-induced activation of Akt and Erk
Cardiac hypertrophy involves upregulation of key signaling kinases including PKA, Akt, and Erk [7]. Although it has long been established that under adrenergic stress, these kinases are activated by the classical second messenger system, recent studies also suggest a role of ROS in their induction [28]. To understand if the regression of hypertrophy by Apo involves these kinases, we measured their levels by western blotting (Fig. 4). Iso treatment increased pPKA level by ~ 1.4-fold and pretreatment with Apo prevented that increase. The level of pAkt increased by ~ 2.0-fold under Iso treatment and Apo brought it to ~ 1.2-fold to the baseline. Similarly, in Iso-treated rats, pErk1/2 level was increased by ~ 1.2-fold and pretreatment with Apo abrogated the induction. Apart from the signaling kinases, we also assayed the level of the transcription factor CREB, a downstream target of PKA, which was unaffected under Iso treatment. Taken together, these data suggest that the activation of PKA, Akt, and Erk by the stimulation of β-adrenergic receptor is attenuated by the inhibition of Nox by apocynin.
Apocynin prevents isoproterenol-induced activation of downstream kinases. Wistar rats were administered with 5 mg/kg body weight (subcutaneous) of Iso and 10 mg/kg body weight of Apo (intraperitoneal). After 14 days, rats were sacrificed and heart was excised. Total protein was isolated from the left ventricle and equal amounts (80 µg) of lysates were assayed by western blot analysis using antibodies specific for phospho-PKA, phospho-CREB, phospho-Akt, and phospho-ERK1/2. Total PKA, total CREB, total Akt, and total ERK1/2 were used as respective controls. Also GAPDH level was the loading control. The mean density values of pPKA, pCREB, pAkt, and pERK were evaluated as a ratio relative to that of total PKA, total CREB, total Akt, and total ERK, respectively. Fold differences of relative ratio of protein levels over control were calculated. *P ≤ 0.05 vs. control; **P ≤ 0.01 vs. control; ***P ≤ 0.001 vs. control; # P ≤ 0.05 vs. Iso; ## P ≤ 0.01 vs. Iso
Discussion
Cardiac hypertrophy is the common manifestation of several cardiovascular disorders. At the cellular level, there are several hallmarks like increased protein synthesis, increase in myocyte volume but not in number, and partial reactivation of fetal gene expression programs. Although oxidative stress has been attributed to it, failure of antioxidants in ameliorating those conditions necessitated a revisit of the concept of the cause and effect relationship [29]. Such re-examination has also been necessitated by the emergence of reactive oxygen species like superoxide and hydrogen peroxide as the mediators of physiological signals [30].
Noxes are the enzymes solely dedicated to the generation of intracellular superoxide and hydrogen peroxide. With the emerging evidences on their pivotal role in the pathophysiology of various diseases, therapeutic inhibitors of Noxes are also being explored [20, 31]. Apocynin, a non-toxic plant metabolite, has been in use for treating respiratory, metabolic, and cardiovascular disorders even before its mode of action were known [32]. Later studies had described it as a general inhibitor of Nox [20]. In recent years, there has been a flurry of reports showing its beneficial effects under several pathobiological setups [20, 33]. Despite the ambiguities regarding the specificity of its actions, its beneficial effects have largely been well appreciated [34]. Based on our earlier study showing the involvement of Nox2 in mediating adrenergic signaling [16], we have tested whether Apo can ameliorate the hypertrophic response elicited by the adrenergic agonist Iso in male rats. As evident from the gross morphological parameters (HW/BW and HW/TL ratio), echocardiography, histopathology, level of well-established marker of oxidative stress, and expression levels of the transcripts of the conventional markers of hypertrophy (β-MHC, ANP, BNP, and ACTA-1), Apo was quite effective in preventing the hypertrophic responses measured after two weeks of treatment. In full agreement, the activation of key nodal kinases mediating adrenergic signaling, viz., PKA, ERK, and Akt by Iso was also prevented by Apo; reiterating the well-anticipated crosstalk between the redox and kinase pathways [28]. Noticeably, transcription factor CREB, one of the downstream targets of β-AR/PKA signaling and a regulator of cardiac functions [35], was not affected either by Iso or by Iso + Apo. Such result is in agreement with earlier reports and thus suggests the pathway-specific effects of Apo [36].
Abbreviations
- β-MHC:
-
β-Myosin heavy chain
- ACTA-1:
-
Actin alpha skeletal muscle 1
- ANP:
-
Atrial natriuretic peptide
- Apo:
-
Apocynin
- BNP:
-
Brain natriuretic peptide
- CREB:
-
cAMP response element binding protein
- DMSO:
-
Dimethyl sulfoxide
- EF:
-
Ejection fraction
- ERK:
-
Extracellular signal-regulated kinases
- FS:
-
Fractional shortening
- Iso:
-
Isoproterenol
- IVS:
-
Interventricular septal
- LVPW:
-
Left ventricular posterior wall
- Nox:
-
NADPH oxidase
- PKA:
-
Protein kinase A
- ROS:
-
Reactive oxygen species
References
Maillet M, van Berlo JH, Molkentin JD (2013) Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 14:38–48. https://doi.org/10.1038/nrm3495
Shimizu I, Minamino T (2016) Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 97:245–262. https://doi.org/10.1016/j.yjmcc.2016.06.001
Ferrara N, Komici K, Corbi G et al (2014) β-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol 4:396. https://doi.org/10.3389/fphys.2013.00396
Ciccarelli M, Santulli G, Pascale V et al (2013) Adrenergic receptors and me: role in development of cardiovascular disease. Front Physiol 4:265. https://doi.org/10.3389/fphys.2013.00265
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7:589–600. https://doi.org/10.1038/nrm1983
Hou J, Kang YJ (2012) Regression of pathological cardiac hypertrophy: signaling pathways and therapeutic targets. Pharmacol Ther 135:337–354. https://doi.org/10.1016/j.pharmthera.2012.06.006
Tham YK, Bernardo BC, Ooi JYY et al (2015) Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 89:1401–1438. https://doi.org/10.1007/s00204-015-1477-x
Dirkx E, da Costa Martins PA, De Windt LJ (2013) Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832:2414–2424. https://doi.org/10.1016/j.bbadis.2013.07.023
Chen Y-R, Zweier JL (2014) Cardiac mitochondria and reactive oxygen species generation. Circ Res 114:524–537. https://doi.org/10.1161/CIRCRESAHA.114.300559
Sag CM, Santos CXC, Shah AM (2014) Redox regulation of cardiac hypertrophy. J Mol Cell Cardiol 73:103–111. https://doi.org/10.1016/j.yjmcc.2014.02.002
Nabeebaccus A, Zhang M, Shah AM (2011) NADPH oxidases and cardiac remodelling. Heart Fail Rev 16:5–12. https://doi.org/10.1007/s10741-010-9186-2
Zhang M, Perino A, Ghigo A et al (2013) NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18:1024–1041. https://doi.org/10.1089/ars.2012.4550
Brandes RP, Weissmann N, Schröder K (2010) NADPH oxidases in cardiovascular disease. Free Radic Biol Med 49:687–706. https://doi.org/10.1016/j.freeradbiomed.2010.04.030
Lassègue B, San Martín A, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110:1364–1390. https://doi.org/10.1161/CIRCRESAHA.111.243972
Streeter J, Thiel W, Brieger K, Miller FJ (2013) Opportunity nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther 31:125–137. https://doi.org/10.1111/j.1755-5922.2011.00310.x
Saleem N, Goswami SK (2017) Activation of adrenergic receptor in H9c2 cardiac myoblasts co-stimulates Nox2 and the derived ROS mediate the downstream responses. Mol Cell Biochem 436:167–178. https://doi.org/10.1007/s11010-017-3088-8
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165. https://doi.org/10.1038/nprot.2006.378
Thakur A, Alam MJ, Ajayakumar MR et al (2015) Norepinephrine-induced apoptotic and hypertrophic responses in H9c2 cardiac myoblasts are characterized by different repertoire of reactive oxygen species generation. Redox Biol 5:243–252. https://doi.org/10.1016/j.redox.2015.05.005
Grimm M, Ling H, Willeford A et al (2015) CaMKIIδ mediates β-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic β-adrenergic stimulation. J Mol Cell Cardiol 85:282–291. https://doi.org/10.1016/j.yjmcc.2015.06.007
Altenhöfer S, Radermacher KA, Kleikers PWM et al (2015) Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23:406–427. https://doi.org/10.1089/ars.2013.5814
Ismail HM, Scapozza L, Ruegg UT, Dorchies OM (2014) Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PloS ONE 9:e110708. https://doi.org/10.1371/journal.pone.0110708
Youn GS, Cho H, Kim D et al (2017) Crosstalk between HDAC6 and Nox2-based NADPH oxidase mediates HIV-1 Tat-induced pro-inflammatory responses in astrocytes. Redox Biol 12:978–986. https://doi.org/10.1016/j.redox.2017.05.001
Theccanat T, Philip JL, Razzaque AM et al (2016) Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell Signal 28:190–203. https://doi.org/10.1016/j.cellsig.2015.11.013
Liu J, Zhou J, An W et al (2010) Apocynin attenuates pressure overload-induced cardiac hypertrophy in rats by reducing levels of reactive oxygen species. Can J Physiol Pharmacol 88:745–752. https://doi.org/10.1139/y10-063
Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH (2015) Glutathione–linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1164. https://doi.org/10.1016/j.freeradbiomed.2015.09.023
Sentellas S, Morales-Ibanez O, Zanuy M, Albertí JJ (2014) GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol Vitro Int J Publ Assoc BIBRA 28:1006–1015. https://doi.org/10.1016/j.tiv.2014.04.017
Taegtmeyer H, Sen S, Vela D (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 1188:191–198. https://doi.org/10.1111/j.1749-6632.2009.05100.x
Jindal E, Goswami SK (2011) In cardiac myoblasts, cellular redox regulates FosB and Fra-1 through multiple cis-regulatory modules. Free Radic Biol Med 51:1512–1521. https://doi.org/10.1016/j.freeradbiomed.2011.07.008
Sawyer DB (2011) Oxidative stress in heart failure: what are we missing? Am J Med Sci 342:120–124. https://doi.org/10.1097/MAJ.0b013e3182249fcd
Zhang Y, Tocchetti CG, Krieg T, Moens AL (2012) Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radic Biol Med 53:1531–1540. https://doi.org/10.1016/j.freeradbiomed.2012.07.010
Brandes RP, Weissmann N, Schröder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76:208–226. https://doi.org/10.1016/j.freeradbiomed.2014.07.046
’t Hart BA, Copray S, Philippens I (2014) Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed Res Int 2014:298020. https://doi.org/10.1155/2014/298020
Rastogi R, Geng X, Li F, Ding Y (2016) NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci 10:301. https://doi.org/10.3389/fncel.2016.00301
Simonyi A, Serfozo P, Lehmidi TM et al (2012) The neuroprotective effects of apocynin. Front Biosci Elite Ed 4:2183–2193
Liu X, Sun SQ, Hassid A, Ostrom RS (2006) cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol 70:1992–2003. https://doi.org/10.1124/mol.106.028951
Yin Q, Yang C, Wu J et al (2016) Downregulation of β-adrenoceptors in isoproterenol-induced cardiac remodeling through HuR. PloS ONE 11:e0152005. https://doi.org/10.1371/journal.pone.0152005
Acknowledgements
This work was supported by the Department of Biotechnology, Government of India, under Grant (BT/PR4268/BRB/10/1016/2011), awarded to SKG, and the DST-PURSE Scheme of Department of Science & Technology, Government of India, awarded to the School of Life Sciences, JNU. NS was a recipient of a JRF/SRF from the Indian Council of Medical Research, Government of India. We thank Prof. S K Maulik and Dr. Pankaj Prabhakar for assisting in doing the echocardiography at the Department of Pharmacology, AIIMS, New Delhi 110029, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are no conflicts of interest associated with this publication.
Rights and permissions
About this article
Cite this article
Saleem, N., Prasad, A. & Goswami, S.K. Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem 445, 79–88 (2018). https://doi.org/10.1007/s11010-017-3253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3253-0