Abstract
Epidemiological studies strongly suggest an association between high levels of dietary fat intake and an increased risk of developing breast cancer. Linoleic acid (LA) is an essential omega-6 PUFA and the major fatty acid in occidental diets. In breast cancer cells, LA induces expression of plasminogen activator inhibitor-1, proliferation, migration, and invasion. Fascin is an actin crosslinker globular protein that generates actin bundles made of parallel actin filaments, which mediate formation and stability of microspikes, stress fibers, membrane ruffles, and filopodia. However, the role of fascin in migration and invasion induced by LA in MDA-MB-231 breast cancer cells remains to be studied. We demonstrate here that LA induces an increase of fascin expression in MDA-MB-231 and MCF12A mammary epithelial cells. Particularly, LA induces the formation of filopodia and lamellipodia and the localization of fascin in these actin structures in MDA-MB-231 breast cancer cells. However, LA only induces formation of microspikes and the localization of fascin in these actin structures in mammary non-tumorigenic epithelial cells MCF12A. In addition, LA induces migration, invasion, and matrix metalloproteinase-9 secretion through a fascin-dependent pathway in MDA-MB-231 cells. In summary, our findings demonstrate that fascin is required for migration and invasion induced by LA in MDA-MB-231 breast cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent cause of cancer-related death among females worldwide [1]. Epidemiological and animal studies propose a relationship between great amounts of dietary fat intake and an elevated risk of developing breast cancer [2,3,4]. Particularly, free fatty acids (FFAs) are the components of membranes, a source of energy, and the precursors of bioactive components including eicosanoids [5]. In breast cancer cells, FFAs mediate a variety of cell processes including proliferation, migration, and invasion [6,7,8].
Linoleic acid (LA) is the major fatty acid in occidental diets. It is an essential omega-6 polyunsaturated fatty acid (PUFA), which is able to be converted to arachidonic acid (AA). Moreover, AA is the precursor of eicosanoids that mediate inflammatory responses and play a crucial role in tumor initiation and progression [9, 10]. In breast cancer cells, LA promotes expression of plasminogen activator inhibitor-1, proliferation, migration, and invasion through a process that does not require the conversion from LA to AA [11,12,13,14]. In addition, LA promotes an epithelial-mesenchymal-transition like process in mammary non-tumorigenic epithelial cells MCF10A [15].
Fascin is an actin crosslinker globular protein of ~ 55 kDa that has been well conserved throughout animal evolution. It is a member of cytoskeleton proteins that generates actin bundles built of parallel actin filaments, which mediate formation and stability of cellular protrusions including microspikes, stress fibers, membrane ruffles, and filopodia [16,17,18]. In vertebrates, fascin has tree isoforms: (1) Fascin-1, also known as fascin, is extensively present in mesenchymal tissues and nervous system; (2) Fascin-2, which is present in retinal cells; and (3) Fascin-3 is only expressed in testis [19, 20]. Particularly, fascin is expressed in adult human tissues, endothelial cells, fibroblasts, glial cells, neurons, smooth muscle cells, and dendritic cells. In contrast, fascin is expressed at very low levels or absent in normal epithelial cells, but it is upregulated in certain human epithelial tumors such as colon, skin, lung, prostate, and breast cancer [21,22,23].
In the present research, we study whether LA promotes an increase of fascin expression and the role of fascin in migration and invasion induced by LA in MDA-MB-231 breast cancer cells. Our findings show that LA promotes an increase of fascin expression in MDA-MB-231 and MCF12A mammary epithelial cells. Particularly, LA promotes the formation of filopodia and lamellipodia and the localization of fascin in these actin structures in MDA-MB-231 cells. However, LA only induces formation of microspikes and the localization of fascin in these actin structures in mammary non-tumorigenic epithelial cells MCF12A. In addition, LA promotes migration, invasion, and matrix metalloproteinase-9 (MMP-9) secretion through a fascin-dependent pathway in MDA-MB-231 cells.
Materials and methods
Materials
Fascin antibody (Ab), epidermal growth factor (EGF), fascin siRNA, and LA sodium salt were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phalloidin-tetramethylrhodamine B isothiocyanate (TRITC-conjugated phalloidin) and hydrocortisone were obtained from Sigma-Aldrich Co. (St. Louis, MO). BD Matrigel™ basement membrane matrix was obtained from BD Biosciences (Bedford, MA). Actin Ab was kindly provided by Dr. José-Manuel Hernández (Cinvestav-IPN).
Cell culture
The human MDA-MB-231 breast cancer cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 3.7 g/L sodium bicarbonate, and antibiotics. The human non-tumorigenic mammary epithelial cell line MCF12A was cultured in DMEM/F12 medium supplemented with 10% FBS, 10 µg/mL insulin, 0.5 µg/mL hydrocortisone, 20 ng/mL EGF, and antibiotics. Cells were cultured in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C. For experimental purposes, MDA-MB-231 and MCF12A cells were starved for 24 h in DMEM without FBS and supplements (insulin, hydrocortisone, and EGF) before treatment.
Cell stimulation
Cultures of MDA-MB-231 and MCF12A cells were washed twice with phosphate buffered saline (PBS), equilibrated in DMEM for at least 30 min at 37 °C and then treated with LA. Stimulation was terminated by aspirating the medium (conditioned medium) and cells were solubilized in 0.5 mL of ice-cold RIPA buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, 100 mM NaF, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 1.5 mM MgCl2, 0.1% SDS, and 1 mM phenylmethylsulfonyl fluoride). Protein content of each sample was determined using the micro-Bradford protein assay.
Western blotting
Equal amounts of protein were separated by SDS-PAGE using 10% separating gels followed by transfer to nitrocellulose membranes. Membranes were blocked using 5% non-fat dried milk in PBS pH 7.2/0.1% tween 20 (wash buffer), and incubated overnight at 4 °C with primary Ab. Next, membranes were washed three times with wash buffer and incubated with secondary Ab (horseradish peroxidase-conjugated) (1:5000) for 2 h at room temperature. After washing three times with wash buffer, immunoreactive bands were visualized using the Western blotting luminol reagent (Santa Cruz Biotechnology). Autoradiograms were scanned, and the labeled bands were quantified using the ImageJ software (NIH, USA).
Interference RNA
Fascin expression was silenced in MDA-MB-231 cells using the silencer siRNA kit from Santa Cruz Biotechnology, according to the manufacturer’s guidelines. One control of scramble siRNAs was included.
Scratch-wound assay
Cells were grown to confluence in 35-mm culture dishes and starved. After starvation, cultures were treated for 2 h with 40 μM mitomycin C to inhibit proliferation, and they were scratch-wounded using a sterile 200-µL pipette tip, washed twice with PBS, and refed with DMEM without or with LA for 48 h. Progress of migration into the wound space was photographed using an inverted microscope coupled to a camera. Each experimental condition was repeated three times. Cell migration was evaluated using the ImageJ Software (NIH, USA).
Invasion assay
Invasion assays were performed by the modified Boyden chamber method in 24-well plates containing 12 cell culture inserts with 8 µm pore size (Costar, Corning Inc). An amount of 30 µl Matrigel BD was added into culture inserts and kept overnight at 37 °C to form a semisolid matrix. MDA-MB-231 cells were plated at 1 × 105 cells per insert in 100 μL serum-free DMEM on top chamber. Lower chamber contained 600 µL DMEM without or with 90 μM LA. Chambers were incubated for 48 h at 37 °C. After incubation, cells and matrigel were removed from the upper side with cotton swabs, and cells on lower surface of membrane were washed and fixed with methanol for 5 min. The number of invaded cells was estimated by staining with 0.1% crystal violet in PBS. Dye was eluted with 500 μL 10% acetic acid and absorbance was measured at 600 nm. Background value was obtained from wells without cells.
Immunofluorescence confocal microscopy
Cells were grown on coverslips, washed with PBS, equilibrated in FBS-free DMEM, and treated with LA. Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 for 20 min, and blocked for 30 min with 3% bovine serum albumin (BSA) at room temperature. Cells were incubated overnight with anti-fascin Ab, followed by FITC-labeled anti-mouse secondary Ab for 2 h at 4 °C. Next, cells were stained with TRITC-conjugated phalloidin to reveal F-actin. Cells were viewed using a Leica confocal microscope (Model TCS SP2; Leica Microsystems, Wetzlar, Germany). Serial optical sections of 0.8–0.99 µm thick were taken in both xyz and xzy. To prevent interference from the fluorescent probes, images of the same optical section were taken as separate channels and they were analyzed using ImageJ software.
Zymography
Conditioned mediums were collected and concentrated using Amicon® ultra centrifuge filters (Merck Millipore). Equal volume of non-heated conditioned medium samples were mixed with sample buffer (2.5% SDS, 1% sucrose, 4 μg/ml phenol red) without reducing agents, and loaded to 8% polyacrylamide gels copolymerized with gelatin (1 mg/ml). Gels were rinsed twice with 2.5% Triton X-100, and then incubated in assay buffer (50 mM Tris–HCl pH 7.4, 5 mM CaCl2) at 37°C for 48 h. Gels were fixed and stained with 0.25% Coomassie Brilliant Blue G-250 in 10% acetic acid and 30% methanol. Proteolytic activity was detected as clear bands against the background stain of undigested substrate.
Statistical analysis
Results are expressed as the mean ± SD of at least three independent experiments. Data were statistically analyzed using one-way ANOVA and Dunnett’s multiple comparison test. Statistical probability of P < 0.05 was considered significant. Asterisks denote comparisons made to control (unstimulated cells).
Results
LA induces an increase of fascin expression in mammary epithelial cells
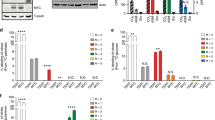
First, we determined whether LA induced an increase of fascin expression levels in MDA-MB-231 breast cancer cells and mammary non-tumorigenic epithelial cells MCF12A. Cultures of MDA-MB-231 and MCF12A cells were stimulated without or with 90 μM LA for 30 and 60 min and lysed. Cell lysates were analyzed by Western blotting with anti-fascin Ab and with anti-actin Ab as loading control. As illustrated in Figs. 1a and 2a (upper panel), MDA-MB-231 cells express fascin and treatment with LA for 30 and 60 min induced an increase of fascin expression levels, whereas MCF12A cells also express fascin, and treatment with LA only induced a little bit of increase of fascin expression levels at 60 min. Western blotting with anti-actin Ab of the same membranes confirmed that similar amounts of protein were recovered after treatment with LA (Figs. 1a, 2a, lower panel).
LA induces an increase of fascin expression in MDA-MB-231 breast cancer cells (a). MDA-MB-231 cells were stimulated without or with 90 μM LA for 30 and 60 min and lysed. Cell lysates were analyzed by Western blotting with anti-fascin Ab and with anti-actin Ab as loading control (b). MDA-MB-231 cells cultured on coverslips were treated with 90 μM LA for 30 and 60 min and fixed. Cells were stained with anti-fascin Ab. F-actin was stained with TRITC-conjugated to phalloidin. Images were obtained by confocal microscopy. Fascin is shown in green and F-actin structures are shown in red. Graphs represent the mean ± SD of fascin or fascin fluorescence intensities and are expressed as fold of control (unstimulated cells). Comparisons were made with control cells. *P < 0.05 and **P < 0.01. (Color figure online)
LA induces an increase of fascin expression in mammary non-tumorigenic epithelial cells MCF12A (a). MCF12A cells were stimulated without or with 90 μM LA for 30 and 60 min and lysed. Cell lysates were analyzed by Western blotting with anti-fascin Ab and with anti-actin Ab as loading control (b). MCF12A cells cultured on coverslips were treated with 90 μM LA for 30 and 60 min and fixed. Cells were stained with anti-fascin Ab. F-actin was stained with TRITC-conjugated to phalloidin. Images were obtained by confocal microscopy. Fascin is shown in green and F-actin structures are shown in red. Graphs represent the mean ± SD of fascin or fascin fluorescence intensities and are expressed as fold of control. Comparisons were made with control cells. *P < 0.05 and ***P < 0.001. (Color figure online)
To further substantiate that LA induced an increase of fascin expression and to determine whether treatment with LA induced relocalization of fascin, we studied fascin expression and its localization by confocal microcopy. MDA-MB-231 and MCF12A cells were cultured on coverslips and treated with 90 μM LA for 30 and 60 min. Cells were fixed and analyzed by immunofluorescence using anti-fascin Ab, whereas F-actin was stained with TRITC-conjugated phalloidin. Our findings showed that LA induced an increase of fascin expression in cytosol and the formation of filopodia and lamellipodia in MDA-MB-231 cells. Moreover, fascin was mainly localized with filopodia and lamellipodia (Fig. 1b). In contrast, treatment with LA induced a little bit of increase of fascin expression in cytosol and the formation of microspikes in MCF12A cells. However, LA did not induce formation of filopodia and lamellipodia and fascin was localized at the edges of cells with microspikes (Fig. 2b).
Migration of MDA-MB-231 cells induced by LA requires fascin expression
Since we have previously demonstrated that LA induced migration of MDA-MB-231 cells [14], we studied the role of fascin on migration. As shown in Fig. 3a, in agreement with our previous findings [14], LA induced migration of MDA-MB-231 cells. Next we determined the role of fascin in migration induced by LA. First, we inhibited fascin expression using siRNAs against fascin. Our results showed a clear inhibition of fascin expression (Fig. 3b). Next, cultures of MDA-MB-231 cells, in which fascin expression was knocked down, were scratch-wounded and stimulated with 90 μM LA for 48 h. Our results showed that migration induced by LA required fascin expression in MDA-MB-231 cells (Fig. 3c).
LA induces migration through a fascin-dependent pathway in MDA-MB-231 cells (a). Confluent cultures of MDA-MB-231 cells were scratch-wounded and treated with 90 μM LA for 48 h (b). Cell lysates of MDA-MB-231 cells transfected with fascin siRNAs or scramble siRNAs were analyzed by Western blotting with anti-fascin Ab. Actin was included as loading control (c). Confluent cultures of MDA-MB-231 cells transfected with fascin siRNAs or scramble siRNAs were scratched and treated with 90 μM LA for 48 h. One control of fetal bovine serum (FBS) was included. Graphs are the mean ± SD and are expressed as the fold of migrated cells or fascin above control. Comparisons were made with control cells. ***P < 0.001, ****P < 0.0001
Cell migration requires formation of focal contacts, whereas paxillin is a protein localized in these structures [24]. We determined whether treatment with LA induced a colocalization of fascin and paxillin. MDA-MB-231 cells cultured on coverslips were stimulated with 90 μM LA for 60 min. Cells were analyzed by immunofluorescence with anti-paxillin Ab and anti-fascin Ab. As we mention above, treatment with LA induced the increase of fascin expression, which was colocalized with paxillin, whereas cells adopted a fibroblast-like structure (Fig. 4a). Our findings suggest that LA also induced an increase of paxillin expression.
LA induces invasion through a fascin-dependent pathway in MDA-MB-231 cells (a). MDA-MB-231 cells cultured on coverslips were stimulated with 90 μM LA for 60 min and fixed. Cells were stained with anti-fascin Ab and anti-paxillin Ab. Images were obtained by confocal microscopy. Fascin is shown in green and paxillin is shown in red (b). Invasion assays were performed using MDA-MB-231 cells transfected with fascin siRNAs or scramble siRNAs and treated with 90 µM LA for 48 h (c). MDA-MB-231 cells transfected with fascin siRNAs or scramble siRNAs were treated with 90 µM LA for 48 h, conditioned medium was obtained, and cells were lysed. MMP-2 and MMP-9 secretions were analyzed on conditioned medium using gelatin-substrate gels. Controls of MMP-2 (PDB) and MMP-9 (EtOH) secretions, and one of fetal bovine serum (FBS) were included. Cell lysates were analyzed by Western blotting with anti-actin Ab. Graph is the mean ± SD and is expressed as the fold of invasion above control. Comparisons were made with control cells. **P < 0.01. (Color figure online)
Invasion of MDA-MB-231 cells induced by LA requires fascin expression
LA induces invasion of MDA-MB-231 cells [11]. We determined the role of fascin on invasion induced by treatment with LA. We performed invasion assays using MDA-MB-231 cells, in which fascin expression was knocked down using siRNAs against fascin, and treated with 90 μM LA for 48 h. As illustrated in Fig. 4b, LA induced a clear invasion and it was dependent on fascin expression in MDA-MB-231 cells.
Next, we determined whether LA induced an increase of matrix metalloproteinase-2 (MMP-2, gelatinase A) and MMP-9 (gelatinase B) secretions and the role of fascin on secretion of these gelatinases. Cultures of MDA-MB-231 cells, in which fascin expression was knocked down, were stimulated with 90 μM LA for 48 h, conditioned medium was obtained, and cells were lysed. Conditioned medium was subjected to gelatin zymography and cell lysates were analyzed by Western blotting with anti-actin Ab. Since ethanol (EtOH) and PDB stimulate secretion of MMP-2 and MMP-9, respectively [25, 26], positive controls of MMP-2 and MMP-9 secretions were included, which were prepared by treatment of MDA-MB-231 cells with 100 ng/ml PDB or 400 mg/dl EtOH for 24 h. Our results showed that LA induced an increase of MMP-9 secretion, but it did not induce an increase of MMP-2 secretion in MDA-MB-231 cells. Interestingly, inhibition of fascin expression blocked the increase of MMP-9 secretion (Fig. 4c). Western blotting with anti-actin Ab of cell lysates confirmed that similar number of cells was present in the different conditions analyzed (Fig. 4c).
Discussion
Epidemiological studies demonstrate that a dietary pattern characterized for high fat choices, including n-6 PUFAs, increase the risk of breast cancer. Particularly, LA is the major fatty acid in occidental diets with an intake of 15–20 g/day/person and a plasma concentration of ~ 275 μM [27,28,29,30]. We demonstrate that LA induces migration and invasion of MDA-MB-231 breast cancer cells [11, 14]. However, the role of LA in the expression and organization of fascin/actin, as well as its role on migration and invasion mediated by treatment with LA in MDA-MB-231 cells, remains to be studied.
Fascin expression has not been reported in normal adult epithelia, but it is expressed in basal layer of epidermis. In contrast, upregulation of fascin expression has been demonstrated in metastatic human carcinomas from different sites [23]. Particularly, elevated levels of fascin correlate with clinically aggressive phenotypes, poor prognosis, and shorter disease-free in breast cancer patients [31]. Here we demonstrate that LA induces an increase of fascin expression at 30 and 60 min of treatment in MDA-MB-231 cells. In agreement with our findings, MDA-MB-231 cells express basal levels of fascin and treatment with IL-6 and TNF-α induces an increase of fascin transcripts at 30 min of treatment [32]. In addition, we demonstrate that mammary non-tumorigenic epithelial cells MCF12A express basal levels of fascin and treatment with LA induces only an increase of fascin expression at 60 min of treatment. Our findings strongly suggest that mammary epithelial cells also express fascin and therefore additional studies are necessary to determine whether fascin is or not expressed in adult mammary epithelial cells.
Fascin contributes to organization of actin-based structures, including cortical cell protrusions and cytoplasmic microfilaments bundles, which mediate migration, cell interactions, architecture, and intracellular movements [19]. Moreover, fascin is in equilibrium between cytosol and cytoskeleton, and its function is modulated by a variety of ligands including extracellular matrix components, growth factors, and cytokines [33, 34]. Our findings demonstrate that one fatty acid (LA) induces formation of filopodia and lamellipodia and the localization of fascin with these structures in MDA-MB-231 cells. Particularly, fascin is mainly localized with lamellipodia. In contrast, LA just induces formation of microspikes and the localization of fascin with these structures; however, it does not induce formation of lamellipodia and filopodia in mammary non-tumorigenic epithelial cells MCF12A. We propose that fascin contributes to formation of specific actin/fascin structures in human mammary cells and they are specific of breast cancer and mammary non-tumorigenic epithelial cells. Supporting our proposal, it has been demonstrated that filopodia are rich in metastatic tumor cells and its number correlates with their invasiveness, whereas filopodium-like structures are critical for colonization of secondary tissues or organs for metastatic tumor cells [35, 36].
Invasion/metastasis process involves cell detachment, migration/invasion, intravasation, survival in circulation, extravasation, and proliferation at distal tissues [37]. In breast cancer, fascin expression correlates inversely with the presence of estrogen and progesterone receptors, but it is not related with HER-2 overexpression [31, 38]. We previously demonstrated that LA induces migration and invasion of MDA-MB-231 cells, but it is not able to induce migration of MCF12A cells. Here we demonstrate that migration and invasion induced by LA require fascin expression in MDA-MB-231 cells. We propose that LA plays an important role in migration and invasion in breast cancer through fascin expression. Supporting our proposal, it has been demonstrated that fascin expression promotes downregulation of breast cancer metastasis suppressor-1 (BRMS1) and upregulation of proteins involved in metastasis including urokinase-type plasminogen activator, MMP-2, and MMP-9 in MDA-MB-231 cells [39].
Matrix metalloproteinases (MMPs) are a family of endopeptidases that collectively are able to degrade all extracellular matrix (ECM) components and are implicated in tumor progression, because they promote angiogenesis, tumor cell growth, and degradation of basement membrane (BM) and interstitial matrices [40, 41]. However, MMPs are synthesized and secreted as zymogens that require activation to become proteolytically active, and therefore activation is a critical step in regulation of MMP-dependent proteolytic activity [42, 43]. Particularly, MMP-2 and MMP-9 are highly expressed in malignant tumors, including breast cancer, and are associated with invasion and metastasis because they are able to degrade type IV collagen, which is the main component of BM [43, 44]. We demonstrate here that LA induces secretion of MMP-9 and it is dependent on fascin expression in MDA-MB-231 cells. Our findings demonstrate a new role of fascin in MMP-9 secretion induced by LA and they support an important role of fascin in migration and invasion of breast cancer cells.
Interestingly, MMP-2 and MMP-9 appear in the supernatant fluid long before an increase in gelatinases mRNA is detected by RT-PCR in Jurkat T and primary T cells transfected with FAK wild-type cDNA. In addition, blocking transcription with actinomycin D does not inhibit the increase of gelatinases secretion, whereas blocking protein secretion with monensin and brefeldin A reduces gelatinases production in supernatant [45]. In addition, we demonstrate that treatment of MCF7 breast cancer cells with type IV collagen induces an increase of MMP-9 secretion, but it does not induce an increase of MMP-9 mRNA at 30 h of treatment [26]. We propose that treatment with LA for 48 h induces secretion of MMP-9, but it does not induce an increase of MMP-9 mRNA in MDA-MB-231 cells.
Fascin is localized in focal adhesion and is considered as an adhesome protein [46, 47]. Moreover, fascin is also localized in paxillin-positive adhesions, whereas the number of mature adhesions is higher in cells depleted of fascin [48]. We demonstrate here that untreated MDA-MB-231 cells do not show colocalization of fascin with paxillin. However, treatment with LA induces an increase of fascin expression, which is accompanied with its colocalization with paxillin. Since reorganization of actin cytoskeleton and focal adhesions is required for migration, invasion, and metastasis and fascin and paxillin play an important role in the formation of these structures, we propose that colocalization of fascin and paxillin induced by LA is required for migration and invasion process in breast cancer cells. Supporting our proposal, fascin and paxillin are expressed in infiltrating duct carcinoma and they correlate with poor prognosis factors [49].
In conclusion, our results demonstrate that fascin mediates migration and invasion induced by LA in MDA-MB-231 breast cancer cells.
References
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomark Prev 25:16–27
Binukumar B, Mathew A (2005) Dietary fat and risk of breast cancer. World J Surg Oncol 3:45
Lopez R, Agullo P, Lakshmanaswamy R (2013) Links between obesity, diabetes and ethnic disparities in breast cancer among Hispanic populations. Obes Rev 14:679–691
Abel S, Riedel S, Gelderblom WC (2014) Dietary PUFA and cancer. Proc Nutr Soc 73:361–367
Tvrzicka E, Kremmyda LS, Stankova B, Zak A (2011) Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:117–130
Yonezawa T, Katoh K, Obara Y (2004) Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun 314:805–809
Ferre P (2004) The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53(Suppl 1):S43–S50
Soto-Guzman A, Navarro-Tito N, Castro-Sanchez L, Martinez-Orozco R, Salazar EP (2010) Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin Exp Metastasis 27:505–515
Fritsche KL (2008) Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot Essent Fatty Acids 79:173–175
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP (2017) Linoleic acid induces migration and invasion through FFAR4- and PI3 K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol 34:111
Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y (2008) Unsaturated fatty acids promote proliferation via ERK1/2 and Akt pathway in bovine mammary epithelial cells. Biochem Biophys Res Commun 367:729–735
Byon CH, Hardy RW, Ren C, Ponnazhagan S, Welch DR, McDonald JM, Chen Y (2009) Free fatty acids enhance breast cancer cell migration through plasminogen activator inhibitor-1 and SMAD4. Lab Invest 89:1221–1228
Serna-Marquez N, Villegas-Comonfort S, Galindo-Hernandez O, Navarro-Tito N, Millan A, Salazar EP (2013) Role of LOXs and COX-2 on FAK activation and cell migration induced by linoleic acid in MDA-MB-231 breast cancer cells. Cell Oncol (Dordr) 36:65–77
Espinosa-Neira R, Mejia-Rangel J, Cortes-Reynosa P, Salazar EP (2011) Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol 43:1782–1791
Courson DS, Rock RS (2010) Actin cross-link assembly and disassembly mechanics for alpha-Actinin and fascin. J Biol Chem 285:26350–26357
Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG (2006) Role of fascin in filopodial protrusion. J Cell Biol 174:863–875
Edwards RA, Bryan J (1995) Fascins, a family of actin bundling proteins. Cell Motil Cytoskelet 32:1–9. doi:10.1002/cm.970320102
Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC (2002) Fascins, and their roles in cell structure and function. BioEssays 24:350–361
Jayo A, Parsons M (2010) Fascin: a key regulator of cytoskeletal dynamics. Int J Biochem Cell Biol 42:1614–1617
Tan VY, Lewis SJ, Adams JC, Martin RM (2013) Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med 11:52
Ma Y, Machesky LM (2015) Fascin1 in carcinomas: its regulation and prognostic value. Int J Cancer 137:2534–2544
Hashimoto Y, Kim DJ, Adams JC (2011) The roles of fascins in health and disease. J Pathol 224:289–300
Schaller MD (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459–6472
Ke Z, Lin H, Fan Z, Cai TQ, Kaplan RA, Ma C, Bower KA, Shi X, Luo J (2006) MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int J Cancer 119:8–16
Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP (2008) Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol 27:220–231
Schulz M, Hoffmann K, Weikert C, Nothlings U, Schulze MB, Boeing H (2008) Identification of a dietary pattern characterized by high-fat food choices associated with increased risk of breast cancer: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr 100:942–946
Lee MM, Lin SS (2000) Dietary fat and breast cancer. Annu Rev Nutr 20:221–248
Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD (2000) Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 71:179S–188S
Anderson SG, Sanders TA, Cruickshank JK (2009) Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 53:839–845
Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, Adams JC, Hicks DG (2005) The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res 11:186–192
Snyder M, Huang J, Huang XY, Zhang JJ (2014) A signal transducer and activator of transcription 3.Nuclear Factor kappaB (Stat3.NFkappaB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha. J Biol Chem 289:30082–30089
Adams JC, Clelland JD, Collett GD, Matsumura F, Yamashiro S, Zhang L (1999) Cell-matrix adhesions differentially regulate fascin phosphorylation. Mol Biol Cell 10:4177–4190
Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B (2003) Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene 22:3616–3623
Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA (2012) The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2:706–721
Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, Segall JE, Condeelis JS (2002) Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res 62:6278–6288
Bashyam MD (2002) Understanding cancer metastasis: an urgent need for using differential gene expression analysis. Cancer 94:1821–1829
Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW Jr, Yu D, Mooney EE, McCrea PD (2000) C-erbB-2/HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene 19:4864–4875
Al-Alwan M, Olabi S, Ghebeh H, Barhoush E, Tulbah A, Al-Tweigeri T, Ajarim D, Adra C (2011) Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS ONE 6:e27339
Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C (2004) Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 48:411–424
Curran S, Murray GI (1999) Matrix metalloproteinases in tumour invasion and metastasis. J Pathol 189:300–308
Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2:252–257
Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM (2004) Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res 10:7621–7628
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, Urbano-Marquez A, Yamada KM, Cid MC (2005) Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. FASEB J 19:1875–1877
Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM (2011) Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13:383–393
Schiller HB, Friedel CC, Boulegue C, Fassler R (2011) Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep 12:259–266
Elkhatib N, Neu MB, Zensen C, Schmoller KM, Louvard D, Bausch AR, Betz T, Vignjevic DM (2014) Fascin plays a role in stress fiber organization and focal adhesion disassembly. Curr Biol 24:1492–1499
Omran OM, Al Sheeha M (2015) Cytoskeletal Focal Adhesion Proteins Fascin-1 and Paxillin Are Predictors of Malignant Progression and Poor Prognosis in Human Breast Cancer. J Environ Pathol Toxicol Oncol 34:201–212
Acknowledgements
We are grateful to Nora Ruiz for her technical assistance. This research was funded by CONACYT (255429).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Gonzalez-Reyes, C., Marcial-Medina, C., Cervantes-Anaya, N. et al. Migration and invasion induced by linoleic acid are mediated through fascin in MDA-MB-231 breast cancer cells. Mol Cell Biochem 443, 1–10 (2018). https://doi.org/10.1007/s11010-017-3205-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3205-8