Abstract
The aim of this study was to evaluate the influence of resveratrol on HG-induced calcium entry in islet microvascular (MS-1) endothelial cells. MS-1 cells were pretreated with resveratrol or 2-APB (an inhibitor of store-operated calcium entry) and then incubated with high glucose. Cell viability was determined using the cell counting kit-8 method. Reactive oxygen species, endothelial apoptosis, and NO production were detected by DHE probe, TUNEL detection, and nitrate reductase assay kit. Protein levels of SOCE were detected by western blotting. Pretreatment with resveratrol significantly attenuated HG-induced endothelial apoptosis and improved cell viability. However, pretreatment with resveratrol and 2-APB abolished this effect, suggesting that the attenuation of HG-induced apoptosis by resveratrol may be associated with SOCE. Subsequent analyses indicated that HG induced the SOCE-related proteins, including TRPC1, Orai1, and Stim1. These results suggest that resveratrol pretreatment is associated with relieved HG-induced endothelial apoptosis at least partly via inhibition of SOCE-related proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis has been well established as a key pathophysiological feature of many cardiovascular diseases, including diabetes-associated vascular complications [1]. The integrity of endothelial cells (ECs) is the basis of overall vascular function, and apoptosis of ECs may be an important underlying mechanism of atherosclerosis [2, 3]. High glucose-induced apoptosis results in endothelial injury, which may eventually lead to microvascular dysfunction, causing cardiovascular and cerebrovascular complications. Indeed, EC apoptosis is not only considered as an initial trigger of the progression of atherosclerosis, but it is also indicated to be responsible for many acute vascular events related to atherosclerotic plaque instability, because loss of ECs can predispose individuals to arterial thrombosis, causing acute coronary occlusion and sudden death [4, 5]. Previous studies have suggested that in patients with diabetes mellitus (DM), a high glucose level is a toxic stimulus that may lead to EC senescence [6] and apoptosis [7, 8]. However, the exact mechanisms underlying HG-induced EC apoptosis remain to be determined.
Cytosolic calcium (Ca2+) is an intracellular messenger that exerts many regulatory functions, including the determination of cell fate [9]. Endoplasmic reticulum (ER) calcium release can be stimulated through a variety of mechanisms. When thestromal interaction molecule (Stim1), as the ER calcium ion receptor, perceives calcium depletion, it initiates a change in the structure of the membrane and, with calcium ion release, activates the calcium channel protein (Orai1) to form a classical SOC channel mediated by calcium influx [10]. Previous evidence indicated that high glucose stimulation of an intracellular calcium influx is an important mechanism of cell apoptosis [11]. Interestingly, the cellular flux can be regulated by the store-operated calcium channels, namely store-operated calcium entry (SOCE). Increasing evidence suggests that SOCE is extensively involved in the regulation of apoptosis of many non-excited cells, such as neuronal cells [12, 13] and hepatoma cells [14], via regulation of SOCE-related proteins, such as TRPC1, Orai1, and Stim1. However, the reported effects of changes in the expression of SOCE-related proteins on cellular apoptosis have been inconsistent in previous studies. For example, enhanced Orai1 and STIM1 expression as well as SOCE was observed in some cancer cells that are resistant to apoptosis [15, 16], whereas Stim1 and Orai1 were previously identified as pro-proliferative factors in vascular smooth muscle cells [17, 18]. Therefore, the potential roles of these proteins in HG-induced EC apoptosis and their exact roles during the pathogenetic process remain to be determined.
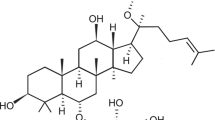
Resveratrol is an anti-inflammatory, antioxidant, and antiapoptotic polyphenol that exhibits many benefits for the cardiovascular system. Previous studies indicated that resveratrol may attenuate HG-related endothelial dysfunction via the regulation of the Akt/endothelial nitric oxide synthase (eNOS) pathway [19]. Moreover, administration of resveratrol may regulate the apoptosis of cancer cells via a SOCE-dependent mechanism [20]. Based on the above facts, we aimed to evaluate the potential effects of resveratrol pretreatment on HG-induced apoptosis in ECs. Moreover, the potential involvement of the SOCE process and changes in SOCE-related proteins were also evaluated.
Materials and methods
Cell culture
Cells of the MS-1 islet microvascular endothelial cell line, which were purchased from the Chinese Academy of Sciences Cell Bank, were cultured in basal medium consisting of Dulbecco’s modified Eagle’s medium (DMEM, Hyklong, USA) with 10% fetal bovine serum (FBS; Gibco, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin under standard conditions (5% CO2 and 37 °C). Cells were synchronized in low-FBS media (0.5%) for 12 h prior to experiments and then exposed to culture medium (CON group) or 30 mmol/L glucose in FBS-containing media for 48 h (HG group). MS-1 cells were first preincubated with 50 µM resveratrol (Sigma, USA) or 100 µM 2-APB (SOC channel inhibitor) for 1 h and then treated with 30 mmol/L glucose (HG + Res) or HG + 2-APB. MS-1 cells were also treated with 50 µM resveratrol alone (Res) or with 2-APB (100 µM).
Cell viability measurement
MS-1 cells were incubated with culture medium or 30 mM glucose for 48 h. Cells were pretreated with either 50 µM resveratrol or 100 µM 2-APB for 1 h. Cell viability was measured with the cell counting kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Briefly, 10 μL CCK8 solution was added to each well of a 96-well plate and incubated for 2 h at 37 °C. The optical density value was measured at an absorption wavelength of 490 nm. Cell viability was calculated and normalized to the control group.
TUNEL staining
MS-1 cells were incubated with culture medium or 30 mM glucose for 48 h. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was used to detect DNA fragmentation in individual cells using a TUNEL fluorescence kit (fluorescein isothiocyanate, FITC) as described previously (Roche, Indianapolis, IN, USA) following the manufacturer’s instructions. Briefly, MS-1 cells grown on coverslips were fixed with 4% paraformaldehyde followed by permeabilization with 0.1% Triton X-100. Then the cells were incubated with the TUNEL reaction mixture at 37 °C for 1 h. The stained cells were examined under a fluorescence microscope (Leica DMI3000 B, Germany).
Dihydroethidium (DHE) staining
Cells were seeded in 6-well plates after 48 h in culture, washed with phosphate-buffered saline (PBS), and mixed with 1 mL DHE probe solution (Sigma) at a concentration of 10 µM in each well. After incubation for 30 min at 37 °C, the cells were washed again with PBS and observed under a fluorescence microscope.
NO detection
NO production was detected by a nitrate reductase assay kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Briefly, the solution (Griess Reagent I 50 µL + Griess Reagent II 50 µL) was added to each well of a 6-well plate and incubated for 5 min at 37 °C. The optical density value was measured at an absorption wavelength of 546 nm. NO production was calculated and normalized to the control group.
Measurement of intracellular Ca2+ ([Ca2+]i)
MS-1 cells were loaded with the calcium indicator Fura-2AM (5 µM) in HEPES-buffered saline. Changes in [Ca2+]i in individual cells were measured using an AquaCosmos system with band-pass filters for 340 and 380 nm. [Ca2+]i was calculated from the Fura-2 fluorescence ratio (F340/F380) using linear regression between adjacent points on a calibration curve generated by measuring F340/F380 in at least seven calibration solutions containing Ca2+ at concentrations ranging between 0 and 854 nM. The store-operated calcium channel (SOCC)-mediated influx of Ca2+ following stimulation with 1 µM thapsigargin (TG, a stimulator of SOCCs) during the change from Ca2+-free conditions to 1.5 mM Ca2+ was measured as described previously [21].
Western blot analyses
Western blot analyses were conducted to measure the SOCE-related protein expression during HG-stimulated apoptosis of ECs. MS-1 cells were collected by centrifugation (700×g, for 10 min at 4 °C) and lysed. Cellular proteins were extracted from MS-1 cells. Total protein concentration was determined by Lowry’s method using bovine serum albumin (BSA) as a standard. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gels. The protein was subsequently transferred onto a polyvinylidenedifluoride membrane by electroblotting for 3 h at 150 mA. Membranes were blocked for 1 h in Tris-buffered saline (TBS)/5% low-fat milk powder and incubated with the primary antibody overnight in TBS/5% BSA or 5% low-fat milk. The secondary antibody, coupled with horseradish peroxidase (HRP), was applied for 1 h at room temperature. Chemiluminescence detection was performed using HRP Juice (PJK) (Thermo, USA) and a CCD camera (Bio-Rad, USA). Densitometric signals were quantified using Quantity One Bioanalysis software (Bio-Rad, Hercules, CA, USA). Anti-TRPC1 (1:1000 dilution), anti-Stim1 (1:1000 dilution), and anti-Orai1 (1:500 dilution) antibodies were purchased from Abcam, and anti-GAPDH (1:1000 dilution) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical analyses
SPSS ver19.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. All experiments were repeated at least thrice. Data are mean ± standard error of the mean (SEM). Groups of data were compared using analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Data that did not follow a normal distribution were analyzed with the Mann–Whitney test or Student’s t test after log normalization. P < 0.05 was considered significant.
Results
Resveratrol inhibited HG-induced apoptosis and NO production in ECs
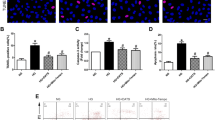
The results of TUNEL analyses are shown in Fig. 1. HG significantly induced apoptosis among MS-1 cells. However, pretreatment with resveratrol (50 μM, 1 h) in HG group significantly attenuated the apoptosis and of the ECs (P < 0.05), suggesting a protective effect of resveratrol against HG-induced EC apoptosis. In addition, resveratrol attenuated HG-mediated NO reduction and the reduction of cell viability. Compared with cells treated with 30 mM HG, the cells incubated with 50 μM resveratrol for 1 h exhibited a significant reversal of NO reduction and cell viability inhibition (P < 0.05; Fig. 1c, d).
Resveratrol suppressed HG-stimulated EC apoptosis and cell viability. a Representative images of TUNEL staining showing apoptotic cells (stained in green). Nuclei were stained blue with DAPI (magnification, ×100). b Quantification of the TUNEL staining. c Quantification of NO production. d Quantification of cell viability. Data are presented as mean ± SEM from five experiments. *P < 0.05 versus control group, #P < 0.05 versus HG-treated group. (Color figure online)
Changes in reactive oxygen species (ROS) levels during HG-induced apoptosis of ECs
The measured cellular ROS levels are shown in Fig. 2. Culture in HG medium was associated with a significant increase in cellular ROS levels in MS-1 cells (P < 0.05). However, this increase in cellular ROS levels was inhibited to control levels in ECs by pretreatment with resveratrol (50 μM, 1 h). These results further confirm the potential antioxidant role of resveratrol in ECs.
Resveratrol significantly suppressed HG-stimulated ROS production. a Representative images of DHE detection of ROS (stained in red, magnification, ×100). b Quantification of ROS levels. Data are presented as mean ± SEM from five experiments. *P < 0.05 versus control group, #P < 0.05 versus HG-treated group. (Color figure online)
Changes in [Ca2+]i during HG-induced apoptosis of ECs
Changes in the cellular [Ca2+]i in ECs from each group are shown in Fig. 3. [Ca2+]i was increased significantly in MS-1 cells after stimulation with TG, even with no Ca2+ present in the culture medium. After Ca2+ was added to the culture medium, HG exposure significantly increased [Ca2+]i in MS-1 cells compared with levels in the control group (P < 0.05). However, 2-APB significantly reversed the HG-induced [Ca2+]i increase. Moreover, 2-APB significantly increased cell viability and reversed HG-induced MS-1 cell apoptosis, indicating that SOCE may be involved in the induction of EC apoptosis by HG.
HG-stimulated apoptosis of ECs via exaggeration of SOCC-mediated [Ca2+]i. a MS-1 ECs were preincubated with HG, 2-APB (a blocker of SOCC), or HG + 2-APB for different time periods. After the specified incubation periods, TG (1 μM)-induced store Ca2+ release and Ca2+ entry were measured. b and c 2-APB significantly suppressed HG-stimulated apoptosis of ECs (magnification, ×100). d Treatment with 2-APB significantly increased cell viability. Data are presented as mean ± SEM from five experiments. *P < 0.05 versus control group, #P < 0.05 versus HG-treated group
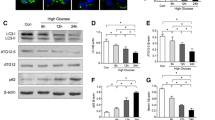
Resveratrol inhibited HG-stimulated [Ca2+]i via suppression of SOCE-related proteins
The changes in [Ca2+]i following resveratrol pretreatment and HG exposure are shown in Fig. 4a. HG significantly increased [Ca2+]i in ECs. However, resveratrol significantly reversed the HG-stimulated [Ca2+]i in ECs, indicating that resveratrol might attenuate HG-induced EC apoptosis via abrogation of [Ca2+]i in ECs. Changes in the expression of SOCE-related proteins are shown in Fig. 4b–d. HG significantly induced EC expression of Orai1, TRPC1, and Stim1, and pretreatment with resveratrol significantly reversed the upregulation of Orai1 and TRPC1. However, Stim1 protein expression was not significantly affected by resveratrol pretreatment. Taken together, these results indicate that resveratrol might inhibit the HG-induced [Ca2+]i in ECs at least partly via regulation of the expression of SOCE-related proteins, especially TRPC1 and Orai1.
Resveratrol inhibited HG-stimulated [Ca2+]i via suppressing the expression of TRPC1 and Orai1. a Arrow indicates time at which the cells were stimulated with TG. Data are presented as mean ± SEM from five experiments. b and c Resveratrol significantly reversed HG-induced expression of TRPC1 and Orai1. d Resveratrol had no effect on the expression of total Stim1. Representative plot is shown from five independent experiments. *P < 0.05 versus control group, #P < 0.05 versus HG-treated group
Discussion
In the present study, we demonstrated that HG induced apoptosis among ECs via stimulation of a SOCE-related increase in [Ca2+]i, as well as excessive ROS production and NO reduction. Interestingly, pretreatment with resveratrol attenuated the HG-induced EC apoptosis via restoration of cellular calcium homeostasis and expression of SOCE-related proteins, such as TRPC1 and Orai1 (supplemental figure). These results suggest that the SOCE-related increase in [Ca2+]i may be an important underlying mechanism of the pathogenesis of DM-induced EC apoptosis. Moreover, resveratrol may inhibit HG-induced EC apoptosis via regulation of SOCE-related protein expression, indicating the potential protective role of resveratrol against DM-related vascular complications.
Increased oxidative stress is a key contributor to the development and progression of EC apoptosis induced by HG. Accumulating evidence has demonstrated that HG-related ROS production may contribute to cellular DNA damage and apoptosis [22]. We demonstrated that resveratrol may inhibit the production of ROS induced by HG, which is consistent with the general antioxidative characteristics of resveratrol. These results suggest that the benefit of resveratrol on EC apoptosis may be related to its antioxidative effect.
Hyperglycemia due to DM has emerged as a major problem that threatens health and causes vascular dysfunction [22]. As indicated by previous studies, HG (30 mM glucose, for 24 h) has direct toxicity to human umbilical vein ECs [23]. In many chronic metabolic diseases, vascular endothelial integrity is affected by EC proliferation and apoptosis, which assures blood vessel function. The present study showed that HG significantly reduced cell viability and resveratrol abolished these effects. Therefore, restoration of injured ECs via regulation of EC proliferation and apoptosis may be of significance.
Intracellular Ca2+, as a secondary messenger, regulates both cell survival and a massive increase in intracellular Ca2+, which can cause cell apoptosis [24]. The present study suggests that HG induces apoptosis of ECs by causing intracellular Ca2+ overload via the regulation of a SOCE-related increase in intracellular Ca2+ and expression of SOCE-related proteins. This is consistent with the previous finding that SOCE-related calcium regulation may be an important mechanism for determination of the fate of non-excited cells, such as proliferation, apoptosis, and autophagy [25,26,27]. In view of the previous evidence that resveratrol may interact with SOCE-related proteins, we hypothesized that the regulation of SOCE and intracellular calcium levels may be involved in the benefits of resveratrol against HG-induced EC apoptosis. When resveratrol was added together with Ca2+, we did not observe the inhibition of SOCE, suggesting that resveratrol did not function by inhibiting the Ca2+ channel pore. However, increasing the preincubation time with resveratrol before Ca2+ addition did not inhibit SOCE unless resveratrol was added together with TG shortly before triggering the emptying of Ca2+ stores [28], which is consistent with our results. Subsequently, we found that resveratrol had no effects on the expression of total Stim1, but the HG-stimulated Orai1 and TRPC1 upregulation was significantly abrogated by resveratrol pretreatment. Previous evidence confirmed that SOCE is activated by depletion of Ca2+ stores in the endoplasmic reticulum (ER) and is mediated essentially by two classes of proteins, Stim and Orai [29]. Orai1 is a four-transmembrane-spanning domain protein that forms a pore through the coordination of four subunits that acts as a Ca2+ release-activated Ca2+ channel (CRAC). Orai1 silencing decreases the probability of SOC activation. Orai1 may be an essential subunit of the transient receptor protein channel (TRPC) that is required for TRPC proteins to sense calcium store depletion and, hence, to be store-operated [21]. In the present study, Orai1 and TRPC1 expression were significantly suppressed by resveratrol, which may partly explain the mechanism of Ca2+ influx.
Conclusion
Our experiments indicated that resveratrol pretreatment is associated with relieved HG-induced EC apoptosis at least partly via inhibition of SOCE-related proteins, suggesting the potential protective role of resveratrol against DM-related vascular complications. Further in vivo studies are needed to confirm our results.
References
Mylroie H, Dumont O, Bauer A, Thornton CC, Mackey J, Calay D, Hamdulay SS, Choo JR, Boyle JJ, Samarel AM (2015) PKCε-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc Res 106:509–519
Liu ML, Williams KJ (2012) Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes 19:121–127
Rautou P-E, Vion A-C, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM (2011) Microparticles, vascular function, and atherothrombosis. Circ Res 109:593–606
Bornfeldt KE (2014) 2013 Russell Ross memorial lecture in vascular biology: cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Arterioscler Thromb Vasc Biol 34:705–714
Chait A, Bornfeldt KE (2009) Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res 50(Suppl):S335
Hayashi T, Yamaguchi T, Sakakibara Y, Taguchi K, Maeda M, Kuzuya M, Hattori Y (2014) eNOS-dependent antisenscence effect of a calcium channel blocker in human endothelial cells. PLoS ONE 9:e88391
Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A (2001) Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 281:E924
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A (2003) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52:2795–2804
Takeuchi A, Kim B, Matsuoka S (2013) The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci Rep 3:2766
Muik M, Schindl R, Fahrner M, Romanin C (2012) Ca2+ release-activated Ca2+ (CRAC) current, structure, and function. Cell Mol Life Sci 69:4163–4176
Murphy MP (2012) Modulating mitochondrial intracellular location as a redox signal. Sci Signal 5:39
Zhang M, Song J-N, Wu Y, Zhao Y-L, Pang H-G, Fu Z-F, Zhang B-F, Ma X-D (2014) Suppression of STIM1 in the early stage after global ischemia attenuates the injury of delayed neuronal death by inhibiting store-operated calcium entry-induced apoptosis in rats. NeuroReport 25:507–513
Rao W, Zhang L, Su N, Wang K, Hui H, Wang L, Chen T, Luo P, Y-f Yang, Z-b Liu (2013) Blockade of SOCE protects HT22 cells from hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun 441:351–356
Liu H, Jia X, Luo Z, Guan H, Jiang H, Li X, Yan M (2012) Inhibition of store-operated Ca2+ channels prevent ethanol-induced intracellular Ca2+ increase and cell injury in a human hepatoma cell line. Toxicol Lett 208:254–261
Li W, Zhang M, Xu L, Lin D, Cai S, Zou F (2013) The apoptosis of non-small cell lung cancer induced by cisplatin through modulation of STIM1. Exp Toxicol Pathol 65:1073–1081
Schmidt S, Liu G, Liu G, Yang W, Honisch S, Pantelakos S, Stournaras C, Hönig A, Lang F (2014) Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget 5:4799–4810
R-w Guo, L-x Yang, M-q Li, X-h Pan, Liu B, Y-l Deng (2012) Stim1-and Orai1-mediated store-operated calcium entry is critical for angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res 93:360–370
Guo R-W, Wang H, Gao P, Li M-Q, Zeng C-Y, Yu Y, Chen J-F, Song M-B, Shi Y-K, Huang L (2008) An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc Res 81(4):660–668
Taguchi K, Hida M, Matsumoto T, Kobayashi T (2015) Resveratrol ameliorates clonidine-induced endothelium-dependent relaxation involving Akt and endothelial nitric oxide synthase regulation in type 2 diabetic mice. Biol Pharm Bull 38:1864–1872
Selvaraj S, Sun Y, Sukumaran P, Singh BB (2015) Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog
Saul S, Stanisz H, Backes CS, Schwarz EC, Hoth M (2014) How ORAI and TRP channels interfere with each other: interaction models and examples from the immune system and the skin. Eur J Pharmacol 739:49–59
Safi SZ, Qvist R, Yan GOS, Ismail ISB (2014) Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Med Genomics 7:29
Scheen AJ, Esser N, Paquot N (2015) Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabetes Metab 41:183–194
Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H (2015) MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep 5:9401
Sun Y, Cui X, Wang J, Wu S, Bai Y, Wang Y, Wang B, Fang J (2015) Stromal interaction molecule 1 (STIM1) silencing inhibits tumor growth and promotes cell cycle arrest and apoptosis in hypopharyngeal carcinoma. Med Oncol 32:608
Raphaël M, Lehen’Kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden AF, Farsetti L, Germain E, Bokhobza A, Mihalache A (2014) TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci USA 111:3870–3879
Liu H, Hughes JD, Rollins S, Chen B, Perkins E (2011) Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol 91:753
Casas-Rua V, Alvarez IS, Pozo-Guisado E, Martín-Romero FJ (2013) Inhibition of STIM1 phosphorylation underlies resveratrol-induced inhibition of store-operated calcium entry. Biochem Pharmacol 86:1555–1563
Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC (2013) STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol 305:446–458
Acknowledgements
We thank Fang LI for technique assistance in measuring intracellular Ca2+. The study was supported by Chongqing characteristics of specialist construction funds.
Author information
Authors and Affiliations
Contributions
Qiang Xu conceived of the study. Ting Lu carried out cell culture and Western blotting analysis, participated in TUNEL staining, and measurement of intracellular Ca2+. Dayan Zhou performed the statistical analysis. Pan Gao drafted the manuscript. Liangyi Si participated in the design of the study. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2017_3194_MOESM1_ESM.tif
Supplemental Fig. 1 Scheme image of the signaling mechanisms underlying inhibitory effect of resveratrol for high glucose-induced endothelial cell apoptosis via mediation of store-operated calcium entry. Calcium ion receptor (Stim1), which locates in the endoplasmic reticulum, was found to form Ca2+ channel with Orai1 when the TG was induced to release Ca2+ from the endoplasmic reticulum. High-glucose stimulates apoptosis of endothelial cells via induction of calcium influx through the activation of the SOC channel. Resveratrol functions via inhibiting the activation of the SOC channel to reduce calcium influx, and also via reducing the high glucose-induced reactive oxygen species generation, thereby reducing endothelial cell apoptosis. Supplementary material 1 (TIFF 12837 kb)
Rights and permissions
About this article
Cite this article
Lu, T., Zhou, D., Gao, P. et al. Resveratrol attenuates high glucose-induced endothelial cell apoptosis via mediation of store-operated calcium entry. Mol Cell Biochem 442, 73–80 (2018). https://doi.org/10.1007/s11010-017-3194-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3194-7