Abstract
The present study was aimed to investigate the protective effects of 17β-estradiol (E2) and estrogen receptor α (ERα) on isoproterenol (ISO)-treated H9c2 cardiomyoblast cells. In the present study, we treated H9c2 cells with ISO, a β-adrenergic receptor agonist, to induce myocardiac hypertrophy. Pre-administration of E2 or ERα (induced by doxycycline) and E2 plus ERα significantly prevented ISO-induced increase of cell size and cytosolic calcium accumulation, accompanied with increased mRNA of atrial natriuretic peptide and brain natriuretic peptide. However, ICI-ERs antagonist, and melatonin, a specific inhibitor for ERα, reversed the cardioprotective effects, suggesting that E2 action was mediated through ERα. Further evidences showed that E2 and ERα increased the protein level of GSK3β and protein phosphatase 2a inhibitor 2 (I2-PP2A), which subsequently enhanced the activation of I2-PP2A by disrupting PP2A activity and maintains normal calcium outflow. Collectively, E2 and ERα inhibited hypertrophy by preventing cytosol calcium accumulation and by inhibiting the association between PP2A with Na+–Ca2+ exchanger via GSK3β and I2-PP2A activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is one of the leading causes of death in the world, with a 5-year survival rate of approximately 50%. Heart failure is the final stage of many human heart diseases; however, the molecular mechanisms behind this progression remain incompletely understood [1]. Moreover, studies indicate that the prevalence of left ventricle hypertrophy (LVH) is lower in women than in age-matched men. The incidence of LVH increases progressively with age and becomes more common in women after menopause [2]. Left ventricular hypertrophy is intervened not only by the mechanical stress of pressure overload, but also by various neurohormonal substances such as angiotensin II, aldosterone, norepinephrine, and insulin that independently promote myocyte hypertrophy in the heart [3,4,5].
β-Adrenergic receptor plays an major role in cardiac remodeling, which is considered to be an important pathological process in various heart diseases [6]. β-Adrenergic receptor is the major subtype (90%) of adrenoceptors in heart [7]. Isoproterenol (ISO) is a synthetic catecholamine and β-adrenergic agonist that induces apoptosis of cardiac myocytes via the calcineurin pathway [8, 9]. Activation of the β-adrenogenic receptor increases heart rate and contractile force via coordinated post-translational modifications of the L-type Ca2+ channel, Na+–Ca2+ exchanger (NCX), RyR2, phospholamban (PLB), and phosphatases PP1 and PP2A [10,11,12,13,14]. Type 1 protein phosphatase PP1 and type 2 phosphatase PP2A were found to down-regulate sarcoplasmic reticulum (SR) Ca2+ release and contractile performance [15,16,17]. In particular, protein phosphatase 2a (PP2A) has been recognized as one of the major phosphatases in cardiac tissues associated with the protein contraction machinery under β-adrenergic receptor stimulation [18]. PP2A is a ubiquitous and heterotrimeric protein phosphatase involved in the regulation of cell growth, apoptosis, and numerous cell signaling pathways in cardiac myocytes [19]. Similarly, the calcium-dependent phosphatase calcineurin (PP2B) dephosphorylates NFAT3 and enables its translocation to the nucleus, where it participates in the development of myocardial hypertrophy [20, 21]. Moreover, a number of studies have found that GSK3β plays a crucial role in cardiac hypertrophy through the phosphorylation and inhibition of NFATs, GATA4, etc. [22]. In transgenic mice, calcineurin-induced NFAT nuclear translocation was suppressed by GSK3β activation [23]. Similarly, in another experiment, GSK3β was found to regulate calcium flow in the heart by maintaining its diastolic and systolic function [24].
17β-Estradiol (E2) is the most abundant and most active estrogen in women [25], and it regulates various physiological effects by binding to estrogen receptor α (ERα) [26]. Due to estrogen (E2) levels, reduced incidence of cardiovascular disease was found in females [27, 28]. E2 plays an important role in the pathogenesis of heart disease, and it is able to modulate the progression of cardiovascular disease [29]. Furthermore, E2 has been shown to reduce pathological cardiac hypertrophy and heart failure by up-regulating the PI3K/Akt signaling pathway and by suppressing the calcineurin/NFAT3 signaling pathway in female rats [30]. E2 binds with ERα and stimulates cell proliferation by up-regulating cell cycle and anti-apoptotic proteins [31, 32]. In another study it was found that E2 and ERβ significantly inhibit Ang-II-induced cardiac hypertrophy by multiple novel mechanisms [33]. In hepatocellular carcinoma, E2 binds to ERα and blocked its metastatic function [34]. In this present study, we investigated the cardioprotective effect of E2 and ERα, in ISO-induced H9c2 cardiomyoblast cells.
Materials and methods
Cells, antibodies, reagents, and enzymes
All the chemicals used were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) unless stated otherwise. Heart-derived H9c2 cardiomyoblast cells were obtained from the American Tissue Culture Collection. Tet-on/ERα H9c2 cardiomyoblast cells were generated as previously described [35] and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 1 mM HEPES buffer, and 10% Clontech fetal bovine serum in humidified air (5% CO2) at 37 °C. Rhodamine–phalloidin was purchased from Molecular Probes (Eugene, Oregon, USA). The ER antagonist ICI 182780 (ICI) was purchased from Tocris (Ellisville, Missouri, USA).
The Alexa Fluor 568 rabbit anti-goat IgG (H+L) secondary antibody was purchased from Invitrogen (Carlsbad, California, USA). Antibodies against atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), I2-PP2A, PP2A, SERCA, NCX1, p-I-1(Thr34), (Thr75), I-1, GSK3β, and HDAC were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). α-Tubulin was purchased from Lab Vision Corporation (Fremont, California, USA). GAPDH, p-TnI (Ser23/24), protein phosphatase 1 (PP1), and NFAT3 were purchased from Cell Signaling (Danvers, MA, USA). Phosphorylated PLB (p-PLB) and PLB were purchased from Upstate (Lake Placid, NY, USA). Goat anti-mouse IgG antibody conjugated to horseradish peroxidase, goat anti-rabbit IgG antibody conjugated to horseradish peroxidase, and goat anti-rabbit IgG horseradish peroxidase conjugate were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA).
Construction of the pEGFP-C1-PP2Ac expression plasmid
The DNA sequence of PP2Ac was provided by Dr. Hsien-Bin Huang. The full length cDNA of PP2Ac (930 bp) was obtained by polymerase chain reaction (PCR) amplification with primers. The contents of the PCR reaction mixture and the condition of the PCR reaction are listed in Table 1. The PCR products were digested with the restriction enzyme EcoR1 at the 5′-end and Xba1 at 3′-end. The restricted fragment was extracted with the Gel/PCR DNA fragments extraction kit and then ligated with the large EcoR1–Xba1 fragment of pEGFP-C1 by T4 DNA ligase (16 °C for 4 h). Thus, the PP2Ac gene was inserted into the pEGFP plasmid, which carried a C-terminal GFP, and was named pEGFP-PP2Ac.
Transient transfection of the pEGFP-C1-PP2Ac plasmid
pEGFP-C1 and pEGFP-PP2Ac were prepared using the AxyPrep™ Plasmid Maxiprep kit (Axygen Biosciences, CA, USA) and transfected into the cells using Lipofectamine 2000 transfection reagent (Invitrogen, NY, USA) according to the manufacturer’s guidelines. The culture medium was replaced, and the cells were harvested at the indicated experimental time points.
Actin staining
Tet-on/ERα H9c2 cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 1% BSA in phosphate-buffered saline (PBS) for 10 min. Actin filaments were stained using rhodamine-labeled phalloidin (red) for 40 min and washed with PBS. Cells were examined and photographed using a fluorescence microscope, and the cell area was measured using Carl Zeiss AxioVision LE software. More than 10 fields for each condition were quantified, and independent experiments were performed in triplicate.
Immunoprecipitation
Cell extracts were prepared in modified radio-immunoprecipitation (IP) assay buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 1:200-diluted protease inhibitor cocktail (Sigma, MO, USA), 1 mM PMSF, and 10 mM NEM]. IP with anti-NCX1 antibody or anti-GSK3β antibody was performed as described previously [36].
Western blot
Proteins were separated using 10–12% SDS-PAGE and then transferred to PVDF membranes for 2 h at 100 V. PVDF membrane was then incubated in blocking buffer (5% milk in 1× TBS) for 1 h at room temperature. The membranes were incubated with the primary antibodies at the recommended concentrations at 4 °C overnight and then incubated with the secondary antibodies for 1 h at room temperature. The results were visualized with chemiluminescent HRP substrate (Millipore, MA, USA).
Nuclear extraction
Cells were gently scraped by adding ice-cold BUFFER-I [10 mM HEPES (pH 8.0), 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol, and proteinase inhibitor cocktail (Roche Molecular Biochemicals)] and incubated for 15 min on ice, followed by the addition of 20 μl IGEPAL CA-630. After vortexing for 10 s, cells were centrifuged at 12,000×g for 5 min at 4 °C, the supernatant (cytoplasmic fraction) was carefully aspirated, and the pellet was suspended in ice-cold BUFFER-II [20 mM HEPES (pH 8.0), 1.5 mM MgCl2, 25% glycerol, 420 mM NaCl, 0.2 mM EDTA, 1 mM dithiothreitol, and proteinase inhibitor cocktail (Roche Molecular Biochemicals)] and vortexed vigorously. After vortexing, the suspension was placed on ice for 30 min before centrifuging at 15,000×g for 15 min at 4 °C. The supernatant (nuclear extracts) was stored in aliquots at −80 °C.
Immunofluorescence staining
Cells grown in 24-well plates and subjected to various treatments were subsequently washed five times with ice-cold PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. Cells were washed with ice-cold PBS and permeabilized with 0.5% Triton X-100 for 10 min at 4 °C. Non-specific binding of the fixed cells was blocked with PBS containing 2% bovine serum albumin at 37 °C for 30 min, followed by incubation with the primary antibody overnight at 4 °C. After washing, cells were incubated with second antibody at 37 °C for 1 h. Fluorescence was visualized using a fluorescence microscope coupled to an image analysis system.
Intracellular calcium staining
Ca2+ was determined as previously described [37]. Tet-on/ERα H9c2 cells were incubated with serum-free DMEM containing 2 μM Fluo-4 and AM ester (Invitrogen) at 37 °C. Cells were incubated for 30 min and washed twice with PBS before imaging.
Intracellular calcium concentration detection
A flow cytometric assay was used to detect intracellular calcium accumulation in Tet-on/ERα H9c2 cells. Following treatment, cells were trypsinized, washed, and loaded with 4 µg/ml Fluo-3 AM and 10 µg/ml Fura-2/AM u for 30 min at 37 °C. Cells were centrifuged at 180×g for 6 min, and pellets were suspended in HBSS and analyzed using Cytomics TM FC500 flow cytometry. Increases (Fluo-3) and decreases (Fura Red) in fluorescence levels were then recorded for at least 500 s, collecting approximately 100 events per sample.
Total RNA extraction
Total RNA was extracted using the Ultraspec RNA isolation system (Biotecx Laboratories, Houston, Texas, USA) according to directions supplied by the manufacturer. Cardiomyocytes were thoroughly homogenized (1 ml Ultraspec reagent) with a homogenizer in a polypropylene tube, and total RNA was isolated using a standard method. The RNA precipitate was washed twice by gentle vortexing with 70% ethanol, collected by centrifugation at 12,000×g, dried under a vacuum for 5–10 min, dissolved in 50 μl of diethyl pyrocarbonate-treated water, and incubated for 10–15 min at 55–60 °C. The RNA was quantified and checked for purity and condition by spectrophotometry at a wavelength of 260 nm. The extract integrity was assessed by 1.5% agarose gel electrophoresis, and RNA was visualized by ethidium bromide staining.
RT-PCR condition
We first used the NCBI database to search the CDS sequences of our target genes. We then used the Primer3 program to design forward and reverse DNA primers. For RT-PCR, 4 µg of total RNA were reverse transcribed using gene specific primers and SuperScript III reverse transcriptase (Invitrogen), a fraction of the RT reaction products were used in subsequent PCR reactions. Finally, RT-PCR products were stored at −80 °C. Reactions were incubated at 42 °C for 5 min, followed by 42 °C for 1 h, and PCR amplification was carried out after denaturing at 95 °C for 5 min. Finally, RT-PCR products were stored at −80 °C.
Statistical analysis
Data are reported as the mean ± SD. Differences were analyzed with one-way or two-way analysis of variance with repeated measures, followed by Tukey’s multiple comparison test.
Results
ISO enhances the H9c2 cardiomyoblast cell hypertrophy in a time-dependent manner
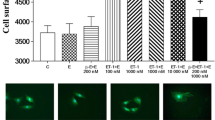
We investigated the ability of ISO to induce hypertrophy we used Tet-on/ERα H9c2 cells. H9c2 cells were starved for 12 h and then treated cells with ISO (50 μM) for 2, 12, 24, and 36 h. Actin filaments were stained, and cell size was analyzed using fluorescence microscopy. The results showed that ISO-treated cells became larger in a time-dependent manner (Fig. 1a, b).
ISO increases the cell size of H9c2 in a time-dependent manner. a Tet-on/ERα H9c2 cardiomyoblast cells were incubated with or without ISO (50 µM) for the indicated times (2, 12, 24, and 36 h) and followed by fluorescent microscope detection. The cells were then fixed and stained with rhodamine-conjugated phalloidin to detect the actin filaments. b Data were quantified by densitometry and are presented as mean ± SD. Statistical significance # p < 0.05, ## p < 0.01, ### p < 0.005, significant differences from control group
To test whether E2 and its receptor (ERα) are important in regulating cardiovascular system [38], we used Tet-on/ERα H9c2 cells. Cells were treated with E2 and/or Dox in the presence or absence of ISO for 24 h and analyzed for cell surface area. Pre-treatment with E2, Dox, or E2 plus Dox effectively inhibited ISO-induced cell surface area in cardiomyoblast cells (Supplemental Fig. 1a, b). To further confirm whether E2 and/or ERα are involved in blocking ISO-induced hypertrophic growth, we treated with ICI to inhibit ER expression. The effects of E2 and the ER on ISO-treated H9c2 cells were effectively blocked by the ER antagonist ICI (Supplemental Fig. 1a, b). Taken together, these data indicate that ISO-induced increases in H9c2 cell size were significantly blocked by E2 and/or ERα overexpression in vitro.
ISO-induced hypertrophic gene expression is regulated by E2 and ERα in H9c2 cells
To confirm the above results we treated H9c2 cells with E2 and ERα and then analyzed for hypertrophic maker BNP mRNA expression. ISO treatment increased BNP mRNA expression was significantly decreased by E2 and/or ERα administrations. However, this decreased expression was blocked by ERs inhibitor, ICI 182780 (ICI; Supplemental Fig. 2).
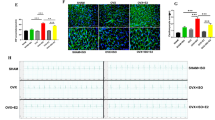
PP2A expression has a co-relationship with myocardial infarction
Previous studies have shown that treatment with β-adrenergic receptor agonists increased PP2A activity in ventricular myocytes [39]. Therefore, we screened for PP2A expression in human heart tissue using a tissue array. As shown in Fig. 2a, PP2A expression was increased in three groups (acute infarction, granulation tissue, and myocardial scar) compared with the control group. To confirm these results, Tet-on/ERα H9c2 cells overexpressing PP2A and treated with E2, Dox, or E2 plus Dox were analyzed with immunofluorescence. Consistent with the tissue array data obtained from H and E staining, E2, Dox, or E2 plus Dox treatment decreased PP2A protein expression levels (Fig. 2b).
E2 and/or ERα inhibit PP2A-induced myocardiac hypertrophy response. Detection of PP2A expression a human heart tissue samples, normal (N), acute infarction (I), granulation tissue (G), and myocardial scar (S) were analyzed for PP2A expression by immunohistochemistry staining. b Tet-on ERα/H9c2 cardiomyoblast cells were transiently transfected with PP2A–GFP and then treated with Dox (1 μg/ml) and/or E2 (10−8 M) for 24 h. Cell were further stained for actin and DAPI staining and then observed under confocal microscope
PP2A participation in ISO-induced cardiac hypertrophy was prevented with E2 and ERα treatment
To understand the functional role of PP2A in the hypertrophic signaling pathway, H9c2 cells were transfected with a vector control or with PP2Ac siRNA and then treated with or without ISO for 24 h. As shown in Fig. 3a, ISO treatment induced PP1 and PP2A expression levels was reduced in siPP2Ac-transfected cells. Silencing PP2Ac expression indeed reduced ANP and BNP mRNA expression in H9c2 cells (Fig. 3b).
Protein phosphatases participate in ISO-induced myocardiac hypertrophy response. Tet-on/ERα H9c2 cardiomyoblasts transfected with non-target or PP2Ac siRNA were incubated with or without ISO (50 µM) for 24 h post-transfection. a PP1 and PP2A, b ANP and BNP mRNA expression were detected by RT-PCR using specific primers. GAPDH was used as an internal control. c H9c2 cells were pre-treated with or without tautomycin (2.5 µM), cyclosporine A (100 nM), and okadaic acid (100 nM) for indicated time and then incubated with ISO (50 µM) for 24 h. BNP and α-tubulin were detected using western blotting; α-tubulin was used as an internal control. The statistical results are shown from three independent experiments; mean ± SD. p < 0.05, **p < 0.01 significant differences from ISO group. # p < 0.05, ### p < 0.005 significantly different from the control group
To further understand the roles of PP1 and PP2A in hypertrophy, H9c2 cells were blocked with tautomycin (PP1 inhibitor), cyclosporine A (PP1 and PP2A inhibitor), or okadaic acid (PP2A inhibitor), and BNP expression was analyzed using western blotting. ISO-induced BNP expression was greatly decreased in OA-treated H9c2 cells (Fig. 3c). Thus, we concluded that activation of PP2A contributed to increased hypertrophic protein expression in ISO-stimulated H9c2 cells.
ISO down-regulates calcium recycling in SR through the PLB protein de-phosphorylation
Previous results showed that high dosage of ISO not only increases intracellular calcium concentration but also increases calcium accumulation in the cytosol. In order to reveal the mechanism, we pre-treated Tet-on ERα H9c2 cells with estrogen and/or Dox and then incubated with ISO for 24 h to check the changes of calcium channel protein. In the Fig. 4a, we observed that even under PP2A stimulation, SERCA and NCX1 calcium channel protein expression has no change. Furthermore, we treated Tet-on ERα H9c2 cells with different dosages of ISO and then incubated for 12 h to detect whether the calcium transport-related proteins were affected. Result showed that p-PLB increases as the dosage of ISO increases from 5 to 15 μM. The PLB protein is a regulatory protein that responds for regulating sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). When PLB is phosphorylated, it has the most activity to promote SERCA to recycle cytosolic calcium back to SR. In contrast, the non-p-PLB closely binds and inhibits SERCA activity, resulting in the calcium accumulation in cytosol.
ISO induces PP2A via I2-PP2A inhibition was blocked by E2 and ERα, but E2 and ERα show no effects on the expression of calcium channel proteins, SERCA and NCX1. Tet-on ERα/H9c2 cardiomyoblasts were pre-treated with E2 (10−8 M) and/or Dox (2 µg/ml) and then treated with ISO (50 µM). Protein lysates were prepared and analysed for I2-PP2A, PP2A, SERCA, and NCX1 expression using western blotting. α-Tubulin was used as an internal control
E2 and/or ERα suppress ISO-induced PP2A via I2-PP2A induction
According to the co-IP experiment, we found that ERα binds with GSK3β protein. This protein–protein interaction effect is blocked by ISO, but restored by E2 and/or ERα treatment. However, the binding ability of ERα and GSK3β is blocked by ERα-specific inhibitor—melatonin treated (Fig. 5a). ISO induces that PP2A bind with NCX1 to regulate intracellular calcium concentration. But pre-treatment with E2, ERα, or E2 plus ERα totally reduces this protein–protein interaction effect (Fig. 5a). On the other hand, ISO treatment reduces both GSK3β and I2-PP2A protein levels and this was further inhibited by E2 and/or ERα treatment. However, this was blocked by ERs inhibitor—ICI (Fig. 5b). Together, all these data indicate that ERα binds with GSK3β and involves I2-PP2A activation.
E2 and ERα enhance GSK3β via protein and protein interaction to induce I2-PP2A and in the inhibition of PP2A and NCX1 protein interaction. Tet-On ERα/H9c2 cardiomyoblasts were incubated with E2 and/or Dox (1 µg/ml), with or without ISO (50 µM) and Melatonin for 24 h. a The cell lysis was first immunoprecipitated by GSK3β antibody and then immunoblot for ERα and GSK3β expression. b Tet-On ERα/H9c2 cardiomyoblasts were incubated with E2 and/or Dox (1 µg/ml), with or without ISO (50 µM) and ICI for 24 h. Cell lysates were collected and then analysed for GSK3β and I2-PP2A expression. α-tubulin was used as an internal control. *p < 0.05, ***p < 0.005 significant differences from ISO treated group. Data were quantified by densitometry and are presented as mean ± SD Statistical significance: # p < 0.05, ## p < 0.01, ### p < 0.005 significant differences from control group
E2 and/or ERα inhibit ISO-induced calcium accumulation in the cytosol of H9c2 cells
In order to observe the distribution of intracellular calcium, we use the specific calcium indicator: Fluo-4 AM. It is used to perform calcium stain for observing the distribution of intracellular calcium by confocal microscope and fluorescent microscope (Fig. 6a, b). The ×400 visions of fluorescent microscope demonstrate that ISO treatment for 12 h induces calcium accumulation in the cytosol of H9c2 cell. And pre-treatment of estrogen and/or ERα effectively prevents ISO-induced calcium accumulation (Fig. 6b). However, pre-treatment of ICI significantly blocks the ability of estrogen and/or ERα in preventing calcium accumulation.
E2 and/or ERα reduced ISO-induced calcium accumulation in H9c2 cardiomyoblast cells. Tet-on/ERα H9c2 cardiomyoblast cells were incubated with Dox (1 µg/ml) and E2 (10−8 M) in presence or absence of ISO (50 µM) and ICI (2 µM) for 12 h, and followed by calcium staining. a The different visions were detected by confocal microscope and b small vision was detected by fluorescent microscope
In the observations of confocal microscope, we use two different filters to get more detailed results, where the color of fluorescence of intracellular calcium changes with the intensity of Fluo-4 from black to yellow which means low intensity to high intensity (Fig. 6a). ISO treatment significantly increases the intensity of fluorescence that was decreased by estrogen and/or ERα treatment. Furthermore the preventive ability of E2 and ERα was blocked by ICI treatment.
To confirm the calcium staining results obtained in a previous experiment, we detected intracellular calcium with flow cytometry. ISO-treated cells showed increased calcium levels compared with the control group. Combined E2 and Dox pre-treatment decreased the calcium levels in ISO-treated H9c2 cells. The effects of E2 and ERα on calcium accumulation were significantly reduced by ICI treatment (Supplemental Fig. 3).
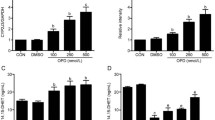
ISO-induced PP1 and PP2A are down-regulated by E2 and/or ERα in H9c2 cells
Previous studies show that ISO is able to induce the PP1 overexpression and regulate downstream signal pathway [40]. In the present study, we show that pre-treatment of estrogen and/or ERα not only effectively reduced ISO-induced PP1 expression but also reduced ISO-induced PP2A expression. E2 and ERα effects on ISO-treated cells were further blocked by ICI treatment (Supplemental Fig. 4a, b). However, the expression of p-PLB significantly decreased when cells were treated with greater than 15 μM ISO (Supplemental Fig. 4b).
E2 and/or ERα inhibit ISO-induced PP1 to stabilize PLB activity via enhanced p-Thr34-I-1 and reduced p-Thr75-I-1
Inhibition of both calcineurin and PP2A was associated with increased Ser16 phosphorylation of PLB, which is controlled by PP1, and thus represents an indirect downstream target of activated I-1 [41]. I-1 becomes active upon phosphorylation of Thr35 by cAMP-dependent protein kinase (PKA), and this activation results in the inhibition of PP1 expression. Therefore, to test this hypothesis, we treated cells with ISO and then analyzed PP1, p-PLB, and p-I-1 expression. The ISO-induced increase in PP1 expression and decreases in p-PLB and p-Thr34-I-1 expression were reversed by E2, Dox, and E2 plus Dox treatment. Notably, another novel phosphorylation site (Thr75) on I-1 has been identified, but phosphorylation at this site has contrary functions to p-Thr34-I-1. The quantification of I-1, p-Thr34-I-1, and p-Thr75-I-1 expression demonstrated the following: (a) the expression levels of p-Thr34-I-1 and p-Thr75-I-1 are inversely related following ISO stimulation; (b) estrogen and ERα inhibit PP1 expression through p-Thr34-I-1 activation and p-Thr75-I-1 inhibition. The ISO-induced increase in PP1 expression and suppression of p-PLB activation was completely restored by E2 and/or ERα treatment (Fig. 7a, b).
E2 and/or ERα inhibit ISO-induced PP1 to stabilize PLB activity through enhanced p-Thr34-I-1 and reduced p-Thr75-I-1. Tet-on/ERα H9c2 cardiomyoblasts were incubated with Dox (1 µg/ml), E2 (10−8 M), or Dox (1 µg/ml) plus E2 (10−8 M) in presence or absence of ISO (50 µM) for 24 h and then analyzed for PP1, p-PLB, p-Thr34-I-1, p-Thr75-I-1, I-1 (total) using western blotting. The statistical results are shown from three independent experiments; mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.005, significant differences from ISO group. # p < 0.05, ## p < 0.01, ### p < 0.005 significant differences from control group
ERα modulates I2-PP2A through GSK3β
To further confirm the role of E2 and ERα in controlling GSK3β-mediated calcium channel signaling, we inhibited GSK3β expression by incubating the cells with LiCl plus ISO and analyzed GSK3β, p-PLB, and NFAT3 expression. Our results indicate that LiCl plus ISO treatment decreased GSK3β and p-PLB expression and increased NFTA3 expression, and these changes in expression were reversed in the E2, Dox, and E2 plus Dox-treated groups (Fig. 8a).
E2 regulated phosphorylated PLB through GSK3β. a Tet-on ERα/H9c2 cardiomyoblasts were pre-treated with LiCl (10 M), Dox (2 µg/ml), E2 (10−8 M) or Dox (2 µg/ml) plus E2 (10−8 M) in presence or absence of ISO (50 µM) for 24 h. GSK3β, I2-PP2A, and α-tubulin were detected by immunoblot analysis using specific antibodies. b Tet-on/ERα H9c2 cardiomyoblasts were incubated with Dox (1 µg/ml), E2 (10−8 M) or Dox (1 µg/ml) plus E2 (10−8 M) in presence or absence of ISO (50 µM) for 24 h. To extract cytosolic and nuclear proteins by nuclei extraction, NFAT3, HDAC1 and α-tubulin were detected by immunoblot analysis. The statistical results were shown from three independent experiments; mean ± SD, n = 3 (*p < 0.05 significant differences from ISO group, # p < 0.05, ## p < 0.01, significant differences from control group)
In our previous study, we demonstrated that LPS induced myocardial hypertrophy through the calcineurin/NFAT3 signaling pathway in H9c2 cells [42]. In the present study, nuclear translocation of NFAT3 was found in LiCl plus ISO-treated cardiomyoblast cells. In contrast, pre-treatment with E2, Dox, or E2 plus Dox inhibited the LiCl plus ISO-induced nuclear translocation of NFAT3. These results suggest that the GSK3β/PP2A/NFAT3 signaling pathway is activated and further modulates the hypertrophic responses induced by ISO treatment in cardiomyoblast cells (Fig. 8b).
Discussion
Previously it was reported that estradiol can protect cardiomyocytes by altering various pathways [43]. Supplementation of female ovariectomized mice with estrogen reduced pressure overload-induced hypertrophy [44]. Patten et al. demonstrated that E2 blocked apoptosis by activating the PI3K/Akt pathway in an animal model of MI [45]. Another study demonstrated that E2 protects cardiomyocytes by inhibiting p38α–p53 signaling in apoptosis [46]. Additionally, it is well understood that up-regulation of the ERs by estrogen inhibits cardiotoxicity through different mechanisms, such as calcineurin/NF-AT3 and the JNK1/2-NFκB pathways [30, 35]. In this study, we demonstrated the mechanism through which estrogen and ERα protects cardiomyoblast cells against ISO-induced injury.
ISO-induced myocardial infraction is commonly used to induce hypertrophy in H9c2 cells as well as in animal models. It is used as a standard model to study the beneficial effect of many drugs and cardiac function [47,48,49]. ANP and BNP are highly up-regulated during myocardial infraction and heart failure and in other human heart diseases [50, 51]. Therefore, we first checked for the protective effect of E2 and ERα in ISO-treated H9c2 cells. In this present study ISO treatment significantly induced cardiomyocyte hypertrophy which is associated with marked increase in cell size and hypertrophy marker BNP expression. Administration of estrogen and/or ERα reduced the phenomenon of ISO-induced cardiomyocyte hypertrophy by decreasing the cell surface area and BNP expression.
ISO promotes the release of calcium ions from the SR, thereby increasing the intracellular calcium level [52]. Intracellular Ca2+/CaM-dependent signaling promotes cardiomyocyte hypertrophic gene expression through various effectors such as PP2B, Ca2+/CaM-dependent kinase II, and NFATs [53]. Thus, a decreased intracellular calcium level may prevent the hypertrophic growth of cardiomyocytes in response to pathological stimuli [48]. We then verified the role of phosphatases in inducing a hypertrophic response in ISO-stimulated cells. PP1 and PP2A antagonists effectively blocked BNP expression, and cells treated with a PP2A inhibitor also effectively blocked BNP expression. These findings clearly demonstrate that the functions of PP1 and PP2A are linked to the modulation of β-adrenergic signaling, which has been suggested to regulate calcium signaling in the failing heart.
Morisco et al. [54] showed that stimulation of β-adrenergic receptors inhibited GSK3β expression [54]. This inhibition was found to play a crucial role during hypertrophy, although the role of GSK3β in regulating PP2A and PP1 is not clearly understood. In this study, we found that inhibition of PP2A increased GSK3β expression and decreased PP2A and ANP expression in ISO-stimulated cells. Similar results were found in E2- and Dox-treated cells. The NFAT3 transcription factor has been shown to negatively regulate GSK3β expression and also play an important role in cardiac hypertrophy [55]. We also examined the effect of GSK3β on NFAT3 expression in vitro. In our experiments, we found that E2 and ERα inhibited LiCl and ISO-induced NFAT3 translocation by increasing GSK3β and p-PLB expression. The direct effect of LiCl on GSK3β, NFAT3, and PLB expression suggests that E2 and ERα reversed the ISO-induced increase in calcium concentration via the GSK3β pathway, which further reduced hypertrophic signaling.
PLB plays a critical role in excitation–contraction coupling by inhibiting Ca2+ uptake by SERCA [56]. Apart from SERCA, the sarcolemma NCX is also important for Ca2+ transport and for maintaining the Ca2+ balance in myocytes [57]. Next, we examined whether PP2A was involved in the regulation of SERCA and NCX1 expression. Notably, ISO treatment decreased I2-PP2A and PP2A expression without altering SERCA or NCX1 expression. Surprisingly, the co-IP data indicate that ISO treatment increased NCX1 binding to PP2A, which was decreased by E2 plus ERα treatment. In addition, PP2A has been reported to directly affect the calcium cycle through direct binding of NCX, which increases NCX activity and increases the ability to transport calcium into the cytosol [58]. We also found that ISO-induced intracellular calcium accumulation was further reduced by estrogen and/or ERα treatment. From these data, it is evident that PP2A binds to NCX1 and removes cytosolic Ca2+ accumulation in cardiomyoblast cells.
Next, we determined the role of PP1 in Ca2+ accumulation and hypertrophy in ISO-stimulated cardiomyoblast cells. I-1 becomes active upon phosphorylation of threonine-35 by PKA, and this activation results in the inhibition of PP1 and reduced phosphorylation of PLB [41, 59, 60]. Moreover, another novel phosphorylation site (Thr75) has been discovered on I1PP1, but phosphorylation at this site has the opposite effect on PP1. Phosphorylation of I-1 at Thr75 converts I-1 into a positive regulator of PP1 activity, which is associated with a marked inhibition of the SR Ca2+-transport function and depressed contractility [61]. The results of the present study suggest that ISO treatment increased PP1 expression by decreasing p-PLB and Thr34 I1PP1 expression [62]. Additionally, the increase in Thr75 I1PP1 protein expression was consistent with the increase in PP1 expression. Blocking PP1 with E2 and ERα increased p-PLB and Thr34 I1PP1 expression and decreased Thr75 I1PP1 expression.
In conclusion, we found the following: (1) ERα closely binds GSK3β and up-regulates I2-PP2A activation [63]; (2) I2-PP2A blocked PP2A expression by preventing PP2A binding to NCX, which normalized the intracellular calcium concentration; and (3) estrogen and/or ERα inhibited NFAT3 nuclear translocation via the GSK3β/PP2A pathway, decreased cardiomyoblast cell size, and down-regulated the expression of pathological hypertrophic markers. However, there are a few points that need to be investigated in future studies.
References
Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT (2007) Altered microRNA expression in human heart disease. Physiol Genomics 31(3):367–373. doi:10.1152/physiolgenomics.00144.2007
Miya Y, Sumino H, Ichikawa S, Nakamura T, Kanda T, Kumakura H, Takayama Y, Mizunuma H, Sakamaki T, Kurabayashi M (2002) Effects of hormone replacement therapy on left ventricular hypertrophy and growth-promoting factors in hypertensive postmenopausal women. Hypertens Res Off J Jpn Soc Hypertens 25(2):153–159
Post WS, Larson MG, Levy D (1994) Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation 90(1):179–185
Johnson DB, Dell’Italia LJ (1996) Cardiac hypertrophy and failure in hypertension. Curr Opin Nephrol Hypertens 5(2):186–191
Ares-Carrasco S, Picatoste B, Camafeita E, Carrasco-Navarro S, Zubiri I, Ortiz A, Egido J, Lopez JA, Tunon J, Lorenzo O (2012) Proteome changes in the myocardium of experimental chronic diabetes and hypertension: role of PPARalpha in the associated hypertrophy. J Proteomics 75(6):1816–1829. doi:10.1016/j.jprot.2011.12.023
Yin Q, Yang C, Wu J, Lu H, Zheng X, Zhang Y, Lv Z, Zheng X, Li Z (2016) Downregulation of beta-adrenoceptors in isoproterenol-induced cardiac remodeling through HuR. PLoS ONE 11(4):e0152005. doi:10.1371/journal.pone.0152005
O’Connell TD, Jensen BC, Baker AJ, Simpson PC (2014) Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol Rev 66(1):308–333. doi:10.1124/pr.112.007203
Saito S, Hiroi Y, Zou YZ, Aikawa R, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I (2000) Beta-adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem 275(44):34528–34533. doi:10.1074/jbc.M002844200
Molojavyi A, Lindecke A, Raupach A, Moellendorf S, Kohrer K, Godecke A (2010) Myoglobin-deficient mice activate a distinct cardiac gene expression program in response to isoproterenol-induced hypertrophy. Physiol Genomics 41(2):137–145. doi:10.1152/physiolgenomics.90297.2008
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415(6868):198–205. doi:10.1038/415198a
Wehrens XH, Marks AR (2004) Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat Rev Drug Discov 3(7):565–573. doi:10.1038/nrd1440
Steenaart NA, Ganim JR, Di Salvo J, Kranias EG (1992) The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys 293(1):17–24
Davare MA, Horne MC, Hell JW (2000) Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem 275(50):39710–39717. doi:10.1074/jbc.M005462200
George CH (2008) Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res 77(2):302–314. doi:10.1093/Cvr/Cvm006
Hu ST, Liu GS, Shen YF, Wang YL, Tang Y, Yang YJ (2011) Defective Ca(2+) handling proteins regulation during heart failure. Physiol Res Acad Sci Bohemoslov 60(1):27–37
Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG (2002) Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol 22(12):4124–4135. doi:10.1128/Mcb.22.12.4124-4135.2002
Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R (2002) Functional coupling of calcineurin and, protein kinase A in mouse ventricular myocytes. J Physiol Lond 544(1):57–69. doi:10.1113/jphysiol.2002.020552
Zhou XW, Mudannayake M, Green M, Gigena MS, Wang G, Shen RF, Rogers TB (2007) Proteomic studies of PP2A-B56gamma1 phosphatase complexes reveal phosphorylation-regulated partners in cardiac local signaling. J Proteome Res 6(9):3433–3442. doi:10.1021/pr060619l
Liu Q, Hofmann PA (2003) Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285(1):H97–H103. doi:10.1152/ajpheart.00956.2002
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93(2):215–228
Molkentin JD, Olson EN (1997) GATA4: a novel transcriptional regulator of cardiac hypertrophy? Circulation 96(11):3833–3835
Kerkela R, Woulfe K, Force T (2007) Glycogen synthase kinase-3beta—actively inhibiting hypertrophy. Trends Cardiovasc Med 17(3):91–96. doi:10.1016/j.tcm.2007.01.004
Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN (2002) Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 99(2):907–912. doi:10.1073/pnas.231619298
Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T (2004) Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem 279(20):21383–21393. doi:10.1074/jbc.M401413200
Lasiuk GC, Hegadoren KM (2007) The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol Res Nurs 9(2):147–160. doi:10.1177/1099800407305600
Totta P, Busonero C, Leone S, Marino M, Acconcia F (2016) Dynamin II is required for 17beta-estradiol signaling and autophagy-based ERalpha degradation. Sci Rep 6:23727. doi:10.1038/srep23727
Wenger NK (2002) Clinical characteristics of coronary heart disease in women: emphasis on gender differences. Cardiovasc Res 53(3):558–567
Urbina EM, Khoury P, Martin LJ, D’Alessio D, Dolan LM (2009) Gender differences in the relationships among obesity, adiponectin and brachial artery distensibility in adolescents and young adults. Int J Obes 33(10):1118–1125. doi:10.1038/ijo.2009.164
Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA (2002) Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res 53(3):709–719
Wu CH, Liu JY, Wu JP, Hsieh YH, Liu CJ, Hwang JM, Lee SD, Chen LM, Chang MH, Kuo WW, Shyu JC, Tsai JH, Huang CY (2005) 17Beta-estradiol reduces cardiac hypertrophy mediated through the up-regulation of PI3K/Akt and the suppression of calcineurin/NF-AT3 signaling pathways in rats. Life Sci 78(4):347–356. doi:10.1016/j.lfs.2005.04.077
Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F (2001) PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J 20(21):6050–6059. doi:10.1093/emboj/20.21.6050
Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M (2005) Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol 203(1):193–201. doi:10.1002/jcp.20219
Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER (2008) Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology 149(7):3361–3369. doi:10.1210/en.2008-0133
Chen YF, Velmurugan BK, Wang HL, Tu CC, Che RJ, Chen MC, Jen LB, Vishwanadha VP, Hsu HH, Huang CY (2017) Estrogen and ERalpha enhanced beta-catenin degradation and suppressed its downstream target genes to block the metastatic function of HA22T hepatocellular carcinoma cells via modulating GSK3beta and beta-TrCP expression. Environ Toxicol 32(2):519–529. doi:10.1002/tox.22256
Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY (2009) Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med 13(9B):3655–3667. doi:10.1111/j.1582-4934.2009.00669.x
Le NT, Heo KS, Takei Y, Lee H, Woo CH, Chang E, McClain C, Hurley C, Wang X, Li F, Xu H, Morrell C, Sullivan MA, Cohen MS, Serafimova IM, Taunton J, Fujiwara K, Abe J (2013) A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation 127(4):486–499. doi:10.1161/CIRCULATIONAHA.112.116988
Shin SY, Yang HW, Kim JR, Heo WD, Cho KH (2011) A hidden incoherent switch regulates RCAN1 in the calcineurin-NFAT signaling network. J Cell Sci 124(Pt 1):82–90. doi:10.1242/jcs.076034
Mahmoodzadeh S, Pham TH, Kuehne A, Fielitz B, Dworatzek E, Kararigas G, Petrov G, Davidson MM, Regitz-Zagrosek V (2012) 17Beta-estradiol-induced interaction of ERalpha with NPPA regulates gene expression in cardiomyocytes. Cardiovasc Res 96(3):411–421. doi:10.1093/cvr/cvs281
Deshmukh PA, Blunt BC, Hofmann PA (2007) Acute modulation of PP2a and troponin I phosphorylation in ventricular myocytes: studies with a novel PP2a peptide inhibitor. Am J Physiol Heart Circ Physiol 292(2):H792–H799. doi:10.1152/ajpheart.00225.2006
Hescheler J, Kameyama M, Trautwein W, Mieskes G, Soling HD (1987) Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem 165(2):261–266
El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didie M, Dobrev D, Eschenhagen T (2006) Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun 346(3):700–706. doi:10.1016/j.bbrc.2006.05.182
Liu CJ, Cheng YC, Lee KW, Hsu HH, Chu CH, Tsai FJ, Tsai CH, Chu CY, Liu JY, Kuo WW, Huang CY (2008) Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells. Mol Cell Biochem 313(1–2):167–178. doi:10.1007/s11010-008-9754-0
Kim JK, Pedram A, Razandi M, Levin ER (2006) Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem 281(10):6760–6767. doi:10.1074/jbc.M511024200
Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP (2003) Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 284(5):H1560–H1569. doi:10.1152/ajpheart.01087.2002
Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH (2004) 17Beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res 95(7):692–699. doi:10.1161/01.RES.0000144126.57786.89
Liu H, Pedram A, Kim JK (2011) Oestrogen prevents cardiomyocyte apoptosis by suppressing p38alpha-mediated activation of p53 and by down-regulating p53 inhibition on p38beta. Cardiovasc Res 89(1):119–128. doi:10.1093/cvr/cvq265
Rathore N, John S, Kale M, Bhatnagar D (1998) Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res 38(4):297–303. doi:10.1006/phrs.1998.0365
Tan X, Li J, Wang X, Chen N, Cai B, Wang G, Shan H, Dong D, Liu Y, Li X, Yang F, Li X, Zhang P, Li X, Yang B, Lu Y (2011) Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway. Int J Biol Sci 7(3):383–389
Yeh YL, Tsai HI, Cheng SM, Pai P, Ho TJ, Chen RJ, Lai CH, Huang PJ, Padma VV, Huang CY (2016) Mechanism of Taiwan Mingjian Oolong Tea to inhibit isoproterenol-induced hypertrophy and apoptosis in cardiomyoblasts. Am J Chin Med 44(1):77–86. doi:10.1142/S0192415X16500051
Hama N, Itoh H, Shirakami G, Nakagawa O, Suga S, Ogawa Y, Masuda I, Nakanishi K, Yoshimasa T, Hashimoto Y et al (1995) Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 92(6):1558–1564
Luchner A, Stevens TL, Borgeson DD, Redfield M, Wei CM, Porter JG, Burnett JC Jr (1998) Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol 274(5 Pt 2):H1684–H1689
Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C (2009) Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res 105(10):956–964. doi:10.1161/CIRCRESAHA.109.198515
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7(8):589–600. doi:10.1038/nrm1983
Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J (2000) The Akt-glycogen synthase kinase 3beta pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem 275(19):14466–14475
Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T (2000) Glycogen synthase kinase-3 beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol 151(1):117–129. doi:10.1083/jcb.151.1.117
Bassani JWM, Yuan WL, Bers DM (1995) Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 268(5):C1313–C1319
Seidler T, Miller SL, Loughrey CM, Kania A, Burow A, Kettlewell S, Teucher N, Wagner S, Kogler H, Meyers MB, Hasenfuss G, Smith GL (2003) Effects of adenovirus-mediated sorcin overexpression on excitation–contraction coupling in isolated rabbit cardiomyocytes. Circ Res 93(2):132–139. doi:10.1161/01.RES.0000081596.90205.E2
Wei SK, Ruknudin AM, Shou M, McCurley JM, Hanlon SU, Elgin E, Schulze DH, Haigney MC (2007) Muscarinic modulation of the sodium–calcium exchanger in heart failure. Circulation 115(10):1225–1233. doi:10.1161/CIRCULATIONAHA.106.650416
Endo S, Zhou X, Connor J, Wang B, Shenolikar S (1996) Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry 35(16):5220–5228. doi:10.1021/bi952940f
Lohse MJ, Engelhardt S, Eschenhagen T (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93(10):896–906. doi:10.1161/01.Res.0000102042.83024.Ca
Rodriguez P, Mitton B, Waggoner JR, Kranias EG (2006) Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem 281(50):38599–38608. doi:10.1074/jbc.M604139200
El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T (2004) Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res 61(1):87–93. doi:10.1016/j.cardiores.2003.11.005
Liu GP, Zhang Y, Yao XQ, Zhang CE, Fang J, Wang Q, Wang JZ (2008) Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging 29(9):1348–1358. doi:10.1016/j.neurobiolaging.2007.03.012
Acknowledgements
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence: MOHW105-TDU-B-212-133019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pai, P., Velmurugan, B.K., Kuo, CH. et al. 17β-Estradiol and/or estrogen receptor alpha blocks isoproterenol-induced calcium accumulation and hypertrophy via GSK3β/PP2A/NFAT3/ANP pathway. Mol Cell Biochem 434, 181–195 (2017). https://doi.org/10.1007/s11010-017-3048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3048-3