Abstract
Protein tyrosine phosphatase 1B (PTP1B) has been shown to regulate multiple cellular events such as differentiation, cell growth, and proliferation; however, the role of PTP1B in differentiation of embryonic stem (ES) cells into cardiomyocytes remains unexplored. In the present study, we investigated the effects of PTP1B inhibition on differentiation of ES cells into cardiomyocytes. PTP1B mRNA and protein levels were increased during the differentiation of ES cells into cardiomyocytes. Accordingly, a stable ES cell line expressing PTP1B shRNA was established. In vitro, the number and size of spontaneously beating embryoid bodies were significantly decreased in PTP1B-knockdown cells, compared with the control cells. Decreased expression of cardiac-specific markers Nkx2-5, MHC-α, cTnT, and CX43, as assessed by real-time PCR analysis, was further confirmed by immunocytochemistry of the markers. The results also showed that PTP1B inhibition induced apoptosis in both differentiated and undifferentiated ES cells, as presented by increasing the level of cleaved caspase-3, cytochrome C, and cleaved PARP. Further analyses revealed that PTP1B inhibition did not change proliferation and pluripotency of undifferentiated ES cells. Taken together, the data presented here suggest that PTP1B is essential for proper differentiation of ES cells into cardiomyocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease is the major cause of death worldwide [1] and heart failure is the final clinical presentation of a variety of cardiovascular diseases [2]. It has been shown that apoptosis of cardiomyocytes is a major contributing factor to the initiation and progression of many forms of cardiac diseases [3, 4]. Apoptosis is also well recognized to play a role in the process of cell death of myocardial infarction following ischemia/reperfusion injury [5]. Pharmacological inhibition of apoptosis improves heart function and survival. Currently used drugs in heart failure therapy such as angiotensin-converting enzyme inhibitors and β-adrenergic receptor blockers prevent apoptosis [6–10].
Recently, more attention has been paid to the beneficial effect of cell therapy in treatment of heart failure. In this regard, embryonic stem (ES) cells are frequently used as in vitro model system to study the molecular mechanisms of various cardiovascular diseases and it seems that ES cells are promising therapeutic agents that can potentially generate an unlimited source for cell therapy. Several lines of evidence have shown that cardiomyocytes derived from ES cells can replenish infarcted heart and rescue heart function [11, 12]. Therefore, improving the conditions leading to a higher differentiation and better survival for cardiomyocytes could produce efficient cells in terms of regenerative medicine.
Protein tyrosine phosphatases represent a large family of enzymes that control many different cellular events. In particular, protein tyrosine phosphatase 1B (PTP1B) regulates many critical events including cell growth, tissue differentiation, intercellular communication, immune response, development, and metabolism [13]. In addition, the significance of PTP1B as a mediator of apoptosis has been reported in a few studies. It has been shown that depending on the cell type, PTP1B can act as a pro-apoptotic or an anti-apoptotic protein. For instance, PTP1B deficiency protected hepatocytes against apoptosis induced by serum withdrawal, whereas the overexpression of PTP1B enhanced cellular events leading to apoptotic cell death [14]. Small interfering RNA targeted to PTP1B also reduced hypoxia/reoxygenation-induced apoptosis in cardiomyocytes [15]. We also demonstrated that PTP1B inhibition ameliorated palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells [16]. On the other hand, PTP1B deficiency enhanced palmitate-induced ER stress and apoptosis in MIN6 β cells [17]. However, the role of PTP1B in differentiation and more specifically apoptosis of ES cell-derived cardiomyocytes remains unexplored. Given the important role of PTP1B in regulating several cellular events, in the present study we aimed to investigate the precise role of PTP1B in differentiation of ES cells into cardiomyocytes. Our results revealed a novel role for PTP1B during differentiation of ES cells into cardiomyocytes. PTP1B inhibition could induce apoptosis without a significant effect on proliferation and pluripotency of the ES cells.
Methods
Cell culture and differentiation
Murine RB1 ES cells (Royan B) were kindly provided by Royan Institute. The cells were seeded on gelatin-coated plates in knockout Dulbecco’s minimum essential medium (Knockout™ DMEM, Gibco) growth media supplemented with 15% fetal bovine serum (Gibco), 1000 U/mL leukemia inhibitory factor (LIF) (Millipore), penicillin/streptomycin (Gibco), glutamine (Gibco), nonessential amino acids (Gibco), and β-mercaptoethanol (Sigma-Aldrich). ES cells were differentiated using hanging drop method, as previously described [18]. Briefly, ES cells were trypsinized and then hanging drops of 1200 cells in 20 mL of cultivation medium without LIF were generated. On day 3 of differentiation, the generated embryonic bodies (EBs) were transferred to petri dishes and re-suspended for further 2 days. On day 5, the EBs were plated on gelatin-coated plates and monitored for the appearance of beating EBs. From day 3 onwards, the cells were treated with 100 mM ascorbic acid (Sigma-Aldrich).
Lentiviral production and infection
Lentiviral production was carried out as previously described [16, 19]. Briefly, 3 × 106 293T cells were seeded onto a 10-cm petri dish and transfected with 13.5 g of transfer vector pCSGW carrying PTP1B shRNA, 9 g of pCMVDR8.91, and 4.5 g of pMDG using polyethyleneimine. The viruses were harvested 48 h after transfection, passed through a 0.45-µm filter, and concentrated by ultracentrifugation at 100,000g for 90 min. Viral particles were re-suspended in serum-free DMEM, snap-frozen in liquid nitrogen, and stored at −80 °C. For infection, the cells were transduced at a multiplicity of infection (MOI) of 8.
Contractility ability assessment
As mentioned before, on day 5, the EBs were plated on gelatin-coated plates. From day 7 onwards, the percentage of beating colonies was calculated by the number of beating colonies divided by the total number of colonies.
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). RNA was reverse transcribed into cDNA using the Revert Aid First-Strand cDNA Synthesis Kit (Fermentas). Quantitative real-time PCR was performed using SYBR Premix Ex TaqTM II (Takara Bio, Inc.) in a Rotor-Gene Q real-time thermocycler. The thermal profile for SYBR Green PCR was 95 °C for 15 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing/extension at 60 °C for 30 s. GAPDH was used as the endogenous control. The comparative Ct method was utilized to calculate the fold change in gene expressions.
Western blot analysis
Cell lysate from mouse ES cells was prepared by homogenization in a modified RIPA buffer (50 mM Tris–HCl pH 7.4, 1% Triton X-100, 0.2% sodium deoxycholate, 0.2% SDS, 1 mM Na-EDTA, 1 mM PMSF) supplemented with a protease inhibitor cocktail (Roche). 30 g of total protein was fractionated by SDS-PAGE and blotted onto PVDF membrane. The antibodies used were as follows: PTP1B (Abcam), cleaved poly (ADP-ribose) polymerase (PARP) [cleaved PARP (Asp214)], cleaved caspase-3, cytochrome c (Cell Signaling Technology), and β-actin (Abcam). Immunoblotted protein bands were visualized with enhanced chemiluminescence and protein bands were quantified using Scion Image software. Each experiment was performed at least three times.
Cell viability
The number of viable ES cells was determined by assessing the mitochondrial function. Metabolically active cells reduce 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) reagent to insoluble formazan. MTT reagent was added to the wells and incubated for 2 h. After the formation of formazan crystals, the culture medium was discarded and DMSO was added to the wells and incubated in dark for 30 min. Absorbance was read at 540 nm in a microplate reader. Results are expressed as the percentage of untreated controls.
Flow cytometry
For cell cycle analysis, ES cells were harvested, washed in PBS, and fixed in cold 70% ethanol. After spinning at 850 g, the cells were suspended in 0.5 mL propidium iodide (PI) staining solution [0.25 mg/mL RNase A and 10 mg/mL PI in PBS]. The cells were then analyzed by a flow cytometer. Single cells were detected by gating for events based on forward scatter and side scatter populations.
Immunofluorescence staining
Contracting EBs were dissociated using collagenase B (Roche) for 40 min in a 37 °C incubator. After dissociation, single cells were plated on gelatin-coated 4-well plates and allowed to grow for 2 days. Cells were fixed with PBS containing 4% paraformaldehyde at 4 °C for 20 min. After washing the cells with PBS containing 0.05% (v/v) Tween-20, blocking was performed in PBS containing 0.2% (v/v) Triton X-100 and 10% serum for 40 min at room temperature. The cells were then incubated with the first antibodies against MHC-α (Abcam), cTnT (Abcam), Nkx2-5 (Abcam), CX43 (Abcam), or respective isotype controls in PBS containing 1% BSA overnight at 4 °C. After incubating the cells with secondary antibodies, immunocytochemically stained images were obtained using a fluorescent microscope (Olympus, IX71). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI).
Statistical analyses
All statistical analyses were performed using SPSS 13.0. (SPSS, Chicago, IL). Comparisons among all groups were performed with the one-way analysis of variance (ANOVA) test. If statistical significance was found, Tukey’s post hoc test was performed. The values of p < 0.05 were considered statistically significant. Results are expressed as mean ± SEM of three independent experiments.
Results
PTP1B is required for ES cell differentiation into cardiac lineage
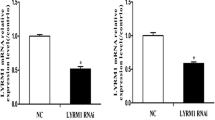
To investigate the function of PTP1B in mouse ES cell differentiation into cardiac lineage, we first measured the expression of PTP1B using quantitative real-time PCR and western blot analyses (Fig. 1a, b). The expression of PTP1B mRNA and protein was significantly increased in differentiated cells compared to undifferentiated ES cells (day 0), while the maximum PTP1B mRNA and protein levels were observed at day 4 of differentiation (73 and 89%, respectively). We then studied the role of PTP1B in differentiation of mouse ES cells into cardiomyocytes using a lentivirus-based short hairpin RNA (shRNA) to knock down PTP1B expression in ES cells. PTP1B mRNA and protein levels in the knockdown (KD) ES cells were significantly reduced by 0.3 and 0.4, respectively, as compared with the scramble control (SC) cells (Fig. 1c, d).
Expression of PTP1B during mouse ES cell differentiation into cardiac lineage. a Quantitative expression analysis of PTP1B in ES cells from day 0 to day 16 of differentiation (D0–D16). The expression levels were calculated relative to day 0 (*P < 0.0001). b Western blot analysis of PTP1B in ES cells during differentiation. The protein levels were calculated relative to day 0 (*P < 0.0001). c PTP1B mRNA level in control (SC) and PTP1B-knockdown (KD) ES cells at day 0 (**P < 0.0005). d Western blot analysis of PTP1B in PTP1B-KD and control ES cells at day 0 (*P < 0.0001)
Cardiac lineage specification and differentiation is regulated by several cardiac-specific transcription factors and a subset of cardiac-specific genes. To examine whether PTP1B inhibition in ES cells has an effect on cardiac myocyte differentiation, we generated EBs using the hanging drop method and examined the expression level of cardiac-specific genes up to D16. As shown in Fig. 2a, the expression of core cardiac-specific transcription factor (Nkx2-5) was significantly reduced in PTP1B-KD EBs. In addition, while an increased expression of cardiac-specific genes such as MYH6 (MHC-α), TNNT2 (Troponin T2), and GJA1 (CX43) was found during the different stages of differentiation of the ES cells, a drastic reduction in the expression of these genes was observed in PTP1B-KD ES cells. We confirmed these findings using immunocytochemistry of Nkx2-5, CX43, and cTnT in PTP1B-KD and SC cells (Fig. 2b).
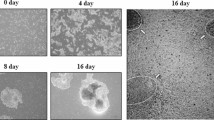
Aberrant differentiation of PTP1B-KD ES cells in vitro. a Quantitative expression analysis of cardiac-specific transcription factor and cardiac-specific genes in control and PTP1B-KD EBs. The expression levels were calculated relative to day 0. b Immunofluorescence staining for CX43, cTnT, and Nkx2-5 in control and PTP1B-KD embryoid bodies (EBs) at day 16. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). Scale bar 10 µm. c EB and cardiomyocyte formation of control and PTP1B-KD ES cells from day 0 (D0) to day 12 (D12). Scale bar 10 µm. d Appearance of spontaneous contractile activity during EB formation
Finally, we sought to evaluate whether there was any morphological change of ES cell colonies in the absence of PTP1B using optical microscopy. As shown in Fig. 2c, the morphology of PTP1B-KD ES cell colonies was not identical to that of the control ES cells and PTP1B-KD ES cells displayed untypical colony morphology. The growth of PTP1B-KD EBs was retarded with a notably fewer and smaller in size and this trend continued until the end of differentiation. In addition, we found the suppression of the contractile activity in PTP1B-KD EBs compared to SC-EBs. As shown in Fig. 2d, more than 50% of control EBs were contracting at day 9 after the initiation of differentiation, and the rate of beating EBs declined after day 10.
In contrast, a significantly smaller number of EBs derived from PTP1B-KD ES cells developed contractile activity during the entire differentiation process. Taken together, these results illustrate that PTP1B is required for proper differentiation of ES cells into cardiac lineage in vitro.
Knockdown of PTP1B leads to increased apoptosis in ES cells
Impaired cardiac differentiation of PTP1B-KD ES cells could be attributed to changes in proliferation and apoptosis of ES cells. Therefore, we investigated these characteristics in undifferentiated and differentiated (day 4) SC and PTP1B-KD cells. Flow cytometry analysis using PI staining revealed no difference in cell cycle distribution among the SC and PTP1B-KD ES cells, suggesting no effect of PTP1B inhibition on ES cell proliferation (Fig. 3a). Then, to check the effect of PTP1B inhibition on pluripotency of the ES cells, we examined the expression of pluripotency transcription factors (SOX2, NANOG, and Oct-4). As shown in Fig. 3b, the expression of these factors did not change in PTP1B-KD ES cells, suggesting that PTP1B might not be involved in the maintenance of pluripotency of the ESCs.
We then investigated whether PTP1B inhibition could affect apoptosis of ES cells. MTT assay showed that undifferentiated PTP1B-KD ES cells had lower viability compared to control SC cells (Fig. 4a). To determine whether reduced cell viability was due to apoptosis, western blot analysis of the apoptotic markers was performed. The results suggested that the apoptotic markers were increased in undifferentiated PTP1B-KD cells compared to control cells (Fig. 4b). We also confirmed these data by measuring apoptotic markers at day 4 of differentiation. The results demonstrated that the levels of cytochrome C, cleaved PARP, and cleaved caspase-3 proteins significantly were increased in the PTP1B-KD ES cells compared with the control cells (1.7-, 2.2-, and 1.7-fold, respectively) (Fig. 4c). Taken together, these findings suggest that while PTP1B inhibition had no effect on proliferation and pluripotency, it could induce apoptosis in ES cells.
Role of PTP1B in apoptosis of ES cells. a MTT assay in control and PTP1B-KD ES cells at different days of culture (*P < 0.0001). b Western blot analysis of apoptotic factors (cytochrome C, cleaved PARP, and cleaved caspase-3) of control and PTP1B-KD ES cells at day 0 (*P < 0.0001). c Western blot analysis of apoptotic markers in differentiated control and PTP1B-KD ES cells (*P < 0.0001)
Discussion
Stem cell therapy is emerging as a novel therapeutic approach to treat various heart diseases including myocardial infarction. Among various sources, ES cells have a great potential to differentiate into cardiac myocytes. In this regard, the identification of appropriate factors that effectively direct ES cell differentiation into functional cardiomyocytes is crucial in ES cell-based cell therapies for cardiac repair [20–23]. In the present study, we focused on the function of PTP1B during cardiac differentiation of ES cells.
PTP1B, the prototype of protein tyrosine phosphatases, has emerged as an important signaling molecule, regulating the immune system, cell cycle, apoptosis, and differentiation, and also plays a crucial role in cancer development and metabolic diseases [24]. However, its role in differentiation of ES cells into cardiac myocytes has not been examined. Therefore, we designed the present study to determine whether the loss of function of PTP1B in ES cells would affect cardiac myocyte differentiation in the cell culture system.
In our in vitro experiments, we generated EBs using the hanging drop method and evaluated changes in different parameters up to D16. Morphological changes, spontaneous beating, positive staining with specific markers, and increased expression of cardiac markers suggested the presence of functional cardiac myocytes. These findings are consistent with the previous reports [18, 25–27]. To evaluate the role of PTP1B in differentiation of ES cells into myocytes, we first measured PTP1B mRNA and protein levels during the differentiation of ES cells. Differentiation of ES cells led to an increase in PTP1B expression at D4 and this was slightly decreased during D8–D16. The reason for this pattern of PTP1B expression during differentiation of ES cells remains unknown. The alteration in the expression of PTP1B during differentiation suggests a link between PTP1B expression and cardiomyocyte generation from ES cells. As PTP1B expression starts to decrease at day 4, the beating of EBs appears from day 7. On the other hand, as the rate of beating EBs declines after day 10, PTP1B expression begins to rise.
We then established two stable cell lines: PTP1B-KD and SC ES cells, and observed no effect of PTP1B inhibition on proliferation and pluripotency of the ES cells. These findings were in accordance with those reported in a previous study [28]. Interestingly, we noticed that PTP1B-KD cells had significantly lower number of beating EBs, compared with the SC–ES cells. We further confirmed impaired cardiac differentiation of ES cells in the beating EBs using immunofluorescent staining. Similarly, our real-time PCR data from PTP1B-KD-derived EBs revealed a remarkable downregulation of cardiac transcription factors, suggesting a possible role of PTP1B in cardiac myocyte differentiation of ES cells.
Reduction in EB size and number and a lower efficiency of cardiac differentiation of ES cells could be due to apoptosis. To test this idea, we first evaluated the cell viability using MTT method in undifferentiated cells. A reduction in viable cells was observed in PTP1B-KD cells during 4 days of culture of undifferentiated ES cells. To assess whether the loss of cell viability was due to apoptosis, caspase activation (measured by caspase-3 and PARP cleavage) was measured. The data of the present study demonstrate that the death of both undifferentiated and differentiated PTP1B-KD ES cells was due to apoptosis associated with increased levels of caspase-3 and PARP cleavage. PTP1B has been suggested to exert pro- or anti-apoptotic activity in various cell types [15, 17, 29–31]. Our findings are in line with those reported in a previous study where PTP1B deficiency in pancreatic MIN6 cells enhanced ER stress-induced apoptosis. In this study, PTP1B overexpression in MIN6 cells mitigated palmitate- or tunicamycin-induced PERK/eIF2α ER stress signaling. ER stress coincided with the inhibition of Src family kinases (SFKs), which was exacerbated by PTP1B overexpression [17]. However, the current findings contrast with those obtained in previous studies where PTP1B deficiency in fibroblasts [29], skeletal muscle [31], and liver cells [30] attenuated apoptosis induced by ER stress, palmitate, and high-fat diet-induced ER stress, respectively. More importantly, our results contrast with those of Song et al. where PTP1B inhibition could reduce hypoxia/reoxygenation-induced apoptosis of rat cardiomyocytes [15]. The reasons for discrepancies between the studies are currently not clear, but it could be due to different cell types used and/or inducers of ER stress and apoptosis.
In summary, we have examined the role of PTP1B in cardiac myocyte differentiation of the ES cells. Our data collectively suggest that PTP1B expression was increased after differentiation, PTP1B knockdown decreased the differentiation of mouse ES cells into functional cardiac myocytes, and PTP1B inhibition increased apoptosis of both differentiated and undifferentiated cells. These findings suggest that PTP1B is required for proper differentiation of ES cells into cardiac myocytes.
Abbreviations
- ESCs:
-
Embryonic stem cells
- PTP1B:
-
Protein tyrosine phosphatase 1B
- EB:
-
Embryonic body
- Nkx2-5:
-
Homeobox protein Nkx-2.5
- MHC-α:
-
Myosin heavy chain
- cTnT:
-
Cardiac troponin
- CX43:
-
Gap junction alpha-1 protein (GJA1)
- NANOG:
-
Homeobox protein NANOG
- SOX2:
-
SRY (sex-determining region Y)-box 2
- Oct-4:
-
Octamer-binding transcription factor 4
- PARP:
-
Poly (ADP-ribose) polymerase
References
Go DMAS, Roger VL, Benjamin EJ, Berry JD (2013) Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. doi:10.1161/01.cir.0000441139.02102.80
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure the biomechanical model and beyond. Circulation 111:2837–2849
Narula J, Haider N, Arbustini E, Chandrashekhar Y (2006) Mechanisms of disease: apoptosis in heart failure—seeing hope in death. Nat Clin Pract Cardiovasc Med 3:681–688
Anversa P, Olivetti G, Leri A, Liu Y, Kajstura J (1997) Myocyte cell death and ventricular remodeling. Curr Opin Nephrol Hypertens 6:169–176
Pirat B, Muderrisoglu H, Unal MT, Ozdemir H, Yildirir A, Yucel M, Turkoglu S (2007) Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia–reperfusion. Coron Artery Dis 18:61–66
Goussev A, Sharov VG, Shimoyama H, Tanimura M, Lesch M, Goldstein S, Sabbah HN (1998) Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol Heart Circ Physiol 275:H626–H631
Communal C, Singh K, Sawyer DB, Colucci WS (1999) Opposing effects of β1-and β2-adrenergic receptors on cardiac myocyte apoptosis role of a pertussis toxin-sensitive g protein. Circulation 100:2210–2212
Communal C, Singh K, Pimentel DR, Colucci WS (1998) Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation 98:1329–1334
Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui M (2000) β-Adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 102:344–350
Bartling B, Milting H, Schumann H, Darmer D, Arusoglu L, Koerner MM, El-Banayosy A, Koerfer R, Holtz J, Zerkowski H-R (1999) Myocardial gene expression of regulators of myocyte apoptosis and myocyte calcium homeostasis during hemodynamic unloading by ventricular assist devices in patients with end-stage heart failure. Circulation 100(II):216–223
Zhu W-Z, Hauch KD, Xu C, Laflamme MA (2009) Human embryonic stem cells and cardiac repair. Transplant Rev 23:53–68
Hendry SL, van der Bogt KE, Sheikh AY, Arai T, Dylla SJ, Drukker M, McConnell MV, Kutschka I, Hoyt G, Cao F (2008) Multimodal evaluation of in vivo magnetic resonance imaging of myocardial restoration by mouse embryonic stem cells. J Thoracic Cardiovasc Surg 136(4):1028–1037
Arias-Salgado EG, Haj F, Dubois C, Moran B, Kasirer-Friede A, Furie BC, Furie B, Neel BG, Shattil SJ (2005) PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol 170:837–845
González-Rodriguez Á, Escribano Ó, Alba J, Rondinone CM, Benito M, Valverde ÁM (2007) Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J Cell Physiol 212:76–88
Song H, Zhang Z, Wang L (2008) Small interference RNA against PTP-1B reduces hypoxia/reoxygenation induced apoptosis of rat cardiomyocytes. Apoptosis 13:383–393
Nasimian A, Taheripak G, Gorgani-Firuzjaee S, Sadeghi A, Meshkani R (2013) Protein tyrosine phosphatase 1B (PTP1B) modulates palmitate-induced cytokine production in macrophage cells. Inflamm Res 62:239–246
Bettaieb A, Liu S, Xi Y, Nagata N, Matsuo K, Matsuo I, Chahed S, Bakke J, Keilhack H, Tiganis T (2011) Differential regulation of endoplasmic reticulum stress by protein tyrosine phosphatase 1B and T cell protein tyrosine phosphatase. J Biol Chem 286:9225–9235
Rabiee F, Forouzanfar M, Zadegan FG, Tanhaei S, Ghaedi K, Bashi MM, Baharvand H, Nasr-Esfahani MH (2014) Induced expression of Fndc5 significantly increased cardiomyocyte differentiation rate of mouse embryonic stem cells. Gene 551:127–137
Bakhtiyari S, Meshkani R, Taghikhani M, Larijani B, Adeli K (2010) Protein tyrosine phosphatase-1B (PTP-1B) knockdown improves palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Lipids 45:237–244
Yan B, Singla RD, Abdelli LS, Singal PK, Singla DK (2013) Regulation of PTEN/Akt pathway enhances cardiomyogenesis and attenuates adverse left ventricular remodeling following thymosin β4 overexpressing embryonic stem cell transplantation in the infarcted heart. PLoS ONE 8:e75580
Murry CE, Reinecke H, Pabon LM (2006) Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol 47:1777–1785
Guan K, Hasenfuss G (2007) Do stem cells in the heart truly differentiate into cardiomyocytes? J Mol Cell Cardiol 43:377–387
Klimanskaya I, Rosenthal N, Lanza R (2008) Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov 7:131–142
Stenzinger A, Märker D, Koch P, Hoffmann J, Baal N, Steger K, Wimmer M (2009) Protein tyrosine phosphatase interacting protein 51 (PTPIP51) mRNA expression and localization and its in vitro interacting partner protein tyrosine phosphatase 1B (PTP1B) in human placenta of the first, second, and third trimester. J Histochem Cytochem 57:143–153
Shabani P, Ghazizadeh Z, Pahlavan S, Hashemizadeh S, Baharvand H, Aghdami N, Doosti M (2015) Exogenous treatment with eicosapentaenoic acid supports maturation of cardiomyocytes derived from embryonic stem cells. Biochem Biophys Res Commun 461:281–286
Narita N, Bielinska M, Wilson DB (1997) Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development 124:3755–3764
Wang X, Yang P (2008) In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J Vis Exp. doi:10.3791/825
Matulka K, Lin H-H, Hříbková H, Uwanogho D, Dvořák P, Sun Y-M (2013) PTP1B is an effector of activin signaling and regulates neural specification of embryonic stem cells. Cell Stem Cell 13:706–719
Gu F, Nguyên DT, Stuible M, Dubé N, Tremblay ML, Chevet E (2004) Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem 279:49689–49693
Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong E-G, Cho Y-R, Kim JK, Kahn BB, Neel BG, Bence KK (2009) Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58:590–599
Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R (2013) Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med 65:1435–1446
Acknowledgements
This work was financially supported by grants provided by the Deputy of Research, Tehran University of Medical Sciences (Grant 90-02-30-14084), and the Iranian Council of Stem Cell Research and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to declare.
Rights and permissions
About this article
Cite this article
Eshkiki, Z.S., Ghahremani, M.H., Shabani, P. et al. Protein tyrosine phosphatase 1B (PTP1B) is required for cardiac lineage differentiation of mouse embryonic stem cells. Mol Cell Biochem 425, 95–102 (2017). https://doi.org/10.1007/s11010-016-2865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2865-0