Abstract
Calyptranthes tricona is a species (Myrtaceae) native to South Brazil. Plants belonging to this family are folkloric used for analgesia, inflammation, and infectious diseases. However, little is known about the toxic potential of C. tricona. The present study aimed to evaluate the antioxidant activity of C. tricona ethanol and hexane leaf extracts, as well as verify their effect on human lymphocytes and MCF-7 cells. The extracts were subjected to preliminary phytochemical screening, antioxidant activity using DPPH and ORAC methods. Genotoxic and mutagenic effects in cultured human lymphocytes were assessed using the comet assay and the micronucleus assay, respectively. In addition, cell viability by MTT assay and fluorometric analysis of mitochondrial potential and caspases-9 activity were performed in order to verify the possible effects of both extracts on H2O2-induced cell death of MCF-7 cells. Our findings revealed that the phenol content and the antioxidant activity were only present in the ethanol extract. Also, the phytochemical screening presented steroids, triterpenoids, condensed tannins, and flavones as the main compounds. However, both extracts were capable of inducing concentration-dependent DNA damage in human lymphocytes. When treating MCF-7 cells with the extracts, both of them inhibited MCF-7 cell death in response to oxidative stress through a decrease of mitochondrial depolarization and caspases-9 activity. Thus, our results need to be considered in future in vitro and in vivo studies of C. tricona effects. In the meanwhile, we recommend caution in the acute/chronic use of this homemade preparation for medicinal purpose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of vegetal extracts and phytochemicals for medicinal purposes such as prevention, treatment, and cure of diseases is one of the oldest practices of traditional folk medicine. Despite the increased use of synthetic drugs in recent years, about 80 % of the population in developing countries depend on medicinal plants as the only access to basic health care [1, 2].
Studies with various genera of Myrtaceae family report their usage for the most varied medicinal applications [3–5]. Several Myrtaceae species are folkloric employed in numerous disorders, such as gastrointestinal disturbances, infectious, and inflammatory diseases. Also based on folk medicine, previous studies reported that plants belonging to Myrtaceae family have anti-inflammatory, analgesic, antipyretic, antioxidant, and antifungal properties [6, 7].
Calyptranthes genus belongs to Myrtaceae family and clusters about 100 species distributed through tropical America, from Mexico to Uruguay. In Southern Brazil, six species were reported: C. concinna, C. grandifolia, C. lucida, C. pileata, C. rubella, and C. tricona [6, 8]. Regarding C. tricona, there are no reports about its phytoconstituents, effects on cells, and medical applications. For that reason, the aim of the present work was to determine the antioxidant activity of C. tricona ethanol and hexane leaf extracts, as well as their effect on human lymphocytes and MCF-7 cells.
Materials and methods
Chemicals
Roswell Park Memorial Institute (RPMI) 1640 medium was purchased from Biochrom (Cambourne, UK). Fetal bovine serum (FBS) and pen/strep/fungiezone solution were purchased from Hyclone (Northumberland, UK). JC-1 dye was obtained from Molecular Probes (Eugene, USA). Caspase-9 Fluorimetric Assay Kit was purchased from BioVision (Milpitas, USA). Acetonitrile, formic acid, gallic acid, ellagic acid, and caffeic acid were purchased from Merck (Darmstadt, Germany). All remaining chemicals and reagents were purchased from Sigma-Aldrich (Carlsbad, USA).
Phytochemical investigation
Plant material
Leaves of C. tricona D. Legrand were collected in September 2013 in Lajeado, Rio Grande do Sul, Brazil. The plant material was botanically identified by Dr. Elisete Maria de Freitas. The voucher specimen (accession #4996), containing stem, leaves, flowers, and fruits, was deposited at the Herbarium of Centro Universitário UNIVATES.
Preparation of hexane and ethanol extracts of C. tricona
The leaves of the plant specimens were oven-dried for 24 h. The plant materials were then reduced to small fragments to increase the contact surface of the plant with the extraction solution. The total amount of leaves was equally divided and hexane solvent or 90 % ethanol was added to the sample. The portion in contact with the hexane solvent was stored in an amber bottle at room temperature for 72 h. Then, the solution was vacuum-filtered and the filtrate stored at room temperature in amber bottle until use. The static maceration of plant specimen has lasted for 2 weeks with two changes of solvent. The portion in contact with 90 % ethanol was stored in amber bottle at room temperature for 7 days. The solution was vacuum-filtered and the filtrate was concentrated in a rotary evaporator at 40 °C. Finally, the extracts were stored in amber glass vials at 4 °C until use. For the experiments, the extracts were diluted in DMSO and administered according to the required concentration.
Phytochemical screening
Phytochemical analysis was carried out following standard procedures as previously described [9]. Samples of hexane and ethanol extracts were screened for the following phytoconstituents: steroids, triterpenoids, tannins, flavonoids, coumarins, quinones, and alkaloids.
Total phenolic content
The quantitation of total phenolic content of the extracts was determined using the Folin–Ciocalteu colorimetric method [10]. The total phenolic content of each extract was quantified using a standard curve prepared with gallic acid and the results were expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW).
High-performance liquid chromatography (HPLC–DAD)
HPLC–DAD of C. tricona extracts (10 mg/mL) was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software. Separations were carried out using Phenomenex C18 column (4.6 × 250 mm × 5 µm particle size). The mobile phase was water with 1 % formic acid (v/v) (solvent A) and HPLC-grade acetonitrile (solvent B) at a flow rate of 0.6 mL/min and injection volume of 50 µL. The composition gradient was 5 % solvent B reaching 15, 20, 45, 60, and 98 % at 20, 30, 40, 50, and 60 min, respectively, followed by 70 min at isocratic elution until 75 min. At 80 min, the gradient reached the initial conditions again, following the method previously described with slight modifications [11]. The sample and mobile phase were filtered through a 0.45-μm membrane filter and then degassed by ultrasonic bath prior to use. Stock solutions of reference standards were prepared in acetonitrile:water (1:1, v/v) at a concentration range of 0.03–0.50 mg/mL. The quantification was performed by integration of the peaks using the external standard method, at 280 nm for catechin; 254 nm for ellagic and gallic acids; 327 nm for caffeic acid; and 366 nm for quercetin, kaempferol, apigenin, and rutin. The chromatographic peaks were confirmed by comparing their retention time with those of reference standards and by DAD spectra (200–600 nm). All chromatographic operations were performed at RT.
Antioxidant activity determination
DPPH-free radical scavenging activity
The antioxidant potential of the extracts was determined on the basis of their scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH)-free radical as previously described [12] with slight modifications. The spectrophotometric analysis was used in order to determine the half-maximal inhibitory concentration (IC50) of the extracts. Ascorbic acid (AA) was used as standard. A methanol solution of DPPH was prepared and 1 mL of this solution was added to 0.5 mL of the samples. The absorbance reading was done after 30 min of incubation at room temperature, protected from light, in spectrophotometer at 517 nm. The percentage of antioxidant activity of each sample was calculated using the following formula:
Oxygen radical absorption capacity (ORAC)
The ORAC assay was performed as described by Dávalos et al. [13]. ORAC values were expressed as micrograms of Trolox equivalents per gram of extract (µM TE/g of extract).
Genotoxic and mutagenic effects of C. tricona extracts on human lymphocytes
Blood sampling
Approximately 10 mL of human peripheral blood was obtained from six healthy non-smoking donors of both genders aged between 20 and 40 years, who had not been exposed to ionizing radiation, vaccinated or treated with drugs within 6 months prior to blood collection. Human peripheral blood lymphocyte cultures were set up according to Turkez, Aydin, Geyikoglu, and Cetin [14].
This study was approved by the Research Ethics Committee of Centro Universitário Univates (# 17886413.7.0000.5310). Written informed consent was obtained from each volunteer.
Alkaline comet assay
EDTA blood samples (500 μL) were plated in 12-well plate and challenged with different concentrations (25, 50, 100, and 200 μg/mL) of C. tricona extracts for 3 h. Ethyl methanesulfonate (EMS, 200 μg/mL) was used as positive control. Comet assay was performed essentially as described by Singh, McCoy, Tice, and Schneider [15] with some modifications. Briefly, 5 μL of whole blood samples were suspended in 75 μL of low-melting-point agarose. Cell suspension was spread on a slide previously prepared with a layer of normal-melting-point agarose and covered with a coverslip and maintained at 4 °C for 10 min. Then, the slides were immersed into a lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1 % Triton ×100, and 10 % DMSO; pH 10.0) at 4 °C overnight and afterwards subjected to electrophoresis and staining with silver nitrate. Samples were analyzed at ×400 magnification on light microscope. DNA damage in the cells was assessed by quantification of the amount of DNA released from the core of the nucleus. The extension and distribution of DNA damage were evaluated by the analysis of 100 cells which were randomly chosen and non-overlapping. Comets were visually scored and classified into five classes corresponding to the extent of DNA migration according to tail size formed by breaks in the DNA: (class 0) intact, no tail; (class 1) short tail, smaller than the diameter of the head (nucleus); (class 2) medium tail, up to 2 times the diameter of the head; (class 3) long tail, more than twice the diameter of the head; (class 4) very wide tail, comets without head with nearly all of the DNA in tail, maximum DNA damage.
Micronucleus (MN) assay
The assay was performed as previously described by Fenech [16] with some modifications. Briefly, to determine the frequency of MN, 500 µL of heparinized whole blood was seeded in 6-well plate and incubated for two cycles in 5 mL of RPMI medium supplemented with 20 % FBS and 1 % of phytohemagglutinin A. After 24 h, different concentrations (25, 50, 100, and 200 μg/mL) of C. tricona extracts were added. A final concentration of 200 μg/mL EMS was used as positive control. After 44 h of culture, cytokinesis blockage was performed by adding 3 μg/mL of cytochalasin-B. At the end of 72-h incubation period, after hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation, centrifugation, and resuspension, the cell suspension was dropped on microscopic slides, and then stained with 20 % Giemsa solution. At least 1000 binucleated lymphocytes/slides were examined for the presence of MN.
Cell viability and cytotoxicity assays on MCF-7 cells
MCF-7 human breast adenocarcinoma cells were acquired from DMSZ bank (ACC 115). Cells were cultured in RPMI-1640 medium, supplemented with 10 % FBS and 1 % antibiotic/antimycotic solution. Cells were incubated at 37 °C in a humidified atmosphere containing 5 % CO2.
The assessment of MCF-7 cell viability was performed according to the MTT colorimetric assay [17]. MCF-7 cells were challenged with 200 µg/mL ethanol and hexane extracts for 24 h. Then, the preventive effect of ethanol and hexane extracts in hydrogen peroxide (H2O2)-induced cytotoxicity on MCF-7 cells was tested with 200 µM H2O2 and 200 µg/mL extracts for 24 h. After 3 h of incubation with MTT, the absorbance was read at 570 nm using an ELISA microplate reader. Results were expressed as percentage of control.
Analysis of mitochondrial membrane potential (ΔΨm)
Breakdown of ΔΨm was determined using the fluorescent probe JC-1. MCF-7 cells were treated with 200 µM H2O2 in the presence or absence of 200 μg/mL of C. tricona extracts during 24 h. Results were expressed as the ratio of the monomers/aggregates of JC-1 in percentage of control.
Caspase-9 activity
Caspase-9 activity was assessed using the Caspase 9 Fluorimetric Assay Kit according to the manufacturers’ instructions. Cells were treated with 200 µM H2O2 and 300 μg/mL of extracts for 24 h. The caspase activity was calculated by the slope of the linear phase of the fluorescence resulting from substrate accumulation and expressed as fluorescence (arbitrary units) per milligram of protein per minute [Δ fluorescence (a.u.)/mg protein/min]. Final data were presented as percentage of control.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc). All data were expressed as mean ± SEM, except for HPLC–DAD data which were expressed as mean ± SD and the analyses were performed by the free software R version 3.1.1. [18]. All the results were taken from at least three independent experiments performed in triplicate. Statistical significance was evaluated using analysis of variance ANOVA followed by Tukey’s test. A p value < 0.05 was considered statistically significant.
Results and discussion
Phytochemical investigation of C. tricona extracts
Terpenoids, tannins, flavonoids, and steroids are the organic compounds commonly found in Myrtaceae plants [19]. In general, secondary metabolites exhibit a wide range of biological and pharmacological properties favorable to the treatment and prevention of diseases [20]. In recent years, terpenoids, flavonoids, and steroids have received considerable attention due to their diverse pharmacological properties including antioxidant and antitumor activities [21].
Regarding C. tricona, there is only one work published by Menut, Bessiere, Ntalani, Verin, Henriques, and Limberger [22] describing the chemical composition of the essential oil of C. tricona leaves collected in Southern Brazil. Among the 20 compounds found in the essential oil, 18 belonged to the class of terpenes. The other compounds were isolated and characterized as derivatives of chromene structures that had never been identified in other species of Calyptranthes. In recent years, these compounds have received attention due to their important biological activities, such as anticarcinogenic [23] and antioxidant [24] potentials.
In the present work, ethanol and hexane leaf extracts were analyzed qualitatively for the presence or absence of phytochemicals. The screening using common precipitation and coloring reagents revealed the presence of steroids, triterpenoids, condensed tannins, and flavones in the ethanol extract. Phytochemicals were not detected in the hexane extract by this methodology and sample concentration. Tests for alkaloids, coumarins, and quinones were found to be negative in both extracts.
Phenolics are the main secondary metabolites in plants and the species from Myrtaceae family are able to accumulate these compounds. There are no reports about the phenol content of Calyptranthes genus extracts. However, the presence of phenols in leaves of other species belonging to Myrtaceae family was already reported [4, 5, 25]. As expected, our data showed that ethanol extract had higher total phenolic content (239.76 ± 2.16 mg GAE/g DW) than hexane extract (4.33 ± 3.80 mg GAE/g DW). These data corroborate with the content of phenols found in other Myrtaceae plants [4, 25, 26]. According to Tawaha, Alali, Gharaibeh, Mohammad, and El-Elimat [27], the total phenolic content is considered high when the extract has higher values of phenolic content of about 20 mg GAE/g DW. Thus, the ethanol extract showed a significant phenolic content.
The HPLC profile of C. tricona extracts was also acquired (Fig. 1). The samples contained other minor compounds in addition to gallic acid [retention time (tR) = 10.27 min, peak 1], catechin (tR = 15.08 min, peak 2), caffeic acid (tR = 21.83 min, peak 3), ellagic acid (tR = 29.86 min, peak 4), rutin (tR = 39.75 min, peak 5), quercetin (tR = 48.13 min, peak 6), kaempferol (tR = 54.17 min, peak 7), and apigenin (tR = 69.11 min, peak 8). The amount of each compound is shown in Table 1.
Antioxidant activity of ethanol and hexane extracts
Phenolic compounds of plants are extremely important due to their scavenging properties, being flavonoids the main phenolic components, which have potent antioxidant activity [28]. Since a number of studies have emphasized the antioxidant activity of Myrtaceae species [4, 5, 25, 29], we decided to evaluate the antioxidant properties of both ethanol and hexane extracts using the DPPH scavenging method and the ORAC assay.
Our results in the DPPH assay demonstrated that only the hexane extract presented antioxidant activity less than 50 % (low antioxidant activity). Figure 2 shows that ethanol extract has a considerable dose-dependent DPPH radical scavenging capacity, which is superior to that of hexane extract, since the later showed no significant activity at its highest concentration (data not shown). The IC50 of ethanol extract (31.30 ± 0.45 μg/mL), though lower than AA (8.64 ± 0.27 μg/mL), was very satisfactory if compared with another natural antioxidant, Gingko biloba, which is considered an extract with high antioxidant activity (IC50 = 39 µg/mL) [12]. In order to complement the evaluation of antioxidant activity of the extracts, the ORAC assay was also used (Table 2). As expected, ethanol extract presented the same behavior as in the DPPH method. These data may be related to the types of phenolic compounds, such as ellagic acid and some flavonoids as quercetin, kaempferol, and apigenin that were detected only in the ethanol extract.
Genotoxic and mutagenic evaluation using human lymphocytes
The assays that evaluate genotoxicity (e.g., comet assay) and mutagenicity (e.g., micronucleus assay) are fundamental for safety and efficacy assessment of the compounds present in medicinal plants [30]. Usually, the results obtained from comet assay show early or immediate DNA responses. Actually, this DNA damage may be transient and prone to repair [31]. In order to verify the genotoxicity of C. tricona extracts, the comet assay under alkaline conditions was performed on human lymphocytes. Only non-toxic extract concentrations were used. In the evaluation of DNA double-strand breaks, the damage score was higher in all concentrations of both extracts when compared with control group, indicating a substantial genotoxic effect (Table 3). The frequency and intensity of damage was proportional to the concentration of the extracts and the absence or minimal damage was seen in samples exposed to lower concentrations. Also, there was a significant increase in DNA breakage after exposure to EMS as positive control. However, the increase was almost twice the score of the highest concentration of the extracts.
The formation of MN following exposure to genotoxic compounds reflects a serious and irreversible damage of the DNA [30]. The frequency of MN on human lymphocytes from whole blood treated with increasing concentrations (up to 200 µg/mL) of C. tricona extracts is shown in Table 4. The results revealed that DNA damage was evident at all tested concentrations, and no difference in the frequencies of MN between the hexane and ethanol extracts was observed. EMS was used as a positive control and as expected induced an increased DNA damage. Hence, the results suggest that both ethanol extract and hexane extract have the ability to induce gene mutations. The genotoxic concentration-dependent effects of natural products have been previously described. de Souza Filho, Sagrillo, Garcia, Machado, Cadona, Ribeiro, Duarte, Morel, and da Cruz [32] showed that extracts from Astrocaryum aculeatum, an Amazonian fruit, presented time- and dose-dependent genotoxic effects in human lymphocytes regardless of their great antioxidant activity. Since C. tricona extracts present several molecules for which we do not know their specificity, we could not discard the possibility that some could present toxic effects on human lymphocytes.
Protective effect of C. tricona extracts on MCF-7 cell line
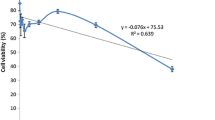
Since C. tricona extracts have genotoxic and mutagenic properties, these may open possibilities of these plant extracts possessing anticancer activity. Thus, we evaluated the possible cytotoxic effect on MCF-7 cells. Figure 3a showed that C. tricona extracts did not affect cell viability after 24 h of exposure. Interestingly, both extracts did not present tumor toxicity, although they showed potent antioxidant activity and genotoxicity in normal cells. Therefore, we decided to assess the cytoprotective effect of both extracts in a model of cell death induced by the oxidizer H2O2. Figure 3b shows that both extracts preserved the viability of the cells co-treated with H2O2 compared with the only H2O2-treated cells.
Effects of ethanol and hexane extracts of C. tricona on MCF-7 cells. a MCF-7 cells were challenged with ethanol extract or hexane extract for 24 h. b MCF-7 cells were challenged with H2O2 in the presence or absence of 200 µg/mL ethanol extract or hexane extract for 24 h. Cell viability was assessed using MTT assay. Mean values ± SEM are shown (n = 8). ***p < 0.001 compared with control; § p < 0.05 compared with H2O2
Cellular mechanisms involved in H2O2 -induced MCF-7 cytotoxicity
Considering the above-mentioned findings, we investigated some mechanisms possibly involved in cellular toxicity induced by H2O2. In order to assess whether ethanol or hexane extract protected mitochondria from injury, we analyzed the impact on mitochondrial transmembrane potential (ΔΨm) of treated cells. Changes of ΔΨm were determined by JC-1 staining of MCF-7 cells co-treated with ethanol/hexane extract and H2O2 for 3 h. As shown in Fig. 4, MCF-7 cells treated with 200 µM H2O2 exhibited a depolarization of mitochondrial membrane around 50 % when compared with the control group. However, both extracts were able to reduce significantly H2O2-induced mitochondrial depolarization.
C. tricona extracts effect on mitochondrial transmembrane potential (ΔΨm) of MCF-7 cells. Cells were exposed to 200 µM H2O2 with the presence or absence of 200 µg/mL ethanol extract or 200 µg/mL hexane extract for 24 h. The results were obtained by the ratio between the monomers/aggregates of JC-1 probe. Mean values ± SEM are shown (n = 8). ***p < 0.001 compared with control; §§§ p < 0.001 compared with H2O2
Mitochondrial disintegration not only leads to a negative impact on ΔΨm as it also causes the release of proapoptotic factors. Therefore, any change in ΔΨm can promote the activation of caspases and thus lead to cell death [33]. In order to understand whether cell death caused by H2O2 was mediated by apoptosis, we prompt to evaluate the initiator caspase-9 activity, since it is an important biomarker of the mitochondrial (intrinsic) apoptosis pathway [34]. Figure 5 showed a significant increase in caspase-9 activity when MCF-7 cells were treated with 200 µM H2O2 compared with control. However, when cells were co-treated with the extracts, a clear reduction of caspase-9 activity was observed. Hexane extract demonstrated the highest and significant decrease when compared with H2O2-treated cells and non-treated cells. These findings suggest that both extracts play important role as inhibitors of cell death during MCF-7 response to oxidative stress.
Effects of C.tricona extracts on caspase-9 activity of MCF-7 cells. Cells were cultured for 24 h in the presence of 200 µg/mL ethanol extract/hexane extract and/or 200 µM H2O2 and caspase-9 activity was assessed. Mean values ± SEM are shown (n = 8). **p < 0.01 compared with control; §§§ p < 0.001 compared with H2O2
Some phenolic compounds identified in the extracts, such as kaempferol and gallic acid, are described as antitumor and chemopreventive molecules acting on cell cycle, apoptosis, and other pathways. Tor et al. [35] showed that a plant extract and its isolated compound, gallic acid, were cytotoxic to MCF-7 cells. However, in our study, C. tricona extracts that also contain gallic acid demonstrated the opposite outcome on MCF-7 cells, cytoprotection. Since the amount of gallic acid (<1 %) found in our extracts is lower than the amount tested in the other study (IC50 = 36 μg/mL), the cytoprotection is probably due to the activity of other compounds not yet identified or by another mechanism of action not evaluated.
Conclusion
In summary, the present study demonstrated that both ethanol and hexane leaf extracts of C. tricona showed lymphocyte genotoxic and mutagenic induction. Our findings also indicate that the ethanol extract is an excellent source of natural antioxidant, mainly phenolics. However, it is not a safe extract to be exploited for nutrition or pharmaceutical uses since it has cytoprotective properties on breast cancer cells (MCF-7 cell line). Our results contribute valuable knowledge about the bioactive properties and biological effects of C. tricona, a Southern Brazil native Myrtaceae species. Further studies should be conducted to isolate, characterize, and clarify the mechanisms involved in the potential mutagens as well as the tumor-protective properties present in both C. tricona extracts. With the intention of defining the potential risks or benefits associated to the ethnopharmacological use of C. tricona extracts, detailed in vitro and in vivo studies are required. In the meanwhile, the folk usage of this plant should be viewed with caution.
References
Mendis S, Fukino K, Cameron A, Laing R, Filipe A Jr, Khatib O, Leowski J, Ewen M (2007) The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ 85:279–288
Cordell GA, Colvard MD (2012) Natural products and traditional medicine: turning on a paradigm. J Nat Prod 75:514–525. doi:10.1021/np200803m
Dastmalchi K, Flores G, Wu SB, Ma C, Dabo AJ, Whalen K, Reynertson KA, Foronjy RF, JM DA, Kennelly EJ (2012) Edible Myrciaria vexator fruits: bioactive phenolics for potential COPD therapy. Bioorg Med Chem 20:4549–4555. doi:10.1016/j.bmc.2012.05.013
Takao LK, Imatomi M, Gualtieri SC (2015) Antioxidant activity and phenolic content of leaf infusions of Myrtaceae species from Cerrado (Brazilian Savanna). Braz J Biol 75:948–952. doi:10.1590/1519-6984.03314
de Figueiroa EO, da Silva LCN, de Melo CM, Neves JK, da Silva NH, Pereira VR, Correia MT (2013) Evaluation of antioxidant, immunomodulatory, and cytotoxic action of fractions from Eugenia uniflora L. and Eugenia malaccensis L.: correlation with polyphenol and flavanoid content. Sci World J. doi:10.1155/2013/125027
Stefanello ME, Pascoal AC, Salvador MJ (2011) Essential oils from neotropical Myrtaceae: chemical diversity and biological properties. Chem Biodivers 8:73–94. doi:10.1002/cbdv.201000098
Borges LL, Conceicao EC, Silveira D (2014) Active compounds and medicinal properties of Myrciaria genus. Food Chem 153:224–233. doi:10.1016/j.foodchem.2013.12.064
Thornhill AH, Ho SY, Kulheim C, Crisp MD (2015) Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Mol Phylogenet Evol 93:29–43. doi:10.1016/j.ympev.2015.07.007
Sagrillo MR, Garcia LF, de Souza Filho OC, Duarte MM, Ribeiro EE, Cadona FC, da Cruz IB (2015) Tucuma fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chem 173:741–748. doi:10.1016/j.foodchem.2014.10.067
Bonoli M, Verardo V, Marconi E, Caboni MF (2004) Antioxidant phenols in barley (Hordeum vulgare L.) flour: comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J Agric Food Chem 52:5195–5200. doi:10.1021/jf040075c
da Silva Brum E, da Rosa Moreira L, da Silva AR, Boligon AA, Carvalho FB, Athayde ML, Brandao R, Oliveira SM (2016) Tabernaemontana catharinensis ethyl acetate fraction presents antinociceptive activity without causing toxicological effects in mice. J Ethnopharmacol. doi:10.1016/j.jep.2016.06.036
Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Dávalos A, Gómez-Cordovés C, Bartolomé B (2004) Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem 52:48–54
Turkez H, Aydin E, Geyikoglu F, Cetin D (2015) Genotoxic and oxidative damage potentials in human lymphocytes after exposure to terpinolene in vitro. Cytotechnology 67:409–418. doi:10.1007/s10616-014-9698-z
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455:81–95
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
RCoreTeam (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Tian T, Olson S, Whitacre JM, Harding A (2011) The origins of cancer robustness and evolvability. Integr Biol (Camb) 3:17–30. doi:10.1039/c0ib00046a
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines. doi:10.3390/medicines2030251
Dinic J, Podolski-Renic A, Stankovic T, Bankovic J, Pesic M (2015) New approaches with natural product drugs for overcoming multidrug resistance in cancer. Curr Pharm Des 21:5589–5604
Menut C, Bessiere JM, Ntalani H, Verin P, Henriques AT, Limberger R (2000) Two chromene derivatives from Calyptranthes tricona. Phytochemistry 53:975–979
Fu X-B, Wang X-F, Chen J-N, Wu D-W, Li T, Shen X-C, Qin J-K (2015) Synthesis, fluorescence properties, and antiproliferative potential of several 3-Oxo-3H-benzo[f]chromene-2-carboxylic acid derivatives. Molecules 20:18565
Salem M, Marzouk M, El-Kazak A (2016) Synthesis and characterization of some new coumarins with in vitro antitumor and antioxidant activity and high protective effects against DNA damage. Molecules 21:249
Salvador MJ, de Lourenco CC, Andreazza NL, Pascoal AC, Stefanello ME (2011) Antioxidant capacity and phenolic content of four Myrtaceae plants of the south of Brazil. Nat Prod Commun 6:977–982
Amensour M, Sendra E, Abrini J, Bouhdid S, Perez-Alvarez JA, Fernandez-Lopez J (2009) Total phenolic content and antioxidant activity of myrtle (Myrtus communis) extracts. Nat Prod Commun 4:819–824
Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T (2007) Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem 104:1372–1378. doi:10.1016/j.foodchem.2007.01.064
Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 12:221. doi:10.1186/1472-6882-12-221
Wang WH, Tyan YC, Chen ZS, Lin CG, Yang MH, Yuan SS, Tsai WC (2014) Evaluation of the antioxidant activity and antiproliferative effect of the jaboticaba (Myrciaria cauliflora) seed extracts in oral carcinoma cells. Biomed Res Int 2014:185946. doi:10.1155/2014/185946
Araldi RP, de Melo TC, Mendes TB, de Sa Junior PL, Nozima BH, Ito ET, de Carvalho RF, de Souza EB, de Cassia Stocco R (2015) Using the comet and micronucleus assays for genotoxicity studies: a review. Biomed Pharmacother 72:74–82. doi:10.1016/j.biopha.2015.04.004
Avishai N, Rabinowitz C, Rinkevich B (2003) Use of the comet assay for studying environmental genotoxicity: comparisons between visual and image analyses. Environ Mol Mutagen 42:155–165. doi:10.1002/em.10189
de Souza Filho OC, Sagrillo MR, Garcia LF, Machado AK, Cadona F, Ribeiro EE, Duarte MM, Morel AF, da Cruz IB (2013) The in vitro genotoxic effect of Tucuma (Astrocaryum aculeatum), an Amazonian fruit rich in carotenoids. J Med Food 16:1013–1021. doi:10.1089/jmf.2012.0287
Nicholls DG (2004) Mitochondrial membrane potential and aging. Aging Cell 3:35–40
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656. doi:10.1101/cshperspect.a008656
Tor YS, Yazan LS, Foo JB, Wibowo A, Ismail N, Cheah YK, Abdullah R, Ismail M, Ismail IS, Yeap SK (2015) Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS One. doi:10.1371/journal.pone.0127441
Acknowledgments
We thank Ms. Andréa Horst from the Physiology Department of Universidade Federal do Rio Grande do Sul (UFRGS) and Dr. Adriane Pozzobon from Centro Universitário UNIVATES for technical assistance. This work was supported by Centro Universitário UNIVATES and the National Counsel of Technological and Scientific Development (CNPq). DMK, DF, and SB are the recipients of Master grants and a postdoctoral grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kich, D.M., Bitencourt, S., Caye, B. et al. Lymphocyte genotoxicity and protective effect of Calyptranthes tricona (Myrtaceae) against H2O2-induced cell death in MCF-7 cells. Mol Cell Biochem 424, 35–43 (2017). https://doi.org/10.1007/s11010-016-2840-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2840-9