Abstract
Whether the DD genotype of the angiotensin-I converting enzyme (ACE) I/D variation contributes to end-stage renal disease (ESRD) risk in type 2 diabetes mellitus (T2DM) remains controversial. Differences in study design, case and control definition, sample size and ethnicity may contribute to the discrepancies reported in association studies. We performed a case–control study to evaluate the association of the ACE I/D variation with ESRD risk in Chinese patients with T2DM receiving hemodialysis and analyzed the genotype–phenotype interaction. Unrelated Chinese patients (n = 432) were classified into the non-diabetic nephropathy (DN) control group (n = 222, duration of diabetes >10 years, no signs of renal involvement) and the DN-ESRD group (n = 210; ESRD due to T2DM, receiving hemodialysis). Polymerase chain reaction was used to genotype ACE I/D for all 432 subjects. The frequencies of the ID + DD genotypes were higher in the DN-ESRD group than non-DN control group (65.2 vs. 50.9 %; adjusted OR 1.98 (95 % CI, 1.31–3.00; P = 0.001). In the DN-ESRD group, the DD genotypic subgroup had significantly elevated HbA1c and diastolic blood pressure (DBP) compared to the II subgroup (both P < 0.05). The DD genotype of the ACE I/D variation may be associated with more elevated blood pressure and HbA1c, and therefore may predict the development, progression and severity of DN-ESRD in Chinese patients with T2DM undergoing hemodialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A recent large-scale epidemiological analysis estimated that the overall prevalence of diabetes in the Chinese adult population was 11.6 % (113.9 million). More than 90 % of patients with diabetes have type 2 diabetes (T2DM) [1]. Approximately one-third of patients with T2DM develop diabetic nephropathy (DN) [2, 3], which is the leading cause of end-stage renal disease (ESRD) in developed countries [4, 5]. Compared to Caucasian populations, Asian patients with T2DM have a higher risk of ESRD [6, 7] and DN is the 2nd common cause of ESRD following IgA nephropathy (IgAN) in patients undergoing dialysis in China [8]. Genetic susceptibility has been proposed as an important risk factor for the development, progression, and severity of DN, and various research efforts are underway worldwide to identify the susceptibility genes such as ACE and KCNQ1 for DN [9–12]. In both humans and experimental models, systemic and glomerular hypertension contributes to the development and progression of DN [13]. Angiotensin-I converting enzyme (ACE) is a key factor in the renin-angiotensin-aldosterone system (RAAS) and converts angiotensin I into angiotensin II and inactivates bradykinin [14]. The human ACE gene is located on chromosome 17q23, and a 287 bp insertion/deletion (I/D) variation (rs179975) has been identified in intron 16 of the gene [15]. This functional I/D variation appears to affect the level of serum ACE activity: individuals homozygous for the deletion (DD genotype) have the highest serum ACE levels, those heterozygous (ID genotype) have intermediate levels, whereas those homozygous for the insertion (II genotype) have the lowest levels [16]. ACE gene I/D variation is not only associated with IgAN [17] but also DN. Whether the DD genotype of the ACE gene is associated with ESRD risk in patients with T2DM among European and Asian populations remains controversial [18–23], which suggested the ethnic heterogeneity contribute to the most differences of association between candidate genes and DN [10]. In China, the prevalence of diabetes has ranked first worldwide, and DN has became to the 2nd common cause of ESRD in patients undergoing dialysis. However, up to date no study has investigated the association between the ACE I/D variation and the risk of ESRD in patients with T2DM from the Chinese mainland. Thus, we performed a case–control study to assess the influence of the ACE gene I/D variation on the risk of ESRD in Chinese patients with T2DM undergoing hemodialysis.
Subjects and methods
This study was approved by the Institutional Review Board of Shanghai Jiaotong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from all participants.
Subjects
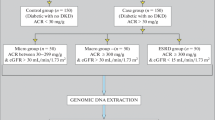
We studied 432 unrelated Chinese Han patients from Shanghai, China with T2DM. Two groups were assessed: (1) the control group (n = 222): patients with a duration of diabetes >10 years, but with no sign of renal involvement, i.e., not receiving antihypertension treatment, absence of albuminuria (urinary albumin excretion rate (UAER) <30 mg/24 h), and a creatinine clearance (using the Cockroft equation) of >60 ml/min per m2 [24]; and (2) the DN-ESRD group (n = 210): patients with ESRD due to T2DM, as indicated by a creatinine clearance rate of <15 ml/min per m2 who were receiving dialysis after ruling out the presence of urinary tract infections, hematuria, nephritis, and other conditions [25]. The non-DN control and DN-ESRD subjects with T2DM were inpatients at the Department of Endocrinology and Metabolism and the Department of Nephrology at Shanghai Jiaotong University Affiliated Sixth People’s Hospital, respectively, between January 2010 and October 2012. Diagnoses of T2DM were made according to the 2010 American Diabetes Association diagnostic criteria [26]. All patients underwent a standardized clinical and laboratory evaluation.
Methods
Genotyping of the ACE gene I/D variation
Genomic DNA was extracted from 2 ml of peripheral blood using the conventional phenol/chloroform method. Polymerase chain reaction (PCR) was used to genotype the 287 bp I/D variation in intron 16 of the ACE gene using previously established procedures [15]. The following primers were used: forward: 5′-CTGGAGA CCACTCCCATCCTTTCT-3′ and reverse: 5′-GATGTGGCCATCACATTCGTCAGAT-3′. PCR was carried out using 10 pmol of each primer, 2 mM dNTPs, 25 mM MgCl2, 5 U/ul Taq DNA polymerase enzyme, 10 × PCR buffer, and 10 ng genomic DNA in total volume of 20 µl. The PCR protocol was 5 min at 95 °C, 40 cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C, and a final extension of 10 min at 72 °C. To avoid mistyping of the ID genotype as DD, we added dimethyl sulfoxide (DMSO) to the PCR reaction mix to enhance amplification of the I allele and repeated the genotyping procedure for samples with a DD genotype. Based on the presence or absence of the 287 bp insertion in the ACE gene, three genotypes (II, ID, and DD) were identified. Five microliters of each PCR product were electrophoresed on a 12 % poly-acrylamide gel and visualized by ethidium bromide staining. Gel photographs were taken using a Gel-Doc gel imaging system (Bio-Rad, Inc., USA).
Statistical analysis
The clinical and laboratory values are expressed as the mean ± SD values or median (interquartile range). Comparisons of the clinical and laboratory parameters of the control group and DN-ESRD group, as well between genotypic groups, were performed using unpaired Student’s t tests or Pearson Chi square tests as appropriate. Data with a skewed distribution, such as the duration of diabetes, triglyceride levels, UAER, and serum creatinine levels, were logarithmically transformed before analysis and are presented as medians (interquartile range). P values <0.05 were considered significant. Multiple logistic regression was used to identify independent risk factors associated with DN-ESRD; odds ratios (OR) and 95 % confidence intervals (CI) were estimated. SPSS11.5 statistical software (SPSS, Chicago, IL) was used for data analysis and processing.
Results
The clinical and laboratory characteristics of the study populations are shown in Table 1. The DN-ESRD group had a significantly higher proportion of men and had a longer duration of diabetes; higher systolic blood pressure (SBP), LDL, UAER, serum creatinine, and BUN; significantly lower age at diagnosis of T2DM; and lower BMI, HbA1c, total cholesterol, HDL, and eGFR compared with the non-DN control group (P < 0.01 or P < 0.05). In addition, as expected, the patients in the DN-ESRD group were more likely to have hypertension (97.1 vs. 55.4 %, P < 0.000), retinopathy (55.5 vs. 42.8 %, P < 0.010), cardiovascular disease (CVD; 47.3 vs. 13.5 %, P < 0.000), and smoke (45.9 vs, 8.6 %, P < 0.000) than the non-DN control group. There were no significant differences in age, diastolic blood pressure (DBP), fasting plasma glucose (FPG), and triglyceride levels between the two groups (all P < 0.05).
As shown in Fig. 1, the three ACE I/D (rs179975) genotypes (II, ID, and DD) could be identified according to the presence of the PCR products: a 478 bp fragment for the II genotype, 191 bp for DD, and both fragments for ID. Table 2 illustrates the frequencies of the ACE I/D genotypes in the non-DN control group and DN-ESRD group in the codominant, dominant, and recessive models, respectively. The genotype frequencies for ACE I/D did not deviate from Hardy–Weinberg equilibrium (P < 0.05) as indicated by the χ2 test. However, there were significant differences in the frequencies of the ACE I/D genotypes between the two groups (χ2 = 13.24, P = 0.001). In multivariate unconditional logistic regression analysis, the ID and DD genotypes were associated with an increased risk of DN-ESRD compared to the II genotype, with adjusted ORs (95 % CI) of 1.72 (1.10–2.68) and 1.73 (1.27–2.36), respectively, after adjusting for age, sex, and BMI. In the dominant model, the frequency of the ID + DD genotype was significantly higher in the DN-ESRD group than the non-DN control group (65.2 vs. 50.9 %) with an adjusted OR of 1.98 (95 % CI, 1.31–3.00; P = 0.001). In the recessive model, the frequency of the genotype DD was significantly higher in the DN-ESRD group compared to the non-DN control group (21.4 vs. 10.8 %) with an adjusted OR of 2.23 (95 % CI, 1.28–3.91, P = 0.005). As shown in Table 2, the frequency of the D allele was higher in the DN-ESRD group than the non-DN control group (43.3 vs. 30.9 %, P = 0.000) with an OR of 1.71 (1.30–2.26), and the frequency of the I allele was lower in the DN-ESRD group than the non-DN control group (56.7 vs. 69.1 %, P = 0.000) with an OR of 0.58 (0.44–0.77).
Table 3 summarizes the clinical characteristics of each ACE I/D genotypic subgroup in the non-DN control group. The DD subgroup had significantly lower FPG and a shorter duration of diabetes (both P < 0.05) as well as a tendency towards higher HbA1C and SBP (both P > 0.05) than the II subgroup. In addition, the ID + DD subgroup had a shorter duration of diabetes than the II group, and the II + ID subgroup had significantly elevated FPG compared to the DD group (P < 0.05, Table 3). Table 4 presents the clinical characteristics of each ACE I/D genotypic subgroup in the DN-ESRD group. The DD subgroup had significantly higher HbA1c and DBP (both P < 0.05) as well as a tendency towards higher SBP than the II group (P < 0.05). Furthermore, the ID + DD subgroup had significantly higher HbA1C and DBP than the II group (all P < 0.05, Table 4).
Discussion
The relationship between the ACE I/D variation (rs179975) and the risk of ESRD in patients with DN varies in different populations and remains inconclusive [18–23]. Analysis of cohorts with varied ethnicities, differences in study design and definition of the case and control groups, as well as insufficient sample size may contribute to the discrepancies reported in association studies. In the present study, we investigated the distribution of the ACE I/D variation and its genotypic phenotypes in patients with ESRD due to T2DM undergoing hemodialysis compared to non-DN control subjects with T2DM from the Chinese mainland. The two major findings of the present study are (1) the ACE DD genotype and D allele were significantly more frequent among patients DN-ESRD on hemodialysis than the non-DN control subjects, and (2) the DD genotype was associated with significantly higher HbA1c and DBP than the II genotype among the DN-ESRD group.
Previous studies indicated the ACE DD genotype has a high prognostic value for progressive deterioration of renal function, and appeared to increase the risk of death once dialysis was initiated in Japanese or Korean patients with DN [18, 19] but not among Caucasian patients [21]. In the present study, the overall analysis revealed a significant association between the ACE I/D variation and the risk of DN-ESRD in all genetic models (ID versus II: OR 1.72, 95 % CI 1.10–2.68; DD versus II: OR 1.73, 95 % CI 1.27–2.36; allele contrast: OR 1.71, 95 % CI 1.30–2.26; dominant model: OR 1.98, 95 % CI 1.31–3.00; and recessive model: OR 2.23, 95 % CI 1.28–3.91, after adjustment for confounders, respectively), which is consistent with the associations reported for Japanese and Korean patients [18, 19] and suggests the ACE I/D variation may also contribute to the progression of DN-ESRD in Chinese patients with T2DM undergoing hemodialysis.
To our knowledge, the sample size of DN-ESRD in our study is more than that of these controversial reports [18–23], which was 3.3-fold of Japanese (208 vs. 63) and 2.5-fold of Korean (208 vs. 83), respectively [18, 19], despite both of their association between ACE I/D variation and DN-ESRD were similar with that of ours.
Hyperglycemia plays a pivotal role in the development of DN, and high plasma glucose levels increase mesangial cell matrix production [27] and mesangial cell apoptosis [28]. A study in South Korean patients revealed that HbA1c levels of 6.50–7.49 % or ≥7.50 % were associated with a significantly increased risk of ESRD compared to a HbA1c level <6.50 % [29]. The DN-ESRD group had a significantly lower HbA1C level than the non-DN control subjects (Table 1), which may be the result of decreased gluconeogenesis in the remnant kidneys, alterations to metabolic pathways, inadequate nutrition, decreased insulin clearance, loss of glucose to the dialysate, and diffusion of glucose into erythrocytes during hemodialysis in patients with DN-ESRD [30, 31] As shown in Table 4, the ACE DD genotype was associated with markedly elevated HbA1C levels compared to the patients with the ACE II genotype in the DN-ESRD group (DD vs. II, 7.5 ± 0.4 % vs. 6.5 ± 0.2 %, P < 0.05), suggesting that ACE DD carrier status may elevate the risk of ESRD or renal impairment in T2DM. Mechanistically, the DD genotype is associated with higher plasma ACE levels [32], suggesting that the DD genotype may have a higher plasma Ang II level [33]. Elevated Ang II impairs glycemic control and leads to β-cell dysfunction [34] and may therefore result in elevated HbA1C among patients with DN-ESRD that carry the DD genotype.
Pharmacogenomic studies have indicated that when genetic variation leads to modified target availability or function, the drug response also modifies [35]. The ACE I/D variation appears to affect ACE activity and the 287 bp deletion (DD genotype) results in higher plasma and tissue ACE levels [16]; therefore, the ACE genotypes may predict the response of patients to the antiproteinuric and renoprotective effects of ACE inhibitors (ACEIs). In fact, the DD genotype reduces the long-term benefit of ACE inhibition on the progression of DN in patients with insulin-dependent diabetes mellitus (IDDM) [36], and angiotensin receptor blockers (ARBs, e.g., losartan) had greatest beneficial effect in the ACE DD genotype group and intermediate effect in the ACE ID genotype group for nearly all composite end-points, i.e., doubling of serum creatinine, ESRD, or death in patients with T2DM with overt nephropathy [37]. In other words, the D allele of the ACE I/D variation was associated with unfavorable renal prognosis in patients with proteinuric T2DM, which could be improved by treatment with losartan [37].
In addition, SBP was significantly higher in the DN-ESRD group than the non-DN control group (Table 1), supporting the suggestion that increased blood pressure promotes the development of DN in patients with T2DM [38]. Actually, higher SBP and renal dysfunction or damage are both a cause and consequence of each other. Several relevant molecular mechanisms may contribute to the promotion of hypertensive renal damage or renal hypertension, such as the activation of renin-angiotensin-aldosterone system (RAAS) or sympathetic nervous system, sodium retention, volume expansion, oxidative stress, endothelial dysfunction, as well as genetic and epigenetic determinants [39, 40]. Interestingly, in the DN-ESRD group, carriers of the ACE DD genotype had higher DBP than carriers of the II genotype (DD vs. II, 81.5 ± 1.9 vs. 75.2 ± 1.6 mmHg, P < 0.05) and non-significant tendency towards higher SBP (150.4 ± 3.6 vs. 144.9 ± 3.1 mmHg, P > 0.05, Table 4). These results suggest the ACE DD genotype may be related to elevated blood pressure and may therefore be associated with the development, progression, and severity of DN-ESRD in Chinese patients with T2DM.
However, no differences of genotypic phenotypes especially elevated DBP and SBP as well as HbA1c were detected in Japanese and Koreans [18, 19].
In conclusion, this study suggests the DD genotype of the ACE I/D variation is associated with a higher risk of ESRD in Chinese patients with T2DM on hemodialysis. Moreover, the DD genotype may be related to more elevated HbA1c and blood pressure; therefore, the ACE DD genotype may predict the development, progression, and severity of DN-ESRD in Chinese patients with T2DM on hemodialysis.
References
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414(6865):782–787
Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators (2006) Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69(11):2057–2063
Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ (2006) The 30-year natural history of type 1 diabetes complications: the Pittsburgh epidemiology of diabetes complications study experience. Diabetes 55(5):1463–1469
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske B, Kutner N, Liu J, Peter W, Ding S, Guo H, Kats A, Lamb K, Li S, Li S, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L (2013) US renal data system 2012 annual data report. Am J Kidney Dis 61(1 Suppl 1):407–476. doi:10.1053/j.ajkd.2012.11.031
Gilg J, Castledine C, Fogarty D, Feest T (2011) UK renal registry 13th annual report (December 2010): chapter 1: UK RRT incidence in 2009: national and centre-specific analyses. Nephron Clin Pract 119(Suppl 2):c1–c25. doi:10.1159/000331741
Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H (2011) Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44(Suppl 2):S14–S21
Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV (2002) Ethnic disparities in diabetic complications in an insured population. JAMA 287(19):2519–2527
Liu ZH (2013) Nephrology in china. Nat Rev Nephrol 9(9):523–528. doi:10.1038/nrneph.2013.146
Placha G, Canani LH, Warram JH, Krolewski AS (2005) Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv Chronic Kidney Dis 12(2):155–169
Mooyaart AL, Valk EJ, van Es LA, Bruijn JA, de Heer E, Freedman BI, Dekkers OM, Baelde HJ (2011) Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 54(3):544–553
Ohshige T, Tanaka Y, Araki S et al (2010) A single nucleotide polymorphism in KCNQ1 is associated with susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes Care 33(4):842–846
Viswanathan V, Zhu Y, Bala K, Dunn S, Snehalatha C, Ramachandran A, Jayaraman M, Sharma K (2001) Association between ACE gene polymorphism and diabetic nephropathy in South Indian patients. JOP 2(2):83–87
Tarnow L (1996) Genetic pattern in diabetic nephropathy. Nephrol Dial Transplant 11(3):410–412
Erdös EG, Skidgel RA (1987) The angiotensin I-converting enzyme. Lab Invest 56(4):345–348
Rigat B, Hubert C, Corvol P, Soubrier F (1992) PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res 20(6):1433
Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F (1992) Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 51(1):197–205
Wiwanitkit V (2006) Angiotensin-converting enzyme gene polymorphism is correlated to the progression of disease in patients with IgA nephropathy: a meta-analysis. Ren Fail 28(8):697–699
Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A, Kubo H, Kawaguchi Y, Kon V, Matsuoka K, Ichikawa I, Sakai O (1996) Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int 50(2):657–664
Ha SK, Park HC, Park HS, Kang BS, Lee TH, Hwang HJ, Kim SJ, Kim DH, Kang SW, Choi KH, Lee HY, Han DS (2003) ACE gene polymorphism and progression of diabetic nephropathy in Korean type 2 diabetic patients: effect of ACE gene DD on the progression of diabetic nephropathy. Am J Kidney Dis 41(5):943–949
Ringel J, Beige J, Kunz R, Distler A, Sharma AM (1997) Genetic variants of the renin-angiotensin system, diabetic nephropathy and hypertension. Diabetologia 40(2):193–199
Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A (2006) Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant 21(4):979–983
Zsom M, Fülöp T, Zsom L, Baráth A, Maróti Z, Endreffy E (2011) Genetic polymorphisms and the risk of progressive renal failure in elderly Hungarian patients. Hemodial Int 15(4):501–508. doi:10.1111/j.1542-4758.2011.00593.x
Schmidt S, Strojek K, Grzeszczak W, Bergis K, Ritz E (1997) Excess of DD homozygotes in haemodialysed patients with type II diabetes. The diabetic nephropathy study group. Nephrol Dial Transplant 12(3):427–429
Liu Limei, Zheng Taishan, Wang Feng, Wang Niansong, Song Yanyan, Li Ming, Li Lifang, Jiang Jiamei, Zhao Weijing (2010) Pro12Ala polymorphism in the PPARG gene contributes to the development of diabetic nephropathy in Chinese type 2 diabetes. Diabetes Care 33(1):144–149
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28(1):164–176
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69
Harris RD, Steffes MW, Bilous RW, Sutherland DE, Mauer SM (1991) Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int 40(1):107–114
Mishra R, Emancipator SN, Kern T, Simonson MS (2005) High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int 67(1):82–93. doi:10.1111/j.1523-1755.2005.00058.x
Oh SW, Kim YC, Koo HS, Jin DC, Na KY, Chae DW, Kim S, Chin HJ (2011) Glycated haemoglobin and the incidence of end-stage renal disease in diabetics. Nephrol Dial Transplant 26(7):2238–2244. doi:10.1093/ndt/gfq707
Riveline JP, Teynie J, Belmouaz S, Franc S, Dardari D, Bauwens M, Caudwell V, Ragot S, Bridoux F, Charpentier G, Marechaud R, Hadjadj S (2009) Glycaemic control in type 2 diabetic patients on chronic haemodialysis: use of a continuous glucose monitoring system. Nephrol Dial Transplant 24(9):2866–2871. doi:10.1093/ndt/gfp181
Abe M, Kalantar-Zadeh K (2015) Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol 11(5):302–313. doi:10.1038/nrneph.2015.38
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86(4):1343–1346
Cambien F (1994) The angiotensin-converting enzyme (ACE) genetic polymorphism: its relationship with plasma ACE level and myocardial infarction. Clin Genet 46(1 Spec No):94–101
Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E (2013) Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab 304(8):E874–E884. doi:10.1152/ajpendo.00490.2012
Evans WE, McLeod HL (2003) Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med 348(6):538–549
Parving HH, Jacobsen P, Tarnow L, Rossing P, Lecerf L, Poirier O, Cambien F (1996) Effect of deletion polymorphism of angiotensin converting enzyme gene on progression of diabetic nephropathy during inhibition of angiotensin converting enzyme: observational follow up study. BMJ 313(7057):591–594
Parving HH, de Zeeuw D, Cooper ME, Remuzzi G, Liu N, Lunceford J, Shahinfar S, Wong PH, Lyle PA, Rossing P, Brenner BM (2008) ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol 19(4):771–779. doi:10.1681/ASN.2007050582
Mogensen CE (1998) Combined high blood pressure and glucose in type 2 diabetes: double jeopardy. British trial shows clear effects of treatment, especially blood pressure reduction. BMJ 317(7160):693–694
Gargiulo R, Suhail F, Lerma EV (2015) Hypertension and chronic kidney disease. Dis Mon 61(9):387–395
Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M (2014) Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 28(2):74–79
Acknowledgments
This work was supported by grants from the Project of National Natural Science Foundation of China (81471012, 81270876, 30771022, and 30971384), the University Innovation Research and Training Program of Shanghai Jiaotong University School of Medicine (2015301), the Shanghai Leading Talent (SLJ15055), the Program of Education Research from Shanghai Jiaotong University of Medicine (YB150612), and Program of Scientific Research from Shanghai Health and Family Planning Commission (20144Y0206). Y. Liu was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant SC1DK104821.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming Lu, Jianzhong Zhang and Ming Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, M., Zhang, J., Li, M. et al. The angiotensin-I converting enzyme gene I/D variation contributes to end-stage renal disease risk in Chinese patients with type 2 diabetes receiving hemodialysis. Mol Cell Biochem 422, 181–188 (2016). https://doi.org/10.1007/s11010-016-2819-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2819-6