Abstract
The study was aimed at describing the mode of action of an innovative drug-like congener of fused azaisocytosine—EIMTC (ethyl 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carboxylate)—on cancer cells in early in vitro oncology-related bioassays. Micromolar concentrations of EIMTC were effective at inhibiting the growth of two types of malignant multiple myeloma cells (including cells resistant to thalidomide) while having less cytotoxic effect on normal HSF cells. Furthermore, EIMTC was disclosed as capable of producing the statistically significant decrease in the number of cells in the S phase (in HeLa, TOV112D, T47D and Vero cells) and in the G2/M phase (in TOV112D cells) as well as evoking the distinctly higher necrosis rates in malignant than normal cells of the same epithelial origin. These results are promising in the sense that the bicyclic nucleobase-like structure related to azaisocytosine may target epithelial cancer cells and inhibit their growth while having less effect on normal cells. This may be due to induction of necrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic fused aza-analogues of isocytosine and isocytidine (mimicking anomalous azanucleobases and azanucleosides, respectively), which are related in structure to the naturally occurring nucleobases and nucleosides, have gained a considerable interest in the field of antitumour search due to their anticipated capability of interacting with the nucleic acids and hence inhibiting their various functions [1]. A number of the designed ribosidated small molecular weight derivatives bearing the imidazo[2,1-c][1,2,4]triazin-4(1H)-one scaffold have been suggested as novel bicyclic nucleosides related in structure to 6-azaisocytosine (the tautomeric 3-amino-1,2,4-triazin-5(2H)-one) and/or betaine-like nucleosides that presumably are capable of participating as transient chemical species in the glycosidation of naturally occurring nucleobases [2].
We have previously described the possible innovative pharmaceutics related in structure to anomalous nucleobases based upon the privileged 7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-one template [3–10] with optimal pharmacokinetic profiles [10]. Numerous modified structures (being differently substituted at the C-3 and N-8 atoms of the common drug-like scaffold) were disclosed as capable of selectively inhibiting the growth of malignant cells [3–9]. Thus, they may be exploited in the design of safer antitumour agents. A range of better bioavailable analogues of this family of compounds have been rationally designed and synthesized with the goal to develop the possible new ester prodrugs of the original compounds with a similarity to the anticipated fraudulent building blocks (such as the abnormal nucleic bases) that are able to efficiently cross the cell biomembranes and may be involved in faulty protein synthesis. Mostly, two small molecules with the common privileged template (bearing the ethoxycarbonyl function at the C-3 and a substituted phenyl moiety by one or two chlorine atoms at the N-8), the structures of which are provided in Table 1, have previously been reported as capable of revealing remarkable cytotoxicity differences between human tumour and non-tumour cells [3, 4]. We have found out that in the case of these anomalous nucleobases introducing substituted phenyl ring at the N-8 of the bicyclic scaffold facilitates their antiproliferative activities in vitro [3, 4], whereas the ester functional group at the C-3 mostly improves their permeability properties through cell membranes of the gut wall.
The objective of our present investigation was to explore and deepen the profile of biological activity (i.e. cytotoxic effects in vitro in certain populations of tumour cells) and to provide an insight into the mode of action of ethyl 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carboxylate (EIMTC), belonging to the class of much less studied fused azaisocytosine-like congeners which should reveal an antagonistic affinity towards the adenosine receptors [11, 12]. For the first time, we try to explain the significant antiproliferative activity of EIMTC (emerged from a rational approach directed towards the obtainment of ester prodrugs of the original compounds). We have focused our attention on its ability of inhibiting different phases of the cell cycle progression and evoking higher rates of necrotic cell death in certain malignant cells than in normal cells of the same epithelial origin.
Materials and methods
The investigated fused azaisocytosine-like congener: ethyl 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carboxylate (EIMTC)
An alternative microwave-assisted route for the synthesis and the spectroscopic characterization of EIMTC are provided as Supplementary information. HPLC analysis was applied to confirm the purity of an analytical sample (i.e. containing 0.01 mg mL−1 EIMTC). EIMTC has previously been employed as an outgoing structure for the synthesis of the possible drug (revealing an antagonistic affinity towards adenosine A2A receptors) which strongly prevents experimentally induced hepatic cirrhosis, e.g. 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (IMT) [11, 12].
Estimation of antiproliferative effects evoked by EIMTC
The in vitro antiproliferative activities of the newly synthesized EIMTC were assessed on the basis of commonly used (in the preliminary stage of antitumoural search for novel compounds [13]) 5-bromo-2′-deoxyuridine (BrdUrd)-based assay [14–17]. In this approach (5-bromo-2′-deoxyuridine labelling and detection kit III, Roche Diagnostics, GmbH, Mannheim, Germany), the incorporation of BrdUrd (a brominated nucleoside biomolecule structurally related to thymidine) into the freshly synthesized cellular DNA during the synthesis phase of the replication is directly proportional to the rate of cell proliferation. The application of this non-radioactive bioassay (in contrast to the standard radioactive [3H]-thymidine-based assay) well agrees with the global tendency and concept of “green chemistry” in the field of anticancer studies.
Antiproliferative activities evoked by an effective concentration of EIMTC (0.158 mM) were assessed after three incubation periods (24, 48 and 72 h) in four populations of recruited human malignant cell lines (that represent human multiple myeloma cells resistant to thalidomide—MM1R as well as susceptible to thalidomide—MM1S, human cervical epithelial carcinoma cells—HeLa and human breast carcinoma cells—T47D) and in one normal cell line of the same epithelial origin (human skin fibroblasts—HSF, derived from the skin of a 25-year-old female, fifth passage). The detailed procedure of antiproliferative studies has been described earlier [18].
The optical densities of malignant and normal cells treated with EIMTC were quantified spectrophotometrically against the untreated controls at three different time points using an ELISA reader (BIO-TEC Instruments, USA). Next, the results of these quantitative measurements (taken from at least three independent evaluations—each with five different cell lines) were converted into the percentage growth inhibition ratios of recruited cells and expressed as means ± SD in order to provide an information about the antiproliferative activity of EIMTC over time.
Analysis of the cells in different phases of the cell cycle
The flow cytometric analysis was employed in order to distinguish and analyse the individual cells in different phases of the cell cycle after 72-h treatment with 0.032 mM EIMTC. The recruited populations of reference malignant (A549, HeLa, TOV112D and T47D) as well as normal (Vero) cells of the same epithelial origin were intentionally used.
The first in preparing cells for an analysis of the cell cycle was the trypsinization of cells from the flask and treating them in phosphate-buffered saline (PBS, Sigma, USA) containing the non-ionic detergent—Triton X-100 (Sigma, USA)—in order to achieve an appropriate level of permeabilization of the cells’ plasma membranes. Next, following staining with a metachromatic fluorochrome, i.e. acridine orange (AO, Sigma, USA) (which differentially stains the cellular double-stranded DNA and a single-stranded RNA), the fluorescence intensity of the stained cells was quantified using a BD FACSCalibur flow cytometer (Becton–Dickinson, CA) with standard settings for green and red detection of fluorescence pulses. The resulting data were presented as the mean percentage content of individual cells in the respective cell cycle phases ± SD. These data were derived from at least three independent evaluations. The detailed experimental procedure has been reported previously by Pożarowski et al. [19].
Quantitative evaluation of apoptosis and necrosis rates
Necrosis and apoptosis rates were established in two different experiments.
The effect of 0.032 mM EIMTC on the apoptosis rates was quantitatively assessed by flow cytometry. Following staining with a fluorescent dye such as AO, the apoptosis rates in all the recruited cell lines treated with EIMTC and in the untreated controls were quantitatively analysed after 72-h incubation period using a BD FACSCalibur flow cytometer (Becton–Dickinson, USA).
In turn, the influence of EIMTC (0.032 mM) on the necrosis rates was quantitatively established employing a confocal microscopy examination. Following staining with propidium iodide (PI, Sigma, USA) and 2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1H-benzimidazole trihydrochloride (e.g. Hoechst 33342, Sigma, USA) [20], all the stained necrotic cell populations in two epithelial cell lines (HeLa, Vero) treated with EIMTC as well as untreated were subjected to a quantitative evaluation after 72 h of incubation, using a FluoView FV 1000 confocal microscope (Olympus, USA). The total number of the analysed cells in each cell line was 1000 cells. The stock concentration of each fluorochrome was prepared as described earlier [18].
Statistical analysis
The results of biological experiments were expressed as mean ± standard deviation. Statistical analyses were performed using Statistica 10.0 software (StatSoft, Poland). The statistical significance level was defined as p < 0.05.
Results
Inhibition of MM1S and MM1R cells’ proliferation by EIMTC
In the sensitive and reliable cell proliferation BrdUrd-based assay, we have found out that the newly synthesized ethyl 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carboxylate (EIMTC) at a concentration of 0.158 mM is not only evidently effective against human multiple myeloma cells susceptible to thalidomide (MM1S), but also towards human multiple myeloma cells resistant to thalidomide (MM1R) after 24-, 48- and 72-h incubation (Table 2). The remarkable anti-multiple myeloma effects of EIMTC were found to be dependent on the period of exposure. Noteworthy is that the investigated small molecular weight congener of fused azaisocytosine at an effective concentration revealed the lower cytotoxic effects (over a threefold to twofold decrease in the percentage growth inhibition after 24 and 48 h of incubation, respectively) towards normal human skin fibroblast (HSF) cells than against malignant MM1R and MM1S cells. This could provide an adequate safety window between normal and malignant cells, justifying further in vivo investigation of EIMTC. This difference was much smaller after a 72-h incubation period (only a 1.4-fold decrease in the per cent of growth inhibition towards HSF cells when compared to the percentage growth inhibition in two types of human multiple myeloma cells).
The IC50 values of EIMTC in MM1R and MM1S cells, obtained from the concentration-dependent inhibition curves, are listed in Table 2.
Influence of EIMTC on additional populations of tumour cells
The obtained results revealed that 0.158 mM EIMTC was also effective at evoking incubation time-dependent antiproliferative effects in human breast carcinoma (T47D) cells and cytotoxic effects in human cervical epithelial carcinoma (HeLa) cells after 24, 48 and 72 h of incubation. Regrettably, at the same concentration and especially after increasing incubation periods, these cancer cell populations of the epithelial origin (HeLa and T47D cells) were as sensitive as normal human skin fibroblast cells to EIMTC exposure. Therefore, the investigated fused azaisocytosine-like congener was identified as revealing a similar cytotoxicity towards these cells, as shown in Table 2. In this case, there is not a safety window (with respect to normal HSF cells) wide enough to encourage further in vivo investigation of EIMTC in human breast and cervical cancer.
Table 2 lists the IC50 values of EIMTC in T47D and HeLa cells that were obtained from the concentration-dependent inhibition curves.
Influence of EIMTC on the cell cycle arrest in human tumour and normal cells of the same epithelial origin
Further efforts were made to check whether the 72-h treatment with 0.032 mM EIMTC influences the cell cycle progression. We have confirmed a remarkable decrease in the number of cells in the S phase of the cell cycle in both the majority of tumour (HeLa, TOV112D, T47D) and normal (Vero) cells of the same epithelial origin. In turn, we have observed a significant decrease in the number of cells in the G2/M phase of the cell cycle in TOV112D cells. The obtained results are shown in Table 3.
Detection of the type of cell death induced by EIMTC
Additional investigations were performed to explore the mode of cell death induced by EIMTC which may be although partially contributed to its remarkable antiproliferative activities in vitro. We have studied, in two different experiments, on cultured epithelial cells whether 0.032 mM EIMTC induces apoptosis and/or necrosis cell death.
The quantitative results of the flow cytometry analysis (Table 3) have clearly indicated that EIMTC treatment did not induce apoptosis in human cancer and normal cells.
Notwithstanding, the experimental results revealed that the remarkable cytotoxicity of EIMTC against cancer cells is strictly related to a pronounced induction of necrosis (Fig. 1a, c vs. Fig. 1b, d, respectively, showing EIMTC-mediated morphological features of necrosis [21]). It should be noted that the 72-h treatment with 0.032 mM EIMTC induced the significantly higher necrosis rate in epithelial HeLa cancer cells (100 % ± 15.0; 2 % ± 0.38 in the untreated control—Fig. 1a vs. Fig. 1b, respectively) than in normal Vero cells of the same origin (75 % ± 6.8; 6 % ± 0.72 in the untreated control—Fig. 1c vs. Fig. 1d, respectively). Furthermore, it has been established that indices of necrosis in HeLa cells were strictly dependent on EIMTC concentration that was used. In a tenfold lower concentration (e.g. 0.0032 mM), EIMTC was not capable of inducing the necrotic cell death, whereas in a twofold lower concentration (0.0158 mM), the percentage content of cells undergoing necrosis was found to be only 10 % ± 1.8 (Fig. S3A and B and Fig. S4 in Supplementary material). Simultaneously, the investigated fused azaisocytosine-like congener at two above-mentioned lower concentrations did not induce necrosis in normal Vero cells (see Fig. S5A and B and Fig. S6 in Supplementary material).
Necrosis rates (when 1000 cells were subjected to the quantitative evaluation) in HeLa and Vero cell lines after staining with propidium iodide and Hoechst 33342: 100 % ± 15.0 of necrotic cells in HeLa cell line after 72-h treatment with 0.032 mM EIMTC (a); 2 % ± 0.38 of necrotic cells in a control of HeLa cell line (b); 75 % ± 6.8 of necrotic cells in Vero cell line after 72-h treatment with 0.032 mM EIMTC (c); and 6 % ± 0.72 of necrotic cells in a control of Vero cell line (d). All these fluorescence microscopy images are provided with magnification ×200
Predicted bioavailability, permeability properties and toxicity risks
The molecular structure of EIMTC is shown in Fig. 2.
We have decided to calculate a set of important molecular descriptors of the investigated small molecular weight fused azaisocytosine-like congener employing the Advanced Chemistry Development (ACD/Labs) software version 11.02 as well as the accessible Molinspiration Property Calculator (http://molinspiration.com). This enables to predict a drug-like behaviour in order to assess the chance for an optimal absorption of EIMTC after oral administration and at once its ability to permeate through the cell biomembranes. The results are provided in Table 4. EIMTC showed all precious drug-relevant structural descriptors and properties (employed by Lipinski in formulating the “rule of five” [22]) in the range that allows to anticipate although hypothetically its optimal bioavailability in vivo after per os administration [23]. This is an important finding taking into account a number of the experimental reports showing that a twofold violation of the “rule of five” in the case of known medicines may lead to serious bioavailability problems. Moreover, EIMTC was identified as being passively permeable by cell membranes. Its predicted topological polar surface area and molecular flexibility (expressed by the number of freely rotatable bonds) were in the optimal range for small molecular weight medicines that have a tendency to be easily transported across the cell biomembranes, including intestinal walls [24, 25]. Furthermore, dissociation constant and water solubility values for this possible ester prodrug at physiological conditions were also in a suitable range which allows to anticipate hypothetically a good bioavailability [26].
In turn, based on the in silico data taken from the OSIRIS Property Explorer (http://www.organic-chemistry.org/prog/ped/), EIMTC predicted to possess a drug-conform behaviour, being devoid of some undesired toxic effects like mutagenicity, tumorigenicity and irritant capacity.
Discussion
We have previously synthesized, fully characterized and thoroughly investigated numerous antiproliferative active agents bearing the privileged 7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-one scaffold with the anticipated affinity towards adenosine receptors (ARs). A number of them (the structures A1–A10 in Fig. 3) revealed the explicitly higher cytotoxicity for cancer over non-cancer cells [3–9] as well as lipophilic/hydrophobic parameters and molecular descriptors relevant to optimal pharmacokinetics [10]. Furthermore, related in structure, 3-aryl-[1,2,4]triazinobenzimidazol-4(10H)-ones (the structures B1–B4 in Fig. 3) have been reported by Taliani et al. [27] as selective A2B adenosine receptor antagonists that in the future may be of benefit in the therapy of cancer, diabetic retinopathy and inflammatory diseases. We have also focused particular attention on the designed and synthesized small molecular weight carbohydrazides containing fused azaisocytosine scaffold, disclosing 8-(4-methoxyphenyl)-4-oxo-6,7-dihydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (IMT) (the structure C1 in Fig. 3) as a novel A2A adenosine receptor antagonist in experimentally activated liver stellate cells and accordingly of benefit in the therapy of liver diseases [11, 12].
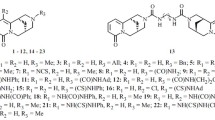
Structures of known antitumour active small molecules revealing the explicitly lower cytotoxicity for normal cells with the anticipated affinity towards ARs (A1–A10), related in structure selective A2B AR antagonists (B1–B4) and the described earlier non-selective A2A AR antagonist (C1). A1: R1 = 4-Cl; R2 = C6H5; A2: R1 = 2-CH3; R2 = fur-2-yl; A3: R1 = 2,3-(CH3)2; R2 = fur-2-yl; A4: R1 = 2-CH3O; R2 = fur-2-yl; A5: R1 = 2-Cl; R2 = fur-2-yl; A6: R1 = 2,6-Cl2; R2 = fur-2-yl; A7: R1 = 3-Cl; R2 = COOC2H5; A8: R1 = 3,4-Cl2; R2 = COOC2H5; A9: R1 = H; R2 = CONHNH2; A10: R1 = 3-Cl; R2 = CONHNH2; B1: R1 = H; R2 = C6H5; X = Cl; B2: R1 = H; R2 = C6H5; X = H; B3: R1 = 4-Cl; R2 = C6H5; X = H; B4: R1 = 4-Cl; R2 = fur-2-yl; X = H
Adenosine receptors have received attention as possible targets to disrupt adenosine-mediated immunosuppression in the tumour microenvironment, since adenosine was found to enhance the polarization of myeloid and T cell subsets towards proangiogenic and immunosuppressive phenotypes, enhancing tumour growth and survival. Furthermore, the blocking of adenosine-mediated signalling was found to aid the function of NK and CD8+ lymphocyte cells, which are responsible for the cancer immune surveillance [28]. There are studies in the literature describing the expression of some types of ARs on cancer cells, and in some of these research works, adenosine signalling is reported to induce apoptosis [29, 30].
In the present study, we have confirmed that 0.158 mM EIMTC reveals not only remarkable inhibitory effects on the proliferative capacity of human malignant multiple myeloma cells but also the lower toxicity for normal HSF cells. We have demonstrated that EIMTC reveals cytotoxic effects even in cells resistant to the anti-multiple myeloma agent—thalidomide—such as MM1R cells. These in vitro preliminary experimental findings justify a potential rationale for further evaluation of EIMTC as a possible anti-multiple myeloma agent, especially by reason of the fact that human multiple myeloma cells in clinical studies are still regarded as capable of becoming resistant to a conventional therapy [31], including thalidomide. Thalidomide, which entered phase III clinical trials, has emerged as a great hope for the treatment of the incurable patients with multiple myeloma. Nevertheless, some multiple myeloma cells resistant to thalidomide (MM1R) have already been isolated.
There is an ongoing search for novel agents targeting malignant cells since the majority of commonly authorized cytotoxic chemotherapeutics are equally as toxic to healthy tissues. So, among the available structural libraries of numerous antiproliferative agents, only the lead structures with the most profitable toxicity profile in vitro are still being extensively investigated. Considering the above facts, we hope that the modified small molecular weight fused azaisocytosine-like congener—EIMTC—may be exploited as a lead structure with the useful in vitro anticancer activity and a profitable toxicity profile. This possible ester prodrug may be employed for the design and development of potent and more selective anti-multiple myeloma agents targeting to malignant cells (also cells resistant to conventional therapies, including thalidomide). These agents in the future may represent an innovation in the field of cancer research. However, in order to assess whether EIMTC is of benefit in the therapy of multiple myeloma further more detailed in vivo experiments including animal models should be performed, which lay outside the scope of this paper.
Firstly, the remarkable antiproliferative effects of EIMTC were found to be connected with the induction of the cell cycle arrest in some human tumour cells. Moreover, the obtained results of the cell cycle analysis suggest that EIMTC is capable of arresting different phases of the cell cycle (S and G2/M) in human carcinoma cells of uterus, ovary and breast and therefore may supposedly be successfully used to limit the tumour progression in these severe cancers. Secondly, the EIMTC treatment did not induce apoptosis but necrosis. Hence, we speculate that an alternative necrosis cell death pathway induced by EIMTC may be of benefit in the chemotherapy of severe tumours that are not sensitive to the apoptosis (because of mutation faults that are capable of effectively inactivating the normal apoptosis signalling pathways) [32, 33].
It has been proved in present studies that EIMTC possesses a certain level of selectivity towards normal epithelial cells which allows to assume a lower damage to the epithelium of the gastrointestinal tract, justifying its further in vivo testing. This finding is significant in the field of anticancer research.
It should be noted that this is the first communication reporting that the synthetically modified congener of fused azaisocytosine—EIMTC—induces the cell cycle arrest in human tumour cells and accordingly prevents the cell proliferation. The inhibition of cell proliferation would be predictably due to a competition for nucleotides required for DNA synthesis. Subsequent studies are being continued to completely explain the mechanisms mediating the biogenic effects of that promising molecule. On the other hand, we would like to believe that the identified lead structure (EIMTC) may be of benefit in designing novel anticancer agents inducing an alternative necrosis-death pathway (that in accordance with newest significant findings [34] may unfold also in a highly regulated and controlled manner). It has been confirmed in our investigations that EIMTC (that should interact with the adenosine receptors) not only evokes the necrosis-dependent cell death but also causes the explicitly higher levels of necrotic cell populations in tumour HeLa cells than in normal Vero cells of the same epithelial origin, what makes this molecule promising for further more extensive investigations in the field of antitumoural search.
Noteworthy is that the cytotoxic effects caused by 0.158 mM EIMTC in T47D and HeLa cells were distinctly higher than those evoked by 0.234 mM pemetrexed—an approved folate antimetabolite—displaying 10 % ± 0.8, 20 % ± 1.2 and 30 % ± 2.2 growth inhibition in T47D cells and 5 % ± 0.7, 10 % ± 0.9 and 20 % ± 1.5 growth inhibition in HeLa cells after 24-, 48- and 72-h incubation periods, respectively, when investigated in vitro [35].
In summary, based on the above-mentioned findings we propose the designed and synthetically modified congener of fused azaisocytosine, that is, EIMTC (inducing the cell cycle arrest in some human neoplastic cells, evoking higher rates of necrotic cells in tumour than in normal cells of the same epithelial origin and also causing significant anti-multiple myeloma effects combined with a lower toxicity towards normal cells) as a lead structure for the design and development of more selective antineoplastic agents with a lower toxicity for normal cells (that in the future may represent an innovation in the field of cancer chemotherapy). The present report also describes the advantages of an alternative microwave-assisted synthetic approach leading to EIMTC which was developed to improve on existing method for the obtainment of that anomalous nucleobase-like congener.
References
Rusinov VL, Ulomskii EN, Chupakhin ON, Charushkin VN (2008) Azolo[5,1-c]-1,2,4-triazines as a new class of antiviral compounds. Russ Chem Bull Int Ed 57:985–1014
Farras J, del Mar LM, Vilarrasa J, Castillon S, Matheu M, Solans X, Font-Bardia M (1996) New bicyclic nucleosides related to 6-azaisocytidine. Tetrahedron Lett 37:901–904
Sztanke K, Rzymowska J, Niemczyk M, Dybała I, Kozioł AE (2006) Synthesis, crystal structure and anticancer activity of novel derivatives of ethyl 1-(4-oxo-8-aryl-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)formate. Eur J Med Chem 41:539–547
Sztanke K, Pasternak K, Rzymowska J, Sztanke M, Kandefer-Szerszeń M (2008) Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives. Eur J Med Chem 43:1085–1094
Sztanke K, Pasternak K, Sztanke M, Kandefer-Szerszeń M, Kozioł AE, Dybała I (2009) Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1,2,4-triazinones. Bioorg Med Chem Lett 19:5095–5100
Sztanke K, Tuzimski T, Sztanke M, Rzymowska J, Pasternak K (2011) Synthesis, structure elucidation, determination of the lipophilicity and identification of antitumour activities in vitro of novel 3-(2-furanyl)-8-aryl-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-ones with a low cytotoxicity towards normal human skin fibroblast cells. Bioorg Med Chem 19:5103–5116
Sztanke K, Sztanke M, Pasternak K (2012) 3-(2-Furanyl)-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-ones substituted with mono- or dichlorophenyl and process for the preparation thereof. Polish Patent PL 212442
Sztanke K, Sztanke M, Pasternak K (2012) Derivatives of 3-(2-furanyl)-7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-one substituted with phenyl, alkylphenyl, alkoxyphenyl and process for the preparation thereof. Polish Patent PL 212447
Sztanke M, Rzymowska J, Sztanke K (2013) Synthesis, structure elucidation and in vitro anticancer activities of novel derivatives of diethyl (2E)-2-[(2E)-(1-arylimidazolidin-2-ylidene)hydrazono]succinate and ethyl (4-oxo-8-aryl-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetate. Bioorg Med Chem 21:7465–7480
Janicka M, Sztanke M, Sztanke K (2013) Reversed-phase liquid chromatography with octadecylsilyl, immobilized artificial membrane and cholesterol columns in correlation studies with in silico biological descriptors of newly synthesized antiproliferative and analgesic active compounds. J Chromatogr A 1318:92–101
Kandefer-Szerszeń M, Szuster-Ciesielska A, Sztanke K, Pasternak K (2014) 8-(4-Methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-formic acid hydrazide used as a drug for liver diseases. Polish Patent PL 216264
Szuster-Ciesielska A, Sztanke K, Kandefer-Szerszeń M (2012) A novel fused 1,2,4-triazine aryl derivative as antioxidant and nonselective antagonist of adenosine A2A receptors in ethanol-activated liver stellate cells. Chem Biol Interact 195:18–24
Vega-Avila B, Pugsley M (2011) An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc 54:10–14
Ellwart J, Dormer P (1985) Effect of 5-fluoro-2′-deoxyuridine (FdUrd) on 5-bromo-2′-deoxyuridine (BrdUrd) incorporation into DNA measured with a monoclonal BrdUrd antibody and by the BrdUrd/Hoechst quenching effect. Cytometry 6:513–520
Huong PL, Kolk AH, Eggelte TA, Verstijnen CP, Gilis H, Hendriks JT (1991) Measurement of antigen specific lymphocyte proliferation using 5-bromo-deoxyuridine incorporation. An easy and low cost alternative to radioactive thymidine incorporation. J Immunol Methods 140:243–248
Magaud JP, Sargent I, Mason DY (1988) Detection of human white cell proliferative responses by immunoenzymatic measurement of bromodeoxyuridine uptake. J Immunol Methods 106:95–100
Muir D, Varon S, Manthorpe M (1990) An enzyme-linked immunosorbent assay for bromodeoxyuridine incorporation using fixed microcultures. Anal Biochem 185:377–382
Sztanke M, Rzymowska J, Sztanke K (2015) Synthesis, structure elucidation and identification of antiproliferative activities of a novel class of thiophene bioisosteres bearing the privileged 7,8-dihydroimidazo[2,1-c][1,2,4]triazin-4(6H)-one scaffold. Bioorg Med Chem 23:3448–3456
Pożarowski P, Halicka D, Darzynkiewicz Z (2003) NF-κB inhibitor sesquiterpene parthenolide induces concurrently atypical apoptosis and cell necrosis: difficulties in identification of dead cells in such cultures. Cytometry A 54:118–124
Fiołka MJ, Grzywnowicz K, Rzymowska J, Lewtak K, Szewczyk R, Mendyk E, Keller R (2015) Antitumour and apoptotic effects of a novel Tris-peptide complex obtained after isolation of Raoultella ornithinolytica extracellular metabolites. J Appl Microbiol 118:1357–1369
Ziegler V, Groscurth P (2004) Morphological features of cell death. News Physiol Sci 19:124–128
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26
Palm K, Stenberg P, Luthman K, Artursson P (1997) Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm Res 14:568–571
Eartl P, Rhode B, Seltzer P (2000) Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 43:3714–3717
Veber DE, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623
Keserű GM, Makara GM (2009) The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discov 8:203–212
Taliani S, Pugliesi I, Barresi E, Simorini F, Salerno S, La Motta C, Marini AM, Cosimelli B, Cosconati S, Di Maro S, Marinelli J, Daniele S, Trincavelli ML, Greco G, Novellino E, Martini C, Da Settimo F (2012) 3-Aryl-[1,2,4]triazinobenzimidazol-4(10H)-one: a novel template for the design of highly selective A2B adenosine receptor antagonists. J Med Chem 55:1490–1499
Young A, Mittal D, Stagg J, Smyth MJ (2014) Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 4:1–10
Hajiahmadi S, Panjehpour M, Aghaei M, Shabani M (2015) Activation of A2b adenosine receptor regulates ovarian cancer cell growth: involvement of Bax/Bcl-2 and caspase 3. Biochem Cell Biol 93:321–329
Kanno T, Nakano T, Fujita Y, Gotoh A, Nishizaki T (2012) Adenosine induces apoptosis in SBC-3 human lung cancer cells through A3 adenosine receptor-dependent AMID upregulation. Cell Physiol Biochem 30:666–677
Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai Y-T, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC (2000) Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 96:2943–2950
Edinger LE, Thompson BC (2004) Death by design: apoptosis, necrosis and authophagy. Curr Opin Cell Biol 16:663–669
Siddik ZH (2014) In: Neidle S (ed) Cancer drug design and discovery. Elsevier Academic Press, London, pp 357–390
Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic death pathways. Nat Rev Mol Cell Biol 15:135–147
Sztanke M, Rzymowska J, Janicka M, Sztanke K (2016) Synthesis, structure elucidation, determination of antiproliferative activities, lipophilicity indices and pharmacokinetic properties of novel fused azaisocytosine-like congeners. Arab J Chem. doi:10.1016/j.arabjc.2016.04.002
Acknowledgments
Authors gratefully acknowledged Dr. Katarzyna Skórzyńska (Medical University of Lublin, Poland) for the generous gifts of MM1R and MM1S cell lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sztanke, M., Rzymowska, J. & Sztanke, K. In vitro effects of a new fused azaisocytosine-like congener on relative cell proliferation, necrosis and cell cycle in cancer and normal cell cultures. Mol Cell Biochem 418, 179–188 (2016). https://doi.org/10.1007/s11010-016-2744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2744-8