Abstract
Adiponectin is an anti-diabetic and anti-atherogenic adipokine; its plasma levels are decreased in obesity, insulin resistance, and type 2 diabetes. An adiponectin-interacting protein named disulfide bond A-like protein (DsbA-L) plays an important role in the assembly of adiponectin. This study examined the hypothesis that l-cysteine (LC) regulates glucose homeostasis through the DsbA-L upregulation and synthesis and secretion of adiponectin in diabetes. 3T3L1 adipocytes were treated with LC (250 and 500 µM, 2 h) and high glucose (HG, 25 mM, 20 h). Results showed that LC supplementation significantly (p < 0.05) upregulated the DsbA-L, adiponectin, and GLUT-4 protein expression and glucose utilization in HG-treated adipocytes. LC supplementation significantly (p < 0.05) promoted the secretion of total and HMW adiponectin secretion in HG-treated adipocytes. In addition, LC significantly (p < 0.05) decreased ROS production and MCP-1 secretion in HG-treated cells. We further investigated whether MCP-1 has any role of LC on DsbA-L expression and adiponectin levels in 3T3-L1 cells. Treatment with LC prevented the decrease in DsbA-L, adiponectin, and GLUT-4 expression in 3T3L1 adipocyte cells exposed to MCP-1. Thus, this study demonstrates that DsbA-L and adiponectin upregulation mediates the beneficial effects of LC on glucose utilization by inhibiting MCP-1 secretion in adipocytes and provides a novel mechanism by which LC supplementation can improve insulin sensitivity in diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adiponectin is a major adipocyte-secreted protein and is downregulated in obesity and diabetes [1]. Epidemiological evidence has indicated that circulating adiponectin levels are reduced in patients with insulin resistance, type 2 diabetes, obesity, or cardiovascular disease [2–4]. Adiponectin is considered a potent modulator of lipid and glucose metabolism with anti-diabetic, anti-atherogenic, and anti-inflammatory properties, and it plays an important role in the pathogenesis of metabolic diseases. Circulating adiponectin exists in three oligomeric forms: trimer, hexamer, and high molecular weight (HMW), the latter being the major bioactive isoform. DsbA-L protein was recently identified as a chaperone protein that catalyzes two trimers into a disulfide-linked hexamer, which further helps assemble the HMW form of adiponectin; [5]. The level of DsbA-L is significantly reduced in obese mice and human subjects [5]. Studies showed that fat-specific overexpression of DsbA-L promoted adiponectin multimerization and protected mice against diet-induced obesity and insulin resistance [6] suggesting a potential mechanism by which DsbA-L promotes adiponectin biosynthesis and function. DsbA-L has been identified as a key regulator for adiponectin multimerization and secretion in adipocytes. Thus, targeting adiponectin synthesis and secretion has been regarded as an important therapeutic tool for preventing insulin resistance associated with obesity and type 2 diabetes.
Diabetic patients have lower blood levels of l-cysteine and altered cysteine homeostasis [7–10]. Supplementation with cysteine-rich proteins (whey protein and α-lactalbumin) [11–14], l-cysteine (LC) or N-acetylcysteine [15–23], or the cysteinate form of different compounds is better than the non-cysteinate form [24–27] at increasing glucose utilization and lowering glycemia in diabetic animal studies. There is epidemiological evidence suggesting that overweight subjects with a high intake of milk and dairy products are at lower risk of developing diseases related to the insulin resistance syndrome [14]. Recognition of l-cysteine deficiency has led to many supplementation studies using N-acetylcysteine (NAC) as a source to replenish cysteine levels in diabetes. Recent reviews have discussed various studies that provide evidence for the benefits of LC supplementation in lowering oxidative stress and insulin resistance biomarkers in type 2 diabetic patients and diabetic animals [7, 28]. There is no study that has examined whether DsbA-L plays any role in the beneficial effect of l-cysteine on improved glucose metabolism in diabetes.
This study has examined the hypothesis that l-cysteine upregulates the DsbA-L and therefore could promote synthesis and secretion of adiponectin in adipocytes through DsbA-L upregulation mediated by monocyte chemoattractant protein-1 inhibition in 3T3-L1 adipocytes exposed to high glucose.
Materials and methods
3T3L1 cell culture and differentiation
The murine 3T3L1 fibroblast cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). These cells were cultured in high-glucose (HG) DMEM containing 10 % (v/v) FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin and maintained at 37 °C in a humidified atmosphere containing 5 % (v/v) CO2. Three days after achieving confluence, to allow for differentiation into adipocytes, cells were incubated in HG DMEM containing 10 % (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin supplemented with 100 milliunits/ml insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 250 nM dexamethasone for 2 days. The cells were then placed in the same medium containing insulin but lacking any other supplements for an additional 2 days. The media were replaced every 2 days thereafter until >85 % of the cells contained lipid droplets. Seven to 10 days after the induction of differentiation, 3T3L1 adipocytes were ready to be used in experiments [29]. The cells were incubated with serum-free low-glucose DMEM during the experimental incubation period.
Treatment with high glucose (HG) and l-cysteine (LC)
Cells were treated with normal glucose (5.5 mM) and HG (25 mM) with and without LC. In this study, cells were exposed to 25 mM HG. Many previous studies have reported that glucose concentrations as high as 50 mM have been found in the blood of patients with uncontrolled diabetes [30]. It is true that blood glucose levels in patients are not likely to stay as high as 25 mM for 24 h. However, tissue damage in diabetic patients occurs over many years of countless hyperglycemic episodes. Thus, the glucose concentration of 25 mM used in this cell-culture study does not seem unreasonable. Cells were pretreated with two different concentrations (250 and 500 μM) of LC for 2 h, followed by HG exposure for the next 20 h. After treatment, cells were lysed in RIPA supplemented with protease and phosphatase inhibitors. Lysates were cleared by centrifugation and total protein concentrations were determined using a BCA assay kit (Pierce/Thermo Scientific, Rockford, IL, USA).
MCP-1 treatment of adipocytes
3T3-L1 cells were treated with recombinant human MCP-1 (SRP3109, Sigma-Aldrich, St. Louis, MO, USA) dissolved in phosphate buffered saline solution containing 1 mg/mL bovine serum albumin at a concentration of 1 and 2.5 ng/ml for 24 h under serum-free conditions. After treatment, cells were lysed in RIPA supplemented with protease and phosphatase inhibitors. Lysates were cleared by centrifugation and total protein concentrations were determined using a BCA assay kit (Pierce/Thermo Scientific, Rockford, IL, USA).
Glucose utilization and cytokine secretion
Glucose assays were done at 0 h and at the end of the experiments. The glucose utilization level was determined by subtracting glucose values at the end of the experiments (leftover glucose) from the 0 h glucose level. All assays were done in duplicate at each time point. Bayer Contour Next EZ Glucose Meter (Bayer HealthCare LLC, Mishawaka, IN, USA) was used for the glucose utilization assay. The MCP-1 (monocyte chemoattractant protein-1) level in the supernatants of treated cells was determined by the sandwich ELISA method using a commercially available kit from R&D Systems (Minneapolis, MN, USA). Adiponectin levels were determined using a kit and reagents from ALPCO Diagnostics (Salem, NH). All appropriate controls and standards as specified by each manufacturer’s kit were used. In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analyses.
ROS assay in adipocytes
For the ROS measurement, cells were pretreated with two different concentrations (250 and 500 μM) of LC for 2 h, followed by HG exposure for the next 20 h. After treatment, cells were labeled with 2′, 7′-dichlorofluorescein diacetate (H2DCFDA, Sigma Chemical Co., St. Louis, MO, USA) at a concentration of 20 µM and incubated at 37 °C for 30 min using a plate reader, and the fluorescence was determined at filter settings of 485 nm excitation and 528 nm emission.
Immunoblotting
All samples contained approximately the same amount of protein (~15–20 µg) and were run on 10 % SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked at room temperature for 2 h in a blocking buffer containing 1 % BSA to prevent non-specific binding and then incubated with anti-DsbA-L (1:2000 dilution), anti-adiponectin (1:1000), and anti-GLUT4 (1:1000) primary antibodies at 4 °C overnight. The membranes were washed in TBS-T (50 mmol/L Tris–HCl, pH 7.6, 150 mmol/L NaCl, 0.1 % Tween 20) for 30 min and incubated with the appropriate HRP-conjugated secondary antibody (1:5000 dilution) for 2 h at room temperature and developed using the ultrasensitive ECL substrate (Millipore, MA). The intensity of each immunoblotting band was measured using the histogram tool of Adobe Photoshop CS5. Antibodies against DsbA-L, adiponectin, GLUT-4, and β-actin were purchased from Abcam, Inc. (Cambridge, MA, USA). All other chemicals were purchased from Sigma unless otherwise mentioned.
Statistical analysis
Results are expressed as mean ± SE. Data were analyzed using Student’s t test or ANOVA followed by Duncan’s multiple range test (DMRT with SPSS version 15.00.; SPSS Inc., Chicago, IL, USA). In all cases, p < 0.05 was considered to be statistically significant. In all tests, p < 0.05 was considered significant.
Results
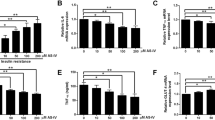
Figure 1 illustrates the effect of LC supplementation on DsbA-L, adiponectin levels, and total and HMW adiponectin secretion in HG-treated 3T3L1 adipocytes. It shows that HG exposure caused a reduction in the expression of both DsbA-L (Fig. 1a) as well as adiponectin (Fig. 1b). In addition, HG exposure also caused a significant decrease in total (Fig. 1d) and HMW adiponectin secretion (Fig. 1e) in adipocytes. Supplementation with LC caused the upregulation of DsbA-L and adiponectin protein expression, and increased the secretion of total and HMW adiponectin in HG-treated adipocytes. LC did not have any effect in control glucose-treated adipocytes.
Effect of LC supplementation on DsbA-L, adiponectin, GLUT-4 protein expression, total and HMW adiponectin secretion, and glucose utilization in 3T3L1 adipocytes exposed to high glucose. a DsbA-L protein expression, b adiponectin protein expression, c GLUT-4 protein expression, d total adiponectin secretion, e HMW adiponectin secretion, and f glucose utilization. Cells were pretreated with LC for 2 h followed by high-glucose (25 mM) exposure for the next 20 h. Values are mean ± SE (n = 3)
GLUT4 is a major player in the regulation of glucose metabolism. To determine whether upregulation of adiponectin by LC also upregulates the glucose metabolism, we investigated the effect of LC supplementation on GLUT4 and glucose utilization in adipocytes exposed to high glucose. Figure 1c, f shows the effect of LC supplementation on GLUT4 total protein expression and glucose utilization in HG-treated 3T3L1 adipocytes. HG treatment caused a significant decrease in GLUT4 protein expression (Fig. 1c) in adipocytes. Treatment with LC upregulated the GLUT4 total protein expression and glucose utilization in HG-treated adipocytes. The beneficial effect on glucose utilization level (Fig. 1f) was greater with higher concentration of LC.
ROS levels are elevated in diabetes and in high-glucose-treated cell and are implicated to play a role in impaired glucose metabolism [31, 32]. Figure 2 demonstrates the effect of LC supplementation on ROS production and MCP-1 secretion in HG-treated 3T3L1 adipocytes. We also observed that HG was capable of producing more ROS in adipocytes and LC pretreatment of cells resulted in significantly lower levels of ROS than those seen in the cells treated with only HG (Fig. 2a). Similarly, HG stimulated the secretion of MCP-1 and pretreatment with LC significantly lowered the secretion of MCP-1 in HG-treated adipocytes (Fig. 2b).
Since HG has increased the secretion of MCP-1, we analyzed whether HG affects adiponectin synthesis and secretion and DsbA-L and GLUT-4 expression are mediated through MCP-1. Figure 3 illustrates the effect of MCP-1 on adipocytes at two different concentrations (1.0 and 2.5 ng/ml). Treating 3T3-L1 adipocytes with MCP-1 significantly and concentration dependently inhibited the expression of DsbA-L (Fig. 3a), adiponectin (Fig. 3b), and GLUT-4 (Fig. 3c) expression in adipocytes.
Effect of MCP-1 treatment on DsbA-L, adiponectin, and GLUT-4 protein expression in 3T3L1 adipocytes. a DsbA-L protein expression, b adiponectin protein expression, and c GLUT-4 protein expression. Cells were pretreated with LC for 2 h followed by MCP-1 exposure for the next 24 h. Values are mean ± SE (n = 3)
To determine whether LC has the potential to overcome MCP-1 effect on adiponectin synthesis and secretion and GLUT-4 expression in adipocytes, we pretreated the cells with LC for 2 h before treatment with MCP-1 (2.5 ng/ml) for next 24 h. Figure 4 shows that adipocytes exposed to MCP-1 significantly attenuated DsbA-L (Fig. 4a) expression and reduction in adiponectin (Fig. 4b) expression. In addition, MCP-1 exposure also caused a significant decrease in total (Fig. 4d) and HMW adiponectin secretion (Fig. 4e) in adipocytes. Pretreatment with LC prevented the deleterious effects on the DsbA-L and adiponectin protein expression and the secretion of total and HMW adiponectin in MCP-1 treated adipocytes. Similarly, pretreatment with LC prevented the inhibition of GLUT4 (Fig. 4c) in adipocytes exposed to MCP-1.
Effect of LC supplementation on DsbA-L, adiponectin, and GLUT-4 protein expression and total and HMW adiponectin secretion in 3T3L1 adipocytes exposed to MCP-1. a DsbA-L protein expression, b adiponectin protein expression, c GLUT-4 protein expression, d total adiponectin secretion, and e HMW adiponectin secretion. Cells were pretreated with LC for 2 h followed by MCP-1 exposure for the next 24 h. Values are mean ± SE (n = 3). Data in Fig. 4b were analyzed by using Student’s t test
Discussion
The present study demonstrates that LC upregulates the synthesis and secretion of adiponectin in 3T3-L1 adipocytes exposed to high glucose. l-cysteine supplementation also caused inhibition of MCP-1 secretion in high-glucose-exposed adipocytes. The expression of DsbA-L and GLUT-4 and glucose utilization in HG-treated adipocytes were also increased by the LC supplementation. In addition, exogenous MCP-1 treatment inhibited the adiponectin synthesis and secretion and LC restores the synthesis of adiponectin in MCP-1-treated adipocytes.
A strong correlation between adiponectin and systemic insulin sensitivity has been well established both in vivo and in vitro in mice, other animals, and humans [33–42]. Insulin-sensitizing thiazolidinedione (TZD) class of compounds are known to improve lower plasma levels of adiponectin associated with type 2 diabetes [43–45]. Epidemiological studies demonstrate a strong correlation between insulin sensitivity and circulating adiponectin levels [46, 47]. Clinical studies suggest that high-molecular weight form of adiponectin is a better correlate for insulin sensitivity in general than total adiponectin [48, 49]. This study demonstrates that LC supplementation upregulates the DsbA-L expression and increases the synthesis and secretion of total and HMW adiponectin. LC supplementation increased GLUT-4 protein expression and glucose utilization in adipocytes exposed to high glucose. This suggests that LC upregulation of DsbA-L may have a key role in increasing adiponectin level, thereby increasing the glucose utilization and metabolism in adipocytes.
MCP-1 levels are increased in obese subjects and diabetic patients. MCP-1 is an important factor involved in inflammatory processes and induces the inflammation of adipocytes and microvasculature [50]. Our results show that HG significantly increased secretion of MCP-1 and treatment with LC effectively inhibited the MCP-1 secretion in HG-treated adipocytes. On the other hand, treatment with MCP-1 significantly reduces adiponectin synthesis and secretion and DsbA-L and GLUT-4 expression. This study also demonstrates that LC effectively attenuated the MCP-1-mediated decrease in adiponectin synthesis and secretion in 3T3-L1 adipocytes. Furthermore, the MCP-1-induced reduction in the DsbA-L and GLUT-4 protein expression was also prevented by LC. MCP-1 concentration used (2.5 ng/ml) is similar to that reported in the blood of patients [51, 52]. Hence, the concentration of MCP-1 used to treat adipocytes is within a physiological range. The effect of LC on MCP-1 receptors, CCR2, and CCR4 expression levels in cells is not known. These results indicate that LC supplementation can reverse the MCP-1-mediated changes in the synthesis and secretion of adiponectin at DsbA-L level in 3T3-L1 adipocytes. We believe that MCP-1 is not the only contributing factor in the harmful effect of high glucose on adiponectin secretion in adipocytes; factor(s) other than MCP-1 may also contribute to the inhibition of adiponectin secretion by high glucose.
Oxidative stress plays an important role in the pathogenesis of IR [53–58]. High-glucose treatment is known to produce ROS which in turn activates the transcription factor NF-κB and increased expression and secretion of MCP-1 [59], suggesting that ROS generation played a substantial role in the MCP-1 secretion, thereby contributing to impaired glucose metabolism. Other studies also suggest that MCP-1 via MCP-1-induced protein (MCPIP) can enhance ROS generation in cardiomyocytes [60]. This suggests that excess ROS can increase MCP-1 secretion which can cause further increase in ROS and impaired glucose metabolism. Activation of GLUT4 by the insulin-dependent and/or insulin independent signaling pathway plays a critical role in the glucose metabolism and has been suggested as a therapeutic target for pharmacological strategies to control hyperglycemia [61]. The present study shows that LC prevented the ROS accumulation induced by the HG indicating the beneficial effect of LC on DsbA-L and GLUT4 expression and glucose utilization may be related to the inhibition of intracellular oxidative stress.
Adiponectin appears to be a major modulator of insulin action and its levels are reduced in type 2 diabetes, and thus targeting adiponectin synthesis has been regarded as an important therapeutic tool for preventing the insulin resistance associated with type 2 diabetes. Figure 5 shows a schematic representation of the mechanism by which LC supplementation can inhibit the ROS and MCP-1. The inhibition of MCP-1 can prevent the downregulation of DsbA-L and the impairment in adiponectin synthesis and insulin resistance in diabetes. Our study provides a novel biochemical mechanism and the ability of LC to upregulate total and HMW adiponectin synthesis and glucose utilization in adipocyte cell model. Future studies are needed to confirm whether LC supplementation increases total and high-molecular weight adiponectin synthesis and glucose metabolism using type 2 diabetic patients. These studies will provide the biochemical mechanism by which LC or novel molecules containing cysteinyl moiety can be used as an adjuvant therapy to lower glycemia and prevent insulin resistance in diabetes.
References
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599
Lindsay RS, Walker JD, Havel PJ, Hamilton BA, Calder AA, Johnstone FD (2003) Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care 26:2244–2249
Sun H, Zhang Y, Gao P, Li Q, Sun Y, Zhang J, Xu C (2011) Adiponectin reduces C-reactive protein expression and downregulates STAT3 phosphorylation induced by IL-6 in HepG2 cells. Mol Cell Biochem 347:183–189
Dadson K, Liu Y, Sweeney G (2011) Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne) 2:62
Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F (2008) A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci USA 105:18302–18307
Liu M, Xiang R, Wilk SA, Zhang N, Sloane LB, Azarnoush K, Zhou L, Chen H, Xiang G, Walter CA, Austad SN, Musi N, DeFronzo RA, Asmis R, Scherer PE, Dong LQ, Liu F (2012) Fat-specific DsbA-L overexpression promotes adiponectin multimerization and protects mice from diet-induced obesity and insulin resistance. Diabetes 61:2776–2786
Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F (2011) Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34:162–167
Darmaun D, Smith SD, Sweeten S, Hartman BK, Welch S, Mauras N (2008) Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation. Pediatr Diabetes 9:577–582
Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA (2010) Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12:1333–1337
Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC (2010) Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 53:1722–1726
Blouet C, Mariotti F, Mikogami T, Tome D, Huneau JF (2007) Meal cysteine improves postprandial glucose control in rats fed a high-sucrose meal. J Nutr Biochem 18:519–524
Blouet C, Mariotti F, Azzout-Marniche D, Mathe V, Mikogami T, Tome D, Huneau JF (2007) Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic Biol Med 42:1089–1097
Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM (2004) Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 80:1246–1253
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH (2010) Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 91:966–975
Jain SK, Velusamy T, Croad JL, Rains JL, Bull R (2009) l-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic Biol Med 46:1633–1638
Song D, Hutchings S, Pang CC (2005) Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur J Pharmacol 508:205–210
Diniz YS, Rocha KK, Souza GA, Galhardi CM, Ebaid GM, Rodrigues HG, Novelli Filho JL, Cicogna AC, Novelli EL (2006) Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur J Pharmacol 543:151–157
Xia Z, Guo Z, Nagareddy PR, Yuen V, Yeung E, McNeill JH (2006) Antioxidant N-acetylcysteine restores myocardial Mn-SOD activity and attenuates myocardial dysfunction in diabetic rats. Eur J Pharmacol 544:118–125
Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, Giacca A, Fantus IG (2003) N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab 285:E744–E753
Ho E, Chen G, Bray TM (1999) Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J 13:1845–1854
Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M (1999) Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 48:2398–2406
Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP (1999) Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96:10857–10862
Pieper GM, Siebeneich W (1998) Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J Cardiovasc Pharmacol 32:101–105
Adachi Y, Yoshikawa Y, Sakurai H (2007) Antidiabetic zinc(II)-N-acetyl-L-cysteine complex: evaluations of in vitro insulinomimetic and in vivo blood glucose-lowering activities. BioFactors 29:213–223
Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH (2004) Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr 134:3245–3249
Lin CC, Yin MC, Hsu CC, Lin MP (2004) Effect of five cysteine-containing compounds on three lipogenic enzymes in Balb/cA mice consuming a high saturated fat diet. Lipids 39:843–848
Liu Z, Li J, Zeng Z, Liu M, Wang M (2008) The antidiabetic effects of cysteinyl metformin, a newly synthesized agent, in alloxan- and streptozocin-induced diabetic rats. Chem Biol Interact 173:68–75
Iyer SS, Accardi CJ, Ziegler TR, Blanco RA, Ritzenthaler JD, Rojas M, Roman J, Jones DP (2009) Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS ONE 4:e5017
An Z, Wang H, Song P, Zhang M, Geng X, Zou MH (2007) Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem 282:26793–26801
Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O (1995) Decreased erythrocyte membrane fluidity in poorly controlled IDDM Influence of ketone bodies. Diabetes Care 18:549–551
Manna P, Gungor N, McVie R, Jain SK (2014) Decreased cystathionine-gamma-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J Biol Chem 289:11767–11778
Manna P, Jain SK (2015) Obesity, oxidative stress, adipose tissue dysfunction and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord 13:423–444
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE (2002) Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 143:998–1007
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108:1875–1881
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y (2001) Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50:1126–1133
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2001) Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett 507:142–146
Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866
Tajiri Y, Hiramatsu S, Karashima T, Mimura K, Umeda F (2002) Adiponectin as a reliable marker for insulin resistance in type 2 diabetic patients (Abstract). Diabetes 51(Suppl. 2):A305
Matsubara M, Maruoka S, Katayose S (2002) Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol 147:173–180
Haque WA, Shimomura I, Matsuzawa Y, Garg A (2002) Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 87:2395
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099
Hirose H, Kawai T, Yamamoto Y, Taniyama M, Tomita M, Matsubara K, Okazaki Y, Ishii T, Oguma Y, Takei I, Saruta T (2002) Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism 51:314–317
Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, Wang JP, Chen CL, Tai TY, Chuang LM (2002) Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 25:376–380
Tao C, Sifuentes A, Holland WL (2014) Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic beta cells and adipocytes. Best Pract Res Clin Endocrinol Metab 28:43–58
Unger RH, Scherer PE (2010) Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 21:345–352
Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T (2006) Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362
Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162
Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30:1347–1355
Longo PL, Artese HP, Rabelo MS, Kawamoto D, Foz AM, Romito GA, Dib SA, Mayer MP (2014) Serum levels of inflammatory markers in type 2 diabetes patients with chronic periodontitis. J Appl Oral Sci 22:103–108
de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E (2003) Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 107:690–695
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev 9:25–53
Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA (2011) Inflammation, oxidative stress, and obesity. Int J Mol Sci 12:3117–3132
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Jain SK (1989) Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem 264:21340–21345
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Quan Y, Jiang CT, Xue B, Zhu SG, Wang X (2011) High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol Sin 32:188–193
Younce CW, Kolattukudy PE (2010) MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem J 426:43–53
Huang S, Czech MP (2007) The GLUT4 glucose transporter. Cell Metab 5:237–252
Acknowledgments
The authors are supported by grants from NCCAM of the National Institutes of Health (RO1 AT007442), the Malcolm Feist Endowed Chair in Diabetes from LSUHSC, Shreveport. The authors thank Georgia Morgan for excellent editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Achari, A.E., Jain, S.K. l-Cysteine supplementation increases adiponectin synthesis and secretion, and GLUT4 and glucose utilization by upregulating disulfide bond A-like protein expression mediated by MCP-1 inhibition in 3T3-L1 adipocytes exposed to high glucose. Mol Cell Biochem 414, 105–113 (2016). https://doi.org/10.1007/s11010-016-2664-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2664-7