Abstract
The inflammatory cells infiltrating the airways produce several mediators, such as reactive oxygen species (ROS). ROS and the oxidant-antioxidant imbalance might play an important role in the modulation of airways inflammation. In order to avoid the undesirable effects of ROS, various endogenous antioxidant strategies have evolved, incorporating both enzymatic and non-enzymatic mechanisms. Recombinant human deoxyribonuclease (rhDNase) in clinical studies demonstrated a reduction in sputum viscosity, cleaving extracellular DNA in the airways, and facilitating mucus clearance, but an antioxidant effect was not studied so far. Therefore, we evaluated whether the administration of rhDNase improves oxidative stress in a murine model of asthma. Mice were sensitized by two subcutaneous injections of ovalbumin (OVA), on days 0 and 7, followed by three lung challenges with OVA on days 14, 15, and 16. On days 15 and 16, after 2 h of the challenge with OVA, mice received 1 mg/mL of rhDNase in the lungs. Bronchoalveolar lavage fluid and lung tissue were obtained on day 17, for inflammatory and oxidative stress analysis. We showed that rhDNase did not alter the population of inflammatory cells, such as eosinophil cells, in OVA-treated rhDNase group but significantly improved oxidative stress in lung tissue, by decreasing oxygen reactive species and increasing superoxide dismutase/catalase ratio, glutathione peroxidase activity, and thiol content. Our data provide the first evidence that rhDNase decreases some measures of oxidative stress and antioxidant status in a murine model of asthma, with a potential antioxidant effect to be further studied in human asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is a complex inflammatory disorder characterized by airway inflammation, intermittent reversible airway obstruction, airway hyperreactivity (AHR), excessive mucus production, and elevated levels of immunoglobulin E (IgE), Th2 cytokines, and increased levels of reactive oxygen species (ROS) [1–3].
Reactive oxygen species generation occurs through various enzymatic pathways, which are essential in many physiological reactions such as killing invading microorganisms [4]. ROS can be produced endogenously within locations including mitochondria and phagocytes, whereas exogenous sources of ROS production have been linked to ozone, diesel fuels, and cigarette smoke [5].
Studies have shown that increases in ROS that occur during asthma are associated with damage to a wide range of biologic molecules in the lungs, and that oxidative stress plays a role in the pathogenesis of asthma [6–8]. ROS that have biological importance include superoxide anion radical (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·). Nitrogen reactive species (RNS) and derivatives of nitric oxide (NO), including peroxynitrite (ONOO−), have also been implicated in oxidation (nitration) of proteins and lipids. ROS and RNS can lead to cell injury through several mechanisms, including direct damage to DNA, lipid peroxidation generating proinflammatory molecules (such as cytokines), and protein oxidation (primarily in sulfhydryl groups) leading to altered protein activity [9, 10]. Under physiological conditions, reactive species are maintained in balance due in large by the neutralization capacity of the non-enzymatic and/or enzymatic antioxidant defense systems, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). An imbalance between the production of reactive species and antioxidant defense capacity tissue can induce oxidative stress [11]. In this context, it is known that excessive ROS production in asthma trigger key enzymatic and non-enzymatic alterations, leading to an antioxidant-oxidant imbalance in respiratory airways [12]. Therefore, antioxidant treatment of asthma has long been a subject of interest in asthma.
Airway obstruction in severe acute asthma is characterized by bronchoconstriction, airway edema, and mucus plugging [13]. In this context, sputum in asthmatic patients is known to be composed of activated and degenerated inflammatory cells, mucoproteins, and free DNA, released from disintegrated inflammatory cells. Recombinant human DNase (rhDNAse) is a mucolytic agent that reduces sputum viscosity by hydrolyzing extracellular DNA in sputum [14]. The efficacy of rhDNase has been well documented in patients with cystic fibrosis [15], and this therapy may be effective in children with severe acute asthma [16, 17]. Another mucolytic therapy is available, including thiol derivatives such as N-acetylcysteine (NAC). NAC is a thiol derivative endowed with antioxidant and anti-inflammatory properties [18] that reduce oxidant-induced lung damage in a variety of experimental models [19, 20].
This sulfhydryl group present in thiols enables thiol derivatives to break down the gel structure of mucus, by substituting sulfhydryl groups for the disulfide bonds connecting mucin proteins [21], and seems to show antioxidant activity in lung injury [22]. Therefore, the aim of this study is to verify whether rhDNase presents antioxidant effect in asthma, evaluating whether rhDNase administration improves oxidative stress in the lungs of a murine model of asthma.
Materials and methods
Animals
Thirty-two female adult BALB/c mice (6–8 weeks old) were used for all experiments (CeMBE, PUCRS). Animals were fed with a balanced chow diet with access to water ad libitum, housed in cages and maintained on a 12/12-h light/dark cycle.
Mice sensitization and challenge
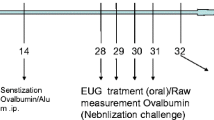
Mice were sensitized by two subcutaneous injections of 20 µg OVA (Grade V, Sigma, USA), diluted in Dulbecco's phosphate-buffered saline (DPBS; 200 µL), on days 0 and 7, followed by three intranasal challenges, under general anesthesia with isoflurane, with OVA (100 µg), in DPBS (50 µL), on days 14, 15, and 16. A control group, replacing OVA by PBS only, was included. On days 15 and 16, after 2 h of the OVA challenge, mice received, intranasally and under general anesthesia with isoflurane, 1 mg/mL of rhDNase (Roche UK, Welwyn Garden City, UK). The protocol of the study is illustrated in Fig. 1.
Bronchoalveolar lavage fluid (BALF)
Bronchoalveolar lavage fluid (BALF) was performed on day 17. Mice were anesthetized with intraperitoneal administration of a solution of ketamine (0.4 mg/g) and xylazine (0.2 mg/g), and the trachea was cannulated with a blunt needle. Lungs were then washed twice with DPBS (1 mL) and aspirated. BALF was centrifuged, and the pellet was resuspended, and total and differential cell counts of BALF were analyzed.
Tissue preparation
Lungs were homogenized in ten volumes (1:10, w/v) of 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl. The homogenates were centrifuged at 750×g for 10 min., at 4 °C, to separate nuclei and cell debris. The pellet was discarded, and the supernatant was immediately separated and used for analysis.
Total and differential cell counts from BALF
BALF was centrifuged at 2.000 rpm, for 4 min., at 4 °C. The supernatant was collected and the pellet was resuspended in 350 µL of DPBS. Total cell count (TCC) and cell viability were determined by trypan blue exclusion test with a Neubauer chamber (BOECO, Hamburg, Germany). For the differential cell analysis, slides of BALF suspension were obtained by cytospin preparations stained with H&E (Panótico Rápido—Laborclin, Brazil).
Reactive oxygen species (ROS) assay
ROS production was measured according to the method of LeBel et al. [23], based on the oxidation of 2′7′-dichlorofluorescein (H2DCF). The sample was incubated in a medium containing 100 μM of 2′7′-dichlorofluorescein diacetate (H2DCF-DA) solution. The reaction produces the fluorescent compound dichlorofluorescein (DCF), which is measured at λem = 488 nm and λex = 525 nm. Results were presented as nmol DCF/mg protein.
Thiol content assay
This assay is based on the reduction of 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) by sulfhydryl groups, which in turn become oxidized (disulfide), generating a yellow derivative, equivalent to the amount of sulfhydryl groups (TNB), whose absorption is measured spectrophotometrically at 412 nm. TNB levels are inversely correlated to oxidative damage to proteins. Results were reported as nmol of TNB levels per milligram of protein [24].
Nitrite assay
Nitrite levels were measured using the Griess reaction. The sample was incubated in a medium contained Griess reagent (1:1 mixture of 1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylenediamine dihydrochloride in water). The absorbance was measured at a wavelength of 543 nm. Nitrite concentration was calculated using sodium nitrite standards [25].
Glutathione peroxidase assay (GPx)
Glutathione peroxidase (GPx) activity was measured using tert-butyl-hydroperoxide as substrate [26]. NADPH disappearance was monitored at 340 nm. The medium contained 2 mM glutathione, 0.15 U/mL glutathione reductase, 0.4 mM azide, 0.5 mM tert-butyl-hydroperoxide and 0.1 mM NADPH. One GPx unit is defined as 1 μmol of NADPH consumed per minute. The specific activity is presented as GPx units/mg protein.
Superoxide dismutase assay (SOD)
Superoxide dismutase (SOD) activity assay is based on the capacity of pyrogallol to autoxidize, a process highly dependent on superoxide, which is a substrate for SOD. The inhibition of autoxidation of this compound occurs in the presence of SOD, whose activity was then indirectly assayed at 420 nm [27]. A calibration curve was performed with purified SOD as standard, in order to calculate the activity of SOD present in the samples. The results were presented as SOD units/mg protein.
Catalase assay (CAT)
Catalase (CAT) activity was assayed using a SpectraMax M5/M5 Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, California, USA). The method used is based on the disappearance of H2O2 at 240 nm, in a reaction medium containing 20 mM H2O2, 0.1 % Triton X-100, 10 mM potassium phosphate buffer pH 7.0, and 0.1–0.3 mg protein/ml [28]. One CAT unit is defined as 1 μmol of hydrogen peroxide consumed per minute, and the results were presented as CAT units/mg protein.
Protein determination
Protein was measured according to Lowry et al. [29] for all techniques. Serum bovine albumin was used as a standard.
Statistical analysis
Data are presented as mean ± SD. Results were analyzed using Statistical Package for the Social Sciences, version 20.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Tukey post hoc test was used. p ≤ 0.05 was considered statistically significant.
Ethics
The study was conducted under ethical standards for research in animal models, following the recommendations of the Brazilian Society of Laboratory Animal Science (SBCAL), advocating the use of fewer animals and adequate management of pain and suffering, during the study procedures and euthanasia. This study was approved by the Animal Ethics Committee of our Institution (#14/00387).
Results
Total and differential cells count in BALF
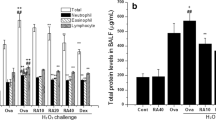
Firstly, we investigated the effect of rhDNase on the total cell count in BALF of mice. Figure 2a shows that rhDNase-treated OVA group did not alter total cell counts from BALF, compared to the OVA group [F(3,28) = 16.72, p > 0.05]. In addition, Fig. 2b shows that treatment with rhDNase did not decrease eosinophil counts [F(3,28) = 16.61, p > 0.05]. Also, Fig. 2c–e shows that treatment with rhDNase did not decrease macrophage [F(3,28) = 13.12, p > 0.05], neutrophil [F(3,28) = 5.82, p > 0.05], and lymphocyte [F(3,28) = 8.51, p > 0.05] counts.
Total and differential cell counts on BALF between the groups studied. Results are expressed as mean ± SD, for eight animals in each group. Different from DPBS group, **p < 0.01; different from DPBS + rhDNase group ***p < 0.001 (one-way ANOVA followed by Tukey test). BALF bronchoalveolar lavage fluid, OVA ovalbumin, rhDNase recombinant human deoxyribonuclease
Oxidative stress parameters and nitric oxide levels in lung tissue
Next, we evaluated the effect of rhDNase on oxidative stress in OVA-induced asthma in mice. Figure 3a demonstrates that OVA significantly increased the levels of reactive species in the lungs, as indicated by DCF formed from the oxidation of H2DCF, compared to the DPBS control group [F(3,28) = 16.20, p < 0.01]. On the other hand, rhDNase-treated mice significantly decreased DCF content, compared to the OVA control group [F(3,28) = 16.20, p < 0.05]. In addition, we investigated the effect of rhDNase on RNS. Figure 3b shows that nitrite levels in lung tissue were not altered by rhDNase treatment, as compared to DPBS and OVA control groups [F(3,28) = 2.17, p > 0.05].
Effect of rhDNase treatment on reactive species levels (a) and nitrite levels (b) in lung tissue between the groups studied. Results are expressed as mean ± SD for eight animals in each group. Different from DPBS group, **p < 0.01; different from OVA group, *p < 0.05 (one-way ANOVA followed by Tukey test). DCF dichlorofluorescein, OVA ovalbumin, rhDNase recombinant human deoxyribonuclease
Antioxidant defenses parameters in lung tissue
We also tested the effect of rhDNase treatment on the enzymatic antioxidant defenses (SOD and CAT) and SOD/CAT ratio. Figure 4a shows that rhDNase treatment did not alter SOD activity [F(3,28) = 1.18, p > 0.05], compared to the OVA group. On the other hand, we demonstrated that rhDNase treatment decreased CAT activity [F(3,28) = 12.32, p < 0.05], compared to the OVA group (Fig. 4b). Also, rhDNase per se decrease CAT activity [F(3,28) = 12.32, p > 0.05], compared to the DPBS group (Fig. 4b). In addition, Fig. 4c shows that rhDNase per se increases SOD/CAT ratio in lung tissue, compared to the control group [F(3,28) = 93.66, p < 0.001]. rhDNase-treated mice also increased SOD/CAT ratio in lung tissue, compared to OVA [F(3,28) = 93.66, p < 0.05]. Finally, Fig. 5 demonstrates the levels of other enzymatic antioxidant defenses in lung tissue. Figure 5a shows that GPx activity was significantly increased in OVA mice treated with rhDNase, compared to the OVA group [F(3,28) = 31.48, p < 0.05] and DPBS group treated with rhDNase [F(3,28) = 31.48, p < 0.001].
Effect of rhDNase treatment on SOD and CAT activity and SOD/CAT ratio in lung tissue between the groups studied. Results are expressed as mean ± SD for eight animals in each group. Different from DPBS group, ***p < 0.001; different from OVA group, *p < 0.05 (one-way ANOVA followed by Tukey test). SOD superoxide dismutase, CAT catalase, OVA ovalbumin, rhDNase recombinant human deoxyribonuclease
Effect of rhDNase treatment on GPx activity (a) and thiol content (b) in lung tissue between the groups studied. Results are expressed as mean ± SD for eight animals in each group. Different from DPBS + rhDNase group, ***p < 0.001; different from OVA group, *p < 0.05 (one-way ANOVA followed by Tukey test). GPx glutathione peroxidase, OVA ovalbumin, rhDNase recombinant human deoxyribonuclease
Figure 5b then shows that rhDNase per se significantly increased thiol content [F(3,28) = 28.06, p < 0.001], compared to the DPBS group. Furthermore, the animals treated with rhDNase also increased thiol content [F(3,28) = 28.06, p < 0.01], compared to the OVA group. On the other hand, OVA-treated mice significantly reduced thiol content [F(3,28) = 28.06, p < 0.05], compared to the DPBS control group.
Discussion
Asthma is a chronic inflammatory lung disease involving complex interactions between numerous cell types and mediators, resulting in bronchial hyperreactivity and airflow limitation. Evidence for the antioxidant imbalance in asthmatic airways has been shown in several studies, which show alterations not only in the non-enzymatic but also in the enzymatic antioxidants, in different components such as BALF and sputum [30–32].
Sputum in asthmatic patients is known to be composed of activated and degenerated inflammatory cells, mucoproteins, and free DNA, released from disintegrated inflammatory cells. rhDNAse is a mucolytic agent that reduces sputum viscosity by hydrolyzing extracellular DNA in sputum. Several studies demonstrated that rhDNAse reduces DNA fragment length and decreases the viscosity of purulent cystic fibrosis secretions [16, 33, 34]. DNA from mucous plugs following lysis of inflammatory cells contributes to increased viscosity and adhesiveness of the mucus. In addition, our group demonstrated that in a murine asthma model, there is a significant increase in DNA extracellular trap production in BALF and lung tissue, and that these traps were dismantled by DNase treatment in vitro [35]. In this context, we investigated the effect of rhDNAse treatment on oxidative stress in an experimental model of eosinophilic pulmonary response. Hence, in the present study, we initially investigated the effect of rhDNase on total cell count in BALF of mice. Interestingly, our results showed that rhDNase treatment did not decrease total cell and inflammatory cell counts in BALF in our murine asthma model. However, oxidative stress analysis showed distinct effects of rhDNAse treatment in lung tissue. A ROS increase was observed, as measured by the DCF fluorescence assay in the OVA group, compared to the control group. These results are in agreement with other studies showing that asthma increased oxidative stress in lung tissue [36–39]. Patients with asthma have shown increased airway ROS generation, such as O ·−2 and H2O2 [8, 40]. Moreover, oxidative stress in asthma increases the production of lipid peroxidation, products, and protein carbonyls in plasma and oxidized glutathione in BALF [41]. ROS also reacts with lipids to liberate isoprostane and ethane. As a result, 8-isoprostane, a biomarker of lipid peroxidation, is elevated in the exhaled breath condensate in adults and children with asthma [42–45]. In addition, ROS-mediated damage may result in increased vascular permeability, mucus hypersecretion and smooth muscle contraction, with impairment in the responsiveness of β-adrenergic receptors [42–45].
Thus, new strategies aimed to decrease ROS and increase endogenous antioxidants through pharmacological interventions to redress oxidant-antioxidant imbalance in asthma is a field of investigation in many research laboratories [39]. In this context, in order to prevent the undesirable effects of ROS, various endogenous antioxidant strategies have evolved, incorporating both enzymatic and non-enzymatic mechanisms. For instance, several non-enzymatic antioxidant species are present within the lung lining fluid such as glutathione, uric acid, albumin, vitamin C, and vitamin E [46]. In our study, rhDNAse reduced DCF fluorescence assay (a measure of reactive species levels) in rhDNase treated, compared to the control group.
Nitrite, which is present in low micromolar concentrations in the airway lining fluid, is a highly diffusible free radical, formed by the conversion of arginine to citrulline, via a family of nitric oxide synthase isoenzymes [47]. We did not find any difference between the animals treated with rhDNase. Asthmatics patients have increased markers of oxidative stress, including nitric oxide, carbon monoxide, xanthine oxidase activities, and 3-nitrotyrosine in sputum, but there are no previous studies about the effect of rhDNase in nitrite levels in a murine model of asthma [48].
Homeostasis of cellular functions during oxidative stress depends on the appropriate induction of protective antioxidant mechanisms. Antioxidants are major in vivo defense mechanisms of the cells against oxidative stress [37, 49–51]. The major enzymatic antioxidants of the lungs are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [52], and the non-enzymatic antioxidants include vitamin C, glutathione, albumin, vitamin E, uric acid, and β-carotene [52, 53]. An imbalance of the airways reducing state is a determinant of asthma initiation and severity [31, 32].
We also evaluated the effect of rhDNase on enzymatic antioxidant systems. SOD catalyzes the dismutation of superoxide anion (O ·−2 ), and CAT catalyzes the reduction of hydrogen peroxide (H2O2) [54]. In our study, we observed that rhDNase decreased CAT activity, but did not alter SOD activity. In this context, rhDNase treatment increased SOD/CAT ratio in rhDNase-treated and OVA-treated rhDNase. We demonstrated for the first time the antioxidant effect of rhDNase in the lungs of a murine asthma model. We suggest that the DCF formed was decreased in the lungs of animals treated with rhDNase, probably because ROS are being effectively neutralized by antioxidant enzymes, shown by the increase in SOD/CAT ratio in this study.
Based on these data, we then investigated the effect of rhDNase on GPx activity and thiol content in our model. We have shown that rhDNase treatment significantly increased GPx activity. Levels of the enzymes GPx and SOD and of the non-enzymatic components of the antioxidant system, including reduced glutathione (GSH), were significantly lower in children with asthma, when compared to healthy controls [55]. In addition, GSH/GSSG homeostasis, which is critical to normal cellular physiological processes, represents one of the most important antioxidant defense systems in lung cells. This system uses GSH as a substrate in the detoxification of peroxides, such as H2O2, a reaction that involves GPx [56]. In our study, rhDNase treatment improved this parameter. Decreased activity of GPx has been well documented [57, 58]. GPX is also essential for removing toxic oxidant products, which are continuously generated as a result of sequestration and infiltration of inflammatory leukocytes in the lung [41].
Previous studies have demonstrated alterations in different antioxidants in clinical and experimental asthma. Interestingly, alterations in antioxidant defenses might be increased or decreased, depending on whether the changes are due to a defense response (increase) or neutralizing effect by oxidants (decrease), whereas if the reserves are sufficient, there might be no alteration [41].
Finally, we investigated the effect of rhDNase treatment on thiol content (equivalent to the amount of sulfhydryl groups) in our model. rhDNase per se increased thiol content in our study. Oxidation of sulfhydryl groups of proteins causes conformational changes, protein unfolding, and degradation, inhibiting their activities [59, 60]. Decreased levels of thiol content is a measure of protein damage and the increase of the sulfhydryl groups is important to antioxidant effect since this group is susceptible to oxidation for free radical. In support of this, protein sulfhydryls were found to be decreased in asthmatic patients [61]. Thus, it might be expected that protein sulfhydryls are the main targets of ROS-mediated attack, reducing their levels [62].
Several mucolytic therapies are available for use in lung disease such as rhDNase [15], hypertonic saline [63], and thiol derivatives (NAC) [18]. NAC enables thiol derivatives to break down the gel structure of mucus, by substituting sulfhydryl groups for disulfide bonds, connecting mucin proteins. In vitro, NAC has been demonstrated to reduce the viscosity and elasticity of mucus, when directly in contact with airway secretions [64]. This effect was similar to the one found with rhDNase in our study, since the sulfhydryl groups interact directly with oxidants, functioning as an oxidant scavenger [65]. The present study is the first to evaluate the changes in some measures of oxidative stress and antioxidant status in rhDNase-treated mice in a model of asthma. Lastly, Fig. 6 summarizes the oxidant-antioxidant status present in the lungs treated with rhDNase.
One limitation of our study is that we did not assess lung function and bronchial hyper-responsiveness to methacholine. Given that the aim of our study was to investigate the antioxidant effect of rhDNase in a model of eosinophilic pulmonary response in mice, and its anti-inflammatory effect, the authors believe that this analysis may be further explored in future studies.
In conclusion, our data provide the first experimental demonstration that rhDNase decreases oxidative stress in the lungs and present a potential antioxidant effect in asthma, which could be further explored in future experimental and clinical studies in asthmatic patients, analyzing different doses and applying in particular clinical situations, such as severe exacerbations.
References
Renauld JC (2001) New insights into the role of cytokines in asthma. J Clin Pathol 54(8):577–589
Elias JA, Lee CG, Zheng T et al (2003) New insights into the pathogenesis of asthma. J Clin Invest 111(3):291–297
Comhair SA, Erzurum SC (2010) Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 12(1):93–124
Henricks PA, Nijkamp FP (2001) Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther 14(6):409–420
Zuo L, Youtz DJ, Wold LE (2011) Particulate matter exposure exacerbates high glucose-induced cardiomyocyte dysfunction through ROS generation. PLoS ONE 6(8):e23116
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5(1):9–19
Andreadis AA, Hazen SL, Comhair SA, Erzurum SC (2003) Oxidative and nitrosative events in asthma. Free Radic Biol Med 35(3):213–225
Sanders SP, Zweier JL, Harrison SJ et al (1995) Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med 151(6):1725–1733
Fialkow L, Chan CK, Grinstein S, Downey GP (1993) Regulation of tyrosine phosphorylation in neutrophils by the NADPH oxidase. Role of reactive oxygen intermediates. J Biol Chem 268(23):17131–17137
Fialkow L, Chan CK, Rotin D, Grinstein S, Downey GP (1994) Activation of the mitogen-activated protein kinase signaling pathway in neutrophils: role of oxidants. J Biol Chem 269(49):31234–31242
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York
Nadeem A, Masood A, Siddiqui N (2008) Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis 2(4):215–235
Silverman RA, Foley F, Dalipi R, Kline M, Lesser M (2012) The use of rhDNAse in severely ill, non-intubated adult asthmatics refractory to bronchodilators: a pilot study. Respir Med 106(8):1096–1102
Boogaard R, Smit F, Schornagel R et al (2008) Recombinant human deoxyribonuclease for the treatment of acute asthma in children. Thorax 63(2):141–146
Fuchs HJ, Borowitz DS, Christiansen DH et al (1994) Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331(10):637–642
Greally P (1995) Human recombinant DNase for mucus plugging in status asthmaticus. Lancet 346(8987):1423–1424
Puterman AS, Weinberg EG (1997) rhDNase in acute asthma. Pediatr Pulmonol 23(4):316–317
Duijvestijn YC, Brand PL (1999) Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatr 88(1):38–41
Cortijo J, Cerdá-Nicolás M, Serrano A et al (2001) Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J 17(6):1228–1235
Leff JA, Wilke CP, Hybertson BM, Shanley PF, Beehler CJ, Repine JE (1993) Postinsult treatment with N-acetyl-l-cysteine decreases IL-1-induced neutrophil influx and lung leak in rats. Am J Physiol 265(5 Pt 1):L501–L506
Dasgupta B, King M (1996) Reduction in viscoelasticity in cystic fibrosis sputum in vitro using combined treatment with nacystelyn and rhDNase. Pediatr Pulmonol 22(3):161–166
Ritter C, da Cunha AA, Echer IC et al (2006) Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med 34(2):471–477
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302(2–3):141–145
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Marklund SL (1984) Pyrogallol autoxidation. Handbook for oxygen radical research. CRC Press, Boca Raton
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Calhoun WJ, Reed HE, Moest DR, Stevens CA (1992) Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis 145(2 Pt 1):317–325
Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC (2000) Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 355(9204):624
Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC (2001) Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J 15(1):70–78
Babinski D, Trawinska-Bartnicka M (2008) Rhinosinusitis in cystic fibrosis: not a simple story. Int J Pediatr Otorhinolaryngol 72(5):619–624
Jones AP, Wallis C (2010) Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev 17(3):CD001127
Cunha AA, Porto BN, Nuñez NK et al (2014) Extracellular DNA traps in bronchoalveolar fluid from a murine eosinophilic pulmonary response. Allergy 69(12):1696–1700
Ciencewicki J, Trivedi S, Kleeberger SR (2008) Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122(3):456–468
Dworski R (2000) Oxidant stress in asthma. Thorax 55:S51–S53
Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O (2011) Oxidative stress in asthma. World Allergy Organ J 4(10):151–158
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26
Al-Harbi NO, Nadeem A, Al-Harbi MM et al (2015) Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int Immunopharmacol 26(1):237–245
Nadeem A, Chhabra SK, Masood A, Raj HG (2003) Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 111(1):72–78
Wedes SH, Khatri SB, Zhang R et al (2009) Noninvasive markers of airway inflammation in asthma. Clin Transl Sci 2(2):112–117
Hanazawa T, Kharitonov SA, Barnes PJ (2000) Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med 162(4 Pt 1):1273–1276
Paredi P, Kharitonov SA, Barnes PJ (2000) Elevation of exhaled ethane concentration in asthma. Am J Respir Crit Care Med 162(4 Pt 1):1450–1454
Dworski R, Roberts LJ II, Murray JJ, Morrow JD, Hartert TV, Sheller JR (2001) Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP(8-iso-PGF2alpha). Clin Exp Allergy 31(3):387–390
Kirkham P, Rahman I (2006) Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther 111(2):476–494
Hunt JF, Fang K, Malik R et al (2000) Endogenous airway acidification. Implications for asthma pathophysiology. J Respir Crit Care Med 161(3 Pt 1):694–699
Olin AC, Rosengren A, Thelle DS, Lissner L, Torén K (2010) Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population. Am J Respir Crit Care Med 181(4):324–327
RiedlMA Nel AE (2008) Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol 8(1):49–56
Bowler RP (2004) Oxidative stress in the pathogenesis of asthma. Curr Allergy Asthma Rep 4(2):116–122
Mak JC, Chan-Yeung MM (2006) Reactive oxidant species in asthma. Curr Opin Pulm Med 12(1):7–11
Rahman I, Biswas SK, Kode A (2006) Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533(1–3):222–239
Rock CL, Rodriguez JL, Khilnani R, Lown DA, Parker RS (1993) Carotenoids and antioxidant nutrients following burn injury. Ann NY Acad Sci 691:274–276
da Cunha AA, Ferreira AG, da Cunha MJ et al (2011) Chronic hyperhomocysteinemia induces oxidative damage in the rat lung. Mol Cell Biochem 358(1–2):153–160
Sackesen C, Ercan H, Dizdar E et al (2008) A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol 122(1):78–85
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760
Pearson DJ, Saurez-Mendez VJ, Day JP, Miller PF (1991) Selenium status in relation to reduced glutathione peroxidase activity in aspirin sensitive asthma. Clin Exp Allergy 21(2):203–208
Powell CV, Nash AA, Powers HJ, Primhak RA (1994) Antioxidant status in asthma. Pediatr Pulmonol 18(1):34–38
Davies KJ (1987) Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem 262(20):9895–9901
Stadtman ER (1990) Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9(4):315–325
Rahman I, Morrison D, Donaldson K, MacNee W (1996) Systemic oxidative stress in asthma, COPD and smokers. Am J Respir Crit Care Med 159(4 Pt 1):1055–1060
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266(7):4244–4250
Elkins MR, Robinson M, Rose BR et al (2006) A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354(3):229–240
Sheffner AL, Medler EM, Jacobs LW, Sarett HP (1964) The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis 90:721–729
Ventresca GP, Cicchetti V, Ferrari V (1989) Acetylcysteine. In: Braga PC, Allegra L (eds) Drugs in bronchial mucology. Raven Press, New York, pp 77–102
Acknowledgments
We thank José Carlos Farias Alves Filho for their technical assistance.
Financial support
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
da Cunha, A.A., Nuñez, N.K., de Souza, R.G. et al. Recombinant human deoxyribonuclease attenuates oxidative stress in a model of eosinophilic pulmonary response in mice. Mol Cell Biochem 413, 47–55 (2016). https://doi.org/10.1007/s11010-015-2638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2638-1