Abstract
The naturally antioxidant and coenzyme, alpha-lipoic acid (α-LA), has gained considerable attention regarding different functions and therapeutically effective in treating oxidative stress-associated diseases in the human body. This study was designed to examine the protective effect of α-LA against H2O2-induced oxidative stress and apoptosis in human lymphoid cells. Human peripheral blood lymphocytes were preincubated with α-LA and then exposed to H2O2. After that, the viability of the cells, rate of apoptosis, oxidative stress biomarkers such as reactive oxygen species (ROS) and level of lipid peroxidation (LPO), and also tumor necrosis factor-α (TNF-α) were studied. Pretreatment of lymphocytes with α-LA, dramatically enhanced viability of the cells and decreased apoptosis. Investigation of caspases gives a clear picture of the mechanism by which α-LA decreases ROS and causes a reduction in apoptosis through caspase-9-dependent mitochondrial pathway. Furthermore, α-LA dose dependently decreased oxidative stress by a reduction in level of LPO, and the dose of 1000 µM indicates a significant decrease (p < 0.01) in TNF-α level. Collectively, the present data show that α-LA is an ideal compound which has profound protective effects on oxidation, inflammation, and apoptosis. As a result, α-LA may indicate a new way toward the development of antioxidant therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing evidence that α-LA is a strong antioxidant [1] produced by plants, humans and either prokaryotic cells [2]. In human’s body, α-LA can be found in organs with an excess of mitochondria, such as heart, kidney, liver, and muscle [3] but the amount of α-LA in those tissues is very low; for example, 10 tons of liver are needed for obtaining 30 mg of α-LA [4]. So, for obtaining this compound from diet, there are different chemically synthesized drugs and food supplements based on α-LA because of its role in a variety of mechanisms like biological and biochemical cellular processes [5].
α-LA or 1,2-dithiolane-3-pentanoic acid is a kind of organosulfur cofactor with an essential role in many enzyme complexes, which produce energy, such as α-ketoacid dehydrogenase through the citric acid cycle and pyrovate dehydrogenase [3, 6]. α-LA acid has also an effective role in regenerating other antioxidants like vitamin C directly and vitamin E indirectly [7]. This small molecule has solubility in aqueous and lipid environment; also it can synchronize and scavenge free radicals inside and outside the cells, so it makes it more powerful than other antioxidants like vitamin C and vitamin E [7, 8]. α-LA repairs oxidative damage in cells and prevents peroxidation of lipids [9]. The small size and lipid solubility of this antioxidant are the features which help α-LA to cross through cell membrane and blood–brain barrier [9]. In vivo and in vitro studies have shown the wide spectrum ability of metal chelating activity by α-LA that result in preventing cells to produce free radicals [10, 11]. Other studies show that α-LA has ability to excrete metals, like mercury, from the body [12].
This valuable compound is widely used and can improve broad spectrum of diseases which are associated with oxidative stress, including Parkinson’s and Huntington’s as neurodegenerative disorders [13], ischemia by producing free radicals after reperfusion of blood flow [14], diabetes type 1 and type 2 by stimulating different cells to uptake glucose and regulating insulin signaling [15, 16], atherosclerosis by protecting LDL exposed to oxidative stress [17], and as well as infectious disease, that are affected by increasing glutathione level which plays an important role in the immune system [18]. Furthermore, α-LA can make process of aging slow [13] and has potential to improve hearing in model of aging by having an impact on mitochondrial function [19].
This study has gained importance by providing analysis of the antiapoptotic effect of α-LA in human lymphocytes as the main goal, and investigating other beneficial protective effects as the antioxidant and anti-inflammatory compound in lymphocytes for the first time by use of very specific methods like fluorescent staining besides flowcytometry. In this study, oxidative stress model was caused by H2O2 and α-LA was used as pre-protective compound against it.
Materials and methods
Chemicals
Human specific tumor necrosis factor-α ELISA kit was obtained from Bender MedSystems (Vienna, Austria; Cat. No. BMS2034) and ApoFlowEx® FITC Kit was purchased from Exbio (Vestec, Czech Republic; Cat. No. ED7044). α-LA and other chemicals, including RPMI 1640 medium, fetal bovine serum (FBS), bovine serum albumin (BSA), 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT), N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-ρNA), N-acetyl-Leu-Glu-His-Asp-p-nitroanilide (Ac-LEHD-ρNA), DNA-binding dyes Acridine orange (AO) and ethidium bromide (EB), ethylenediaminetetraacetic acid (EDTA), 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES), 2′,7′–dichlorodihydrofluorescin diacetate (DCFH-DA), DL-dithiothreitol (DTT), dimethyl sulfoxide (DMSO), and thiobarbituric acid (TBA) were used from Sigma-Aldrich (Munich, Germany).
Isolation of human lymphoid cells
All experimental procedures were approved by the ethics committee of the Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences. Human peripheral blood lymphocytes were isolated from healthy and non-smoking donors. The heparinized blood was diluted with tissue culture medium (RPMI-1640) and then added to Ficoll-Paque and centrifuged at 400 g for 30 min. The lymphocytes, from the interface of plasma and Ficoll-paque, were collected and washed twice with phosphate buffer, then were counted based on the trypan blue exclusion method. After washing and counting, the cells were seeded at a density of 3 × 106 cells/well in the RPMI-1640, which consists of 10 % FBS, 2 mM l-glutamine, 100 u/ml penicillin and 100 µg/ml streptomycin sulfate, and followed by the addition of 50 µl/ml LPS for cell growth stimulation. The lymphocyte cultures were grown in a humidified incubator with 5 % CO2 at 37 °C in 96 microtiter plates.
Investigating the cytotoxic effects of α-LA on human lymphocytes by MTT assay
Before investigating protective effects of α-LA against H2O2, we exposed the human lymphocytes to the logarithmic concentrations of α-LA for finding effective dose for 50 percent of the group (ED50) and to make sure that α-LA doesn’t have toxic effects. Human lymphocytes (3 × 106 cells/well) were incubated with culture medium in combination with different concentrations of α-LA (0, 1, 10, 100, and 1000 μM) for 24 h at 37 °C and 5 % CO2 humidified atmosphere. After treatment, the lymphocytes were processed for cytotoxicity by MTT assay. This assay is based on the reduction of MTT, a yellow tetrazole, to purple insoluble formazon by mitochondrial respiration in viable cells. After 24 h incubation of the cells by α-LA, centrifugation was done and the precipitated lymphocytes were washed twice by phosphate buffer. Then, 50 µl of MTT solution was added, followed by re-incubation for 4 h at 37 °C and 5 % CO2 humidified atmosphere and addition of 150 µl of DMSO solution. Then, the absorbance was determined at 570 nm by ELISA reader. Upon this procedure, the ED50 of α-LA on human lymphocytes was calculated [20].
Investigating the protective effects of α-LA against H2O2-induced oxidative stress
Study design
In this study, H2O2 was used for leading oxidative stress in our cells. In different in vitro and in vivo studies, H2O2 has been widely used as a model, which induces oxidative stress [21]. According to Chiaramonate and his coworkers, we used 100 µM H2O2 for 15 min after treating the lymphocytes by different doses of α-LA [22]. So, for protective investigation, cell suspension (3 × 106 cells/well) was incubated with culture medium in combination with logarithmic concentrations (0, 1, 10, 100, and 1000 μM) of α-LA for 24 h at 37 °C and 5 % CO2 humidified atmosphere. Then, cells were exposed to H2O2 for 15 min in 37 °C. At the end, lymphocytes were prepared for three sets of experiments, including viability assays, oxidative stress assays, and inflammatory assay.
-
1
Viability assays
Mitochondrial activity assay
As described for ED50 determination, the MTT assay was done for investigation of viability of treated lymphocytes by both α-LA and H2O2.
Caspase-3 and -9 activities
Caspase-3 and -9 activities were measured by colorimetric assays based on the identity of specific amino acid sequences by these caspases. The tetrapeptide substrates were labeled with the chromophore r-nitroaniline (ρNA). ρNA is released from the substrate upon cleavage by caspase and produces a yellow color that is monitored by an ELISA reader at 405 nm. The amount of caspase activity present in the sample is proportional to the amount of yellow color produced upon cleavage [23]. Briefly, the pretreated lymphocytes were lysed in the supplied lysis buffer and were incubated on ice for 10 min. The whole cell lysates were incubated in caspase buffer (100 mM HEPES, pH 7.4, 20 % glycerol, 0.5 mM EDTA, 5 mM dithiothreitol) containing 100 mM of caspase-3 and -9 specific substrate (Ac-DEVD-ρNA and Ac-LEHD-ρNA, respectively) for 4 h at 37 °C. Then, absorbance was measured at 405 nm. The caspase-3 and -9 activities of the treatment groups were shown as the percentage of controls which assumed 100 %.
Apoptosis and necrosis evaluation by flow cytometry
Flow cytometry is assumed as a high-quality technique to study physicochemical specifications of the cells, such as homogeneity, viability, cell surface and cytoplasmic antigens, apoptotic percent of viable cells, and cytosolic ROS in viable cells when they pass through a laser beam [24]. This experiment was intended to differentiate the early apoptotic, necrotic, and viable cells. To find out mode of lymphocyte cell death induced by H2O2 in the presence of different α-LA, the Annexin V-FITC/Propidium iodide (PI) staining was carried out. The staining of Annexin V-FITC and PI indicates the type of death induced by the test compound i.e., apoptosis or necrosis. The cells (1 × 106) which were treated by α-LA and H2O2, washed and stained with Annexin V-FITC antibody and PI as each of the instructions given by the manufacturer. The cells were scanned for fluorescence intensity in FL-1 (FITC) and FL-2 (PI) channels. The fraction of cell populations in different quadrants was analyzed using quadrant statistics [25].
Microscopic assay by AO and EB double staining
DNA-binding dyes AO and EB were used for the morphological detection of apoptotic and necrotic cells. AO is taken up by both viable and non-viable cells and emits green fluorescence if intercalated into DNA. EB is taken up only by non-viable cells and emits red fluorescence by intercalation into DNA. After treating lymphocytes with different concentrations of α-LA for 24 h and then exposed to H2O2, the cells were washed and then stained with a mixture of AO (100 µg/ml) and EB (100 µg/ml) at room temperature for 5 min. The stained cells were observed by a fluorescence microscope (Olympus, japan) at ×200 magnifications. The cells were divided into three categories as follows: living cells (normal green nucleus), apoptotic cells (red-stained nuclei with chromatin condensation or fragmentation), and necrotic cells (uniformly red-stained cell nuclei) [26]. An average of 12 randomly selected fields of view in three independent samples was taken.
-
2
Oxidative stress assays
Cytosolic ROS
Cytosolic ROS assay can be done by measuring the generation of dichlorofluorescin after treating by DCFH-DA. To meet this purpose, first the samples were prepared by adding DTT (50 µM) and lysis buffer, containing EDTA, HEPES, KCl, and sucrose, into each vial. Then, after homogenizing, the supernatant was used for measuring the level of cytosolic ROS and protein. For ROS test, the buffer assay and DCFH-DA were added, respectively, into each well of plates containing homogenized samples and incubated for 15 min in 37 °C. Absorbance changes were read by ELISA fluorimeter (excitation 488 nm; emission 525 nm). Standardization of all of the values was done by the amount of total protein in each well [27]. At the end, the results were shown as the percentage of control which assumed 100 %.
Protein assay
To determine total protein concentration of cells, bradford reagent was added to dilute samples and the absorbance was measured by the spectrophotometer at 595 nm after 5 min. The BSA was used as standard.
Determination of LPO
To measure LPO, thiobarbituric acid-reactive substances (TBARS) were measured. TBA reacts with lipid peroxides in the samples producing a measurable pink color that has an absorbance at 532 nm read by the ELISA reader as described in our previous work [28]. The levels of lipid peroxides were expressed as µM.
-
3
Inflammatory assay
Determination of TNF-α
A human-specific ELISA kit was used to quantify TNF-α in the supernatant of lymphocyte culture. To assess the amount of TNF-α, the absorbance of the samples was measured at 450 nm as the primary wavelength and 620 nm as the reference wavelength by an ELISA reader as described in the kit brochure. Data were shown as pg/ml.
Statistical analysis
At least three independent experiments in repetitions were carried out. Data are presented as mean ± SEM. One-way ANOVA and Tukey’s multi-comparison tests were carried out by Stats-Direct 2.7.9 to determine the statistical differences. The p value of <0.05 was considered significant.
Results
The cytotoxic effects of α-LA on human lymphocytes for finding ED50
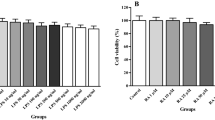
In the first part of experiments, toxic effects of α-LA on lymphocytes were evaluated by MTT assay and found that in some doses, α-LA not only is safe but also can raise the viability of the lymphocytes. As it is shown in Fig. 1, α-LA, at high doses such as 100 and 1000 µM, effectively improved the viability in comparison to the control group (p < 0.01 and p < 0.001). According to our data, ED50 for α-LA was determined as 259.36 µM.
Calculating ED50 of α-LA on human lymphocytes, based on viability assay. After 24 h incubation in the exposure of four different concentrations of α-LA, MTT assay was done and ED50 of α-LA was determined as 259.36 µM. Data are expressed as mean ± standard error of three different experiments (n = 3). Significant difference from control at aaa p < 0.001, aa p < 0.01
Protective effects of α-LA in viability assays
Mitochondrial activity assay
Results of MTT assay after exposing the lymphocytes to H2O2 are shown in Table 1. Data indicate that all concentrations of α-LA can protect cells against H2O2, and doses which were more than ED50 concentration, increase the viability considerably (p < 0.001).
Caspase-3 and -9 activities
Table 1 also presents the activities of caspase-3 and -9. It is shown that α-LA has no significant change in level of caspase-3 activity, but it is represented that level of caspase-9 activity in comparison to H2O2 group reduced significantly (p < 0.05 for doses 1, 10 and 100 µM; and p < 0.01 for 1000 µM).
Flow cytometry assay and fluorescent staining
Results which were obtained from flow cytometry assay and AO/EB staining are depicted in Fig. 2. As it is shown, cells in the control group, which is untreated, are mostly live (84 %) and display uniform green fluorescence. The rate of live cells in the group which was only treated by H2O2, has significantly declined (up to 68 %) and population of early apoptotic cells increased significantly (p < 0.001).
Flow cytometry evaluation of H2O2-induced lymphocytes alone or in combination with different concentrations of α-LA on the percent of live, apoptotic, and necrotic populations of the cells. Lower left quadrant shows live cells with FITC- and PI-, lower right quadrant indicates early apoptotic cells with FITC + and PI-, upper right quadrant represents late apoptotic cells with FITC + and PI + and upper left quadrant expresses necrotic cells with FITC- and PI + . Also changes in the percent of early apoptosis rate are shown in the chart. Significant increasing from control at aaa p < 0.001, a p < 0.05. Significant decreasing from H2O2 at b p < 0.05, bb p < 0.01. AO/EB staining also represents live cells (L), apoptotic cells (A) and necrotic cells (N)
Cells which were incubated with different concentrations of α-LA and then were exposed to H2O2, compensated apoptotic effects of H2O2 and significantly decreased rate of apoptosis as compared with H2O2 group (1 µM α-LA, p < 0.05; 10, 100 and 1000 µM α-LA, p < 0.01).
AO/EB staining shows that the population of the cells in group, which has H2O2 without α-LA, decreased and more necrosis cells with red fluorescence emission were observed. Also, the staining shows that by increasing α-LA concentration, permeability of EB into lymphocyte membrane and staining of DNA decreased, and confirms flow cytometry plots.
Protective effects of α-LA in oxidative stress
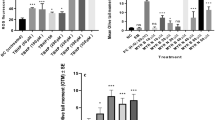
Figure 3a represents significant decreasing in LPO level by doses 100 and 1000 µM (p < 0.01 and p < 0.001 respectively). In addition, in Fig. 3b, it was shown that all doses of α-LA can protect lymphocytes against H2O2 by reduction of ROS level in cells.
Effects of various concentrations of α-LA in LPO level (a) and ROS level (b) of isolated human lymphocytes for 24 h incubation at 37 °C and 5 % CO2 humidified atmosphere, in the present of H2O2. Data are expressed as mean ± standard error of three different experiments (n = 3). Significant increasing from control at aa p < 0.01, aaa p < 0.001. Significant decreasing from H2O2 at b p < 0.05, bb p < 0.01, bbb p < 0.001
Protective effects of α-LA in inflammatory assays
Figure 4 indicates that H2O2 group has significantly increased the level of TNF-α (p < 0.05). On the other hand, the concentration of 1000 µM of α-LA when compared to H2O2 group shows significant reduction in inflammation level of lymphocytes (p < 0.01).
Effects of various concentrations of α-LA in TNF-α level of isolated human lymphocytes for 24 h incubation at 37 °C and 5 % CO2 humidified atmosphere, in the presence of H2O2. Data are expressed as mean ± standard error of three different experiments (n = 3). Significant increasing from control at a p < 0.05. Significant decreasing from H2O2 at bb p < 0.01
Discussion
α-LA is the main component of eukaryotic and prokaryotic cells, mainly consumed in energy metabolism [2]. It has been studied that there are some advantages of α-LA against oxidative stress to treat a number of infections and diseases [29, 30]. It has been reported that ROS produced by oxidative stress caused destruction of lymphocytes DNA that ultimately results in immune system disorders. Also, previous studies demonstrate that oxidative stress produced in lymphocytes is the main cause for further inflammation and infection [31]. In the present study, we investigated antioxidant effects of α-LA in human lymphocytes. α-LA proved to be an excellent antioxidant against infection and oxidative stress, that was produced in lymphocytes by incubation of hydrogen peroxide. Furthermore, different doses of α-LA improved viability as compared to control, as well as decreased inflammation in lymphocytes.
In previous in vivo study, for diabetic rat, ED50 of α-LA is 44 mg/kg/d [32]. We did not find any research of in vitro study related to ED50 of α-LA. So, to support recent study, we determined ED50 of α-LA in an invitro model of human lymphocytes by MTT assay. We used different logarithmic doses of α-LA, for which it was concluded that ED50 of α-LA is 259.36 µM.
In the first phase of our tests, we analyzed the viability of lymphocytes by MTT, caspase-3 and caspase-9 activity, flow cytometry analysis, and fluorescent staining against H2O2. Results of 1st phase showed that, the viability of lymphocytes was improved, while caspase-9 activity was reduced with increasing strength of α-LA, and caspase-3 activity showed no prominent effect (Table 1). In addition to this, in tests of flow cytometry and fluorescent staining analysis, apoptosis and necrosis decreased as compared to H2O2. Figure 2 demonstrates a good correlation between these two tests. Goraca et al. have studied that α-LA suppressed the over expression of genes mainly involved in apoptosis pathways [33]. Other evidence also supports that cell viability has increased for α-LA treated IPEC-J2 cells, as compared to H2O2 group [34]. There are two main mechanisms for justifying pathway of apoptosis, one of them related to caspase enzyme activities and the other one is related to decreased mitochondrial activity which caused increasing levels of cytochrome C and apoptosis inducing factor (AIF) [35, 36]. In this study, diminution of apoptosis may be related to reduction in caspase-9 activity, which is involved in the intrinsic mitochondrial pathway and which is an initiator of AIF [37]. Also, in previous studies, it is proven that α-LA can inhibit the intrinsic pathway of apoptosis in endothelial cells [38].
In phase 2, we studied the antioxidant effects of α-LA against H2O2 by measuring ROS and LPO. Data showed that α-LA has excellent antioxidant effects, while dose of 1000 µM gave maximum results in case of LPO, and doses of 10 µM, 100 µM, and 1000 µM showed maximum effects, with less effective results of 1 µM dose in case of ROS (Fig. 3). It is obvious from the recent study, performed on humans, that DNA protection against oxidative damage by α-LA, developed strong neuroprotection [39]. In another study performed on mouse plasma, α-LA inhibited LPO formation, initiated from ROS production [40]. It has been reported that in rats, thiol group of α-LA played pivotal role in hepatic tissues to eliminate oxidative stress [41].
In phase 3, we evaluated the effect of α-LA, in inflammation by measuring levels of TNFα against H2O2. We found that all doses showed effective results against H2O2 with the maximum significant result of dose of 1000 µM (Fig. 4). α-LA has been studied for positive role in chronic inflammation via inhibition of cytokine-induced inflammation [6]. It has been proposed recently that antioxidant effect may also lead to help anti-inflammatory action [42]. In another study reported by Huang et al., it is clear that deactivation of GSK-3β in BV-2 cells by α-LA played a beneficial role for anti-inflammatory action [43].
Collectively, for all diseases induced by oxidative stress pathway and related mechanisms can be well treated by α-LA and may give more better results in near future [44]. Our data, which correlate well with the other studies, show that α-LA is an ideal antioxidant compound in human lymphocytes which has profound effects on oxidant, inflammation, and apoptosis systems. Though, further investigation for in vivo study is required. It may indicate a new way toward the development of antioxidant therapy.
References
Packer L, Witt EH, Tritschler HJ (1995) Alpha-Lipoic acid as a biological antioxidant. Free Radical Biol Med 19(2):227–250
Nishiura H, Sugimoto K, Akiyama K, Musashi M, Kubota Y, Yokoyama T, Yamashita Y, Kuriki T, Yamaguchi Y (2013) A novel nano-capsule of α-Lipoic Acid as a template of core-shell structure constructed by self-assembly. J Nanomed Nanotechol 4:155–161
Unal F, Taner G, Yuzbasioglu D, Yilmaz S (2013) Antigenotoxic effect of lipoic acid against mitomycin-C in human lymphocyte cultures. Cytotechnology 65(4):553–565
Reed LJ (2001) A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J Biol Chem 276(42):38329–38336
Lee HS, Na MH, Kim WK (2010) alpha-Lipoic acid reduces matrix metalloproteinase activity in MDA-MB-231 human breast cancer cells. Nutr Res 30(6):403–409
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790(10):1149–1160
Evans JL (2000) a-Lipoic Acid: a multifunctional antioxidant that improves insulin sensitivity in patients with Type 2 diabetes. Diabetes Technol Ther 2(3):401–413
Xu J, Gao H, Song L, Yang W, Chen C, Deng Q, Huang Q, Yang J, Huang F (2013) Flaxseed oil and alpha-lipoic acid combination ameliorates hepatic oxidative stress and lipid accumulation in comparison to lard. Lipids Health Dis 12:58–64
Pederzolli CD, Rosa AP, de Oliveira AS, Coelho JG, da Luz Becker D, Dalazen GR, Moraes TB, Dutra-Filho CS (2010) Neuroprotective role of lipoic acid against acute toxicity of N-acetylaspartic acid. Mol Cell Biochem 344(1–2):231–239
Toklu HZ, Hakan T, Celik H, Biber N, Erzik C, Ogunc AV, Akakin D, Cikler E, Cetinel S, Ersahin M, Sener G (2010) Neuroprotective effects of alpha-lipoic acid in experimental spinal cord injury in rats. J Spinal Cord Med 33(4):401–409
Ou P, Tritschler HJ, Wolff SP (1995) Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol 50(1):123–126
Gregus Z, Stein AF, Varga F, Klaassen CD (1992) Effect of lipoic acid on biliary excretion of glutathione and metals. Toxicol Appl Pharmacol 114(1):88–96
Packer L, Cadenas E (2011) Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J Clin Biochem Nutr 48(1):26–32
Cao X, Phillis JW (1995) The free radical scavenger, alpha-lipoic acid, protects against cerebral ischemia-reperfusion injury in gerbils. Free Radic Res 23(4):365–370
Ziegler D, Gries FA (1997) Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes 46(2):62–66
Salinthone S, Yadav V, Bourdette DN, Carr DW (2008) Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets 8(2):132–142
Schleicher E, Wagner E, Nerlich A (1997) Increased accumulation of the glycooxidation product N(epsilon)-carboxymethyl lysine in human tissues in diabetes and aging. J Clin Invest 99:457–468
Baur A, Harrer T, Peukert M, Jahn G, Kalden JR, Fleckenstein B (1991) Alpha-lipoic acid is an effective inhibitor of human immuno-deficiency virus (HIV-1) replication. Klin Wochenschr 69(15):722–724
Seidman MD, Khan MJ, Bai U, Shirwany N, Quirk WS (2000) Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol 21(2):161–167
Rahimifard M, Navaei-Nigjeh M, Mahroui N, Mirzaei S, Siahpoosh Z, Nili-Ahmadabadi A, Mohammadirad A, Baeeri M, Hajiaghaie R, Abdollahi M (2014) Improvement in the function of isolated rat pancreatic islets through reduction of oxidative stress using traditional Iranian medicine. Cell J 16(2):147–163
Gille JJ, Joenje H (1992) Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res 275(3–6):405–414
Chiaramonte R, Bartolini E, Riso P, Calzavara E, Erba D, Testolin G, Comi P, Sherbet GV (2001) Oxidative stress signalling in the apoptosis of Jurkat T lymphocytes. J Cell Biochem 82(3):437–444
Hosseini A, Baeeri M, Rahimifard M, Navaei-Nigjeh M, Mohammadirad A, Pourkhalili N, Hassani S, Kamali M, Abdollahi M (2013) Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum Exp Toxicol 32(5):544–553
Brown M, Witter C (2000) Flow cytometry: principles and clinical applications in hematology. Clin Chem 46(8):1221–1229
Saxena A, Saxena AK, Singh J, Bhushan S (2010) Natural antioxidants synergistically enhance the anticancer potential of AP9-cd, a novel lignan composition from Cedrus deodara in human leukemia HL-60 cells. Chem Biol Interact 188(3):580–590
Attari F, Sepehri H, Delphi L, Goliaei B (2009) Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran Biomed J 13(4):229–236
Shoae-Hagh P, Rahimifard M, Navaei-Nigjeh M, Baeeri M, Gholami M, Mohammadirad A, Abdollahi M (2014) Zinc oxide nanoparticles reduce apoptosis and oxidative stress values in isolated rat pancreatic islets. Biol Trace Elem Res 162(1–3):262–269
Astaneie F, Afshari M, Mojtahedi A, Mostafalou S, Zamani MJ, Larijani B, Abdollahi M (2005) Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch Med Res 36(4):376–381
Podda M, Zollner TM, Grundmann-Kollmann M, Thiele JJ, Packer L, Kaufmann R (2001) Activity of alpha-lipoic acid in the protection against oxidative stress in skin. Curr Probl Dermatol 29:43–51
Biewenga GP, Haenen GR, Bast A (1997) The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29(3):315–331
Bashir S, Harris G, Denman MA, Blake DR, Winyard PG (1993) Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann Rheum Dis 52(9):659–666
Cameron NE, Jack AM, Cotter MA (2001) Effect of α-lipoic acid on vascular responses and nociception in diabetic rats. Free Radic Biol and Med 31(1):125–135
Gorąca A, Asłanowicz-Antkowiak K (2009) Prophylaxis with α-lipoic acid against lipopolysaccharide-induced brain injury in rats. Arch Immunol Ther Exp (Warsz) 57(2):141–146
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L, Zhu S, Xu J (2013) Pre-protective effect of lipoic acid on injury induced by H2O2 in IPEC-J2 cells. Mol Cell Biochem 378(1–2):73–81
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2(8):589–598
Kaufmann SH, Hengartner MO (2011) Programmed cell death: alive and well in the new millennium. Trends Cell Biol 11(12):526–534
Würstle ML, Laussmann MA, Rehm M (2012) The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318(11):1213–1220
Tharakan B, Holder-Haynes JG, Hunter FA, Childs EW (2008) Alpha lipoic acid attenuates microvascular endothelial cell hyperpermeability by inhibiting the intrinsic apoptotic signaling. Am J Surg 195(2):174–178
Jia Z, Zhu H, Vitto MJ, Misra BR, Li Y, Misra HP (2009) Alpha-lipoic acid potently inhibits peroxynitrite-mediated DNA strand breakage and hydroxyl radical formation: implications for the neuroprotective effects of alpha-lipoic acid. Mol Cell Biochem 323(1–2):131–138
Jesudason EP, Masilamoni JG, Jesudoss KS, Jayakumar R (2005) The protective role of DL-α-lipoic acid in the oxidative vulnerability triggered by Aβ-amyloid vaccination in mice. Mol Cell Biochem 270(1–2):29–37
Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P (2005) Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-α-lipoic acid. Mol Cell Biochem 272(1–2):179–185
Koriyama Y, Nakayama Y, Matsugo S, Sugitani K, Ogai K, Takadera T, Kato S (2013) Anti-inflammatory effects of lipoic acid through inhibition of GSK-3β in lipopolysaccharide-induced BV-2 microglial cells. Neurosci Res 77(1):87–96
Huang WC, Lin YS, Wang CY, Tsai CC, Tseng HC, Chen CL, Lu PJ, Chen PS, Qian L, Hong JS, Lin CF (2009) Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 128:275–286
Saeidnia S, Abdollahi M (2013) Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol 273(3):442–455
Acknowledgments
This study was partially supported by TUMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimifard, M., Navaei-Nigjeh, M., Baeeri, M. et al. Multiple protective mechanisms of alpha-lipoic acid in oxidation, apoptosis and inflammation against hydrogen peroxide induced toxicity in human lymphocytes. Mol Cell Biochem 403, 179–186 (2015). https://doi.org/10.1007/s11010-015-2348-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2348-8