Abstract
We have previously shown that PARP-1 inhibition provides protection against lung inflammation in the context of asthma and acute lung injury. Olaparib is a potent new generation PARP inhibitor that has been approved for human testing. The present work was designed to evaluate its beneficial potential against LPS-induced acute lung injury and acute kidney injury upon intratracheal administration of the endotoxin in mice. Administration of olaparib at different doses, 30 min after LPS treatment showed that single intraperitoneal injection of the drug at 5 mg/kg b.wt. reduced the total number of inflammatory cells particularly neutrophils in the lungs. This was associated with reduced pulmonary edema as the total protein content in the bronchoalveolar fluid was found to be decreased substantially. Olaparib provided strong protection against LPS-mediated secondary kidney injury as reflected by restoration of serum levels of urea, creatinine, and uric acid toward normal. The drug restored the LPS-mediated redox imbalance toward normal in lung and kidney tissues as assessed by measuring malondialdehyde and GSH levels. Finally, RT-PCR data revealed that olaparib downregulates the LPS-induced expression of NF-κB-dependent genes namely TNF-α, IL-1β, and VCAM-1 in the lungs without altering the expression of total p65NF-κB. Overall, the data suggest that olaparib has a strong potential to protect against LPS-induced lung injury and associated dysfunctioning of kidney in mice. Given the fact that olaparib is approved by FDA for human testing, our findings can pave the way for testing of the drug on humans inflicted with acute lung injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung injury (ALI) is a syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates that is associated with both pulmonary and nonpulmonary risk factors [1]. ALI and its severe form acute respiratory distress syndrome (ARDS) can result in acute respiratory failure with substantial morbidity and mortality. Even in patients who survive ALI, there is evidence that their long-term quality of life is adversely affected [2, 3]. ALI is characterized by a disruption of the endothelium and alveolar epithelial barriers involving increased micro vascular permeability, followed by an onset of dyspnea, severe hypoxemia, and pulmonary edema [4, 5] ALI can develop numerous devastating complications at later stages, including severe sepsis, severe trauma, and ischemia/reperfusion injury [6]. Sepsis due to nonpulmonary infections, aspiration of gastric contents, and major trauma with shock also commonly precipitates the injury. Less commonly, acute pancreatitis, transfusions, drug reactions, and fungal and parasitic lung infections are linked to ALI and ARDS. The coexistence of two or more of these risk factors can enhance the likelihood of developing ALI or ARDS [1].
Lipopolysaccharide (LPS) is a large molecule consisting of lipid and a polysaccharide composed of O-antigen, found in the outer membrane of the gram-negative bacteria and is known to elicit strong immune response in animals [7]. Inflammatory stimuli from this endotoxin are well recognized for their ability to induce pulmonary inflammation. Experimental administration of LPS, both systemically and intratracheally, has been used to induce pulmonary inflammation in animal models of ALI [8–11]. The acute lung inflammation may have deleterious effects on remote organs such as kidney and may involve crosstalk between the lung and kidney [12]. Survival decreases drastically when acute kidney injury (AKI) and ALI occur together [13, 14]. The development of AKI in the setting of ALI carries an in-hospital mortality rate of 58 %, compared to 28 % in ALI patients without AKI [14].
It has been established that Poly(ADP-ribosyl)ation reactions, carried out by poly (ADP-ribose) polymerases (PARPs) enzymes play an important role in the pathogenesis of oxidative stress-mediated number of human diseases [15–18]. The PARPs are a family of around 18 members whose primary role is to help in the repair of DNA damage [19]. Activation of PARPs results in post-transcriptional modification of enzymes involved in DNA repair process [20, 21] by catalyzing the attachment of ADP-ribose units to acceptor proteins. Such modification of cellular proteins modulates their structure and function, which favors the DNA repair process [22, 23]. DNA damage through excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during inflammatory conditions can cause persistent activation of PARPs resulting in the depletion of their substrate NAD+ and consequently leading to necrotic cell death and organ dysfunction [24, 25]. We and others have shown that PARP-1 also acts as a co-factor to enhance the nuclear factor (NF)-κB-dependent transcription of inflammatory cytokines [26–31]. Treatment with PARP inhibitors have been reported to improve the pathogenesis of septic acute lung inflammation [31, 32] and ventilator-induced lung injury [33]. Recently, PARP has been in the news as number of researchers have shown that PARP inhibition provides a treatment strategy against breast, ovarian, and prostate cancers [34–37]. Olaparib is one such potent PARP inhibitor that has been approved for clinical testing in such cancer patients and has acceptable level of toxicity [38, 39]. A recent study has suggested that the activation of PARP mediates renal inflammation secondary to LPS-induced acute lung inflammation [40]. Since olaparib is approved for testing on humans, our aim for the present work was to evaluate the anti-inflammatory potential of the compound in LPS-induced lung injury and associated kidney injury using mouse model of the disease for its potential application in humans inflicted with ALI/ARDS.
Materials and methods
Animals
Female LACA mice weighing 25–30 g were obtained from central animal house of Panjab University, Chandigarh. All the animals were housed in polypropylene cages and were fed with standard diet and were given water ad libitum. The animals were housed, cared, and used for experiments in accordance with the “Guidelines for the Care and Use of Experimental Animals” approved by University Ethics Committee.
Chemicals
All the chemicals used in the study were of analytical grade. LPS procured from E. coli, serotype O111:B4 (TLRGradeTM) was purchased from Enzo life sciences, Farmingdale, USA. Olaparib was purchased from Selleck chemicals, Houston, USA. All other chemicals needed for several biochemical assays including 2-thiobarbituric acid (TBA), Sulfosalicylic acid, and 5,5′-Dithiobis-2-nitrobenzoic acid (DTNB) were purchased from either Himedia Laboratories, Mumbai, India or Sisco Research Laboratories Pvt. Ltd, Mumbai, India.
Experimental design
The mice were divided randomly into four groups and were given treatment as below:
Control group Mice were given standard diets and no drug was given to them.
Olaparib group Mice were given a single intraperitoneal (i.p.) injection of olaparib at a dose of 5 mg/kg b.wt.
LPS group Mice were administered LPS intratracheally (i.t.) at dose of 50 μg per mouse as reported previously [41].
LPS+ olaparib group Mice were treated with LPS as explained above and olaparib was given as single injection (i.p.) at dose of either 1, 5, or 10 mg/kg b.wt. 30 min post LPS administration. The dose range of drug was chosen on the basis of our earlier study where we have shown that administration of olaparib at different doses 30 min post-allergen (ovalbumin) challenge conferred a robust reduction in lung inflammation using mouse model of asthma [42]. Based on the outcome of different doses of olaparib on LPS-induced lung inflammation, we selected 5 mg/kg b.wt. as final dose for subsequent experiments.
The mice were sacrificed either 6 or 18 h after the administration of LPS by cervical dislocation. The blood was drawn from the heart by using 1 ml syringe and was processed for the preparation of serum. The lungs were subjected to bronchoalveolar lavage (BAL) and fixed on microscopic slides as described [43]. Some groups of mice were sacrificed for procuring whole lung and kidney tissues for preparation of tissue homogenate as explained earlier [44]. The lungs of the mice sacrificed 6 h after LPS treatment were processed for extraction of total RNA and reverse transcribed to cDNA.
Analysis of biochemical parameters
The total protein content was assayed by method of Lowry [45]. Assessment of serum urea, creatinine, uric acid, alkaline phosphatase, glutamate oxaloacetate transaminase (GOT), and glutamate pyruvate transaminase (GPT) was conducted by using commercially available kits from Transasia Bio-medical Limited, Solan. Malondialdehyde (MDA) as a marker of lipid peroxidation was assayed by method of Beuge and Aust [46]. The reduced glutathione (GSH) was measured according to Ellman [47].
Extraction of RNA from lung tissue and RT-PCR analysis
Lung tissue was stored in RNA later for RNA extraction using Qiagen kit, according to the procedure given in the kit manual. The extracted total RNA was used for the generation of cDNA using reverse transcriptase III (Invitrogen) and analyzed by reverse transcriptase polymerase chain reaction (RT-PCR) using primers specific for TNF-a, IL-1β, VCAM-1, p65NF-κB, and β-actin. The amplification program was as follows: 5 min at 95 °C, 30 s at 95 °C, 45 s at 60 °C, and 1 min at 72 °C. The cycle numbers were optimized for each primer pair. The PCR products were then incubated for 15 min at 72 °C. The resulting PCR products were subjected to electrophoresis in a 2 % agarose gel and stained with ethidium bromide. The sequence of primers used are given below: TNF-α: Forward- 5′-TAT GGC TCA GGG TCC AAC TC-3′, Reverse-5′-CTC CCT TTG CAG AAC TCA GG-3′; IL-1 β: Forward-5′-GAC CTT CCA GGA TGA GGA CA-3′, Reverse-5′AGG CCA CAG GTA TTT TGT CG-3′; VCAM-1: Forward-5′-ACA GAC AGT CCC CTC AAT GG-3′, Reverse-5′ACC TCC ACC TGG GTT CTC TT-3′; p65NF-κB: Forward- 5′-CTTGGCAACAGCACAGA CC-3′, Reverse- 5′-GAGAAGTCCATGTCCGCAAT-3′; β-actin: Forward- 5′-TACAGCTTCACCACCACAGC-3′, Reverse- 5′-TCTCCAGGG AGGAAGAGGAT-3′.
Statistical analysis
Results are depicted as mean ± SEM. Statistical analysis was performed by one way Anova test followed by Tukey’s multiple comparison using graph-pad prism software (GraphPad Software, Inc. La Jolla, CA). P < 0.05 was considered as statistically significant.
Results
Olaparib administration markedly reduced the number of inflammatory cells in the lungs upon LPS treatment
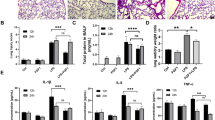
The major components of the inflammatory and immunological reaction in the lungs are phagocytes, in particular, macrophages and polymorphonuclear neutrophils (PMNs). Macrophages are the normal resident cells of the normal lower respiratory tract whereas PMNs are mostly absent. But, PMNs are known to accumulate in the lung in case of injury, trauma, or infection. In order to evaluate the effect of olaparib on LPS-induced recruitment of inflammatory cells in lungs, the drug was given to mice at different doses (i.e., 1, 5, or 20 mg/kg b.wt.) upon LPS administration. Figure 1a depicts that the total number of cells in BAL fluid of LPS-treated mice was increased by 672 % as compared to the mice of control group and statistically found to be highly significant (P < 0.001). When olaparib was given at dose of 1 mg/kg, the number of inflammatory cells in BAL was observed to be similar to that seen in LPS-treated mice. However, when the dose of drug was increased to either 5 or 20 mg/kg, the number of total cells in BAL fluid was restored toward normal by 62.94 and 66.47 %, respectively in comparison to LPS-treated group (P < 0.001). We next quantified the number of neutrophils in the BAL fluid. The data (Fig. 1b) showed that LPS treatment indeed enhanced the number of neutrophils in BAL fluid sharply (P < 0.01 w.r.t. control) and olaparib administration at 1 mg/kg did not cause any major change in such increase. However, olaparib at 5 or 20 mg/kg reverted the neutrophil number in BAL toward normal significantly by 62.85 % (P < 0.01) and 69.7 % (P < 0.01), respectively when compared with LPS-treated group. Since olaparib at a dose of 5 mg/kg ameliorate the neutrophil number in BAL toward normal efficiently, we selected this dose for subsequent experiments.
Olaparib administration markedly reduced the number of inflammatory cells in the lungs upon LPS treatment. Mice were treated with LPS followed by different doses of olaparib as explained in materials and methods section and were subjected to BAL 18 h after LPS treatment. BAL cells thus obtained were differentially stained for counting total cells (a) and neutrophils (b). Supernatant of BAL was assessed for total protein content (c). Results are depicted as mean ± SEM. ***significant w.r.t. control, P < 0.001; **significant w.r.t. control, P < 0.01; ###significant w.r.t. LPS, P < 0.001; #significant w.r.t. LPS, P < 0.05
The pathogenesis of ALI includes injury of endothelial and epithelial barriers leading to protein-rich edema and inflammation induced by cytokines and chemokines released from inflammatory cells, lung epithelial cells, or fibroblasts. Weakening of the endothelial barrier enhances the transendothelial diapedesis of leukocytes into lung tissues, further contributing to pulmonary dysfunction. Our results (Fig. 1c) indeed confirmed the earlier reports that LPS administration leads to pulmonary edema as total protein content in the BAL fluid was found to be elevated by 103.65 % when compared with control (P < 0.001). Olaparib administration at dose of 5 mg/kg after LPS treatment restored BAL protein content by 43.46 % toward normal quite efficiently (P < 0.001 w.r.t. LPS).
Olaparib administration ameliorate kidney function upon intratracheal administration of LPS
Figure 2a shows effect of olaparib on serum urea levels upon intratracheal administration of LPS. LPS treatment in the lungs increased the serum urea content by 288 % (P < 0.01 w.r.t. control) and olaparib administration reverted such increase by 64.61 % toward normal (P < 0.01 w.r.t. LPS). Figure 2b depicts the effect of olaparib administration on LPS-induced changes in serum content of creatinine. As shown in the figure, we noted that creatinine level increased by 230.0 % (P < 0.05 w.r.t. control) upon LPS treatment and olaparib administration suppressed such increase toward normal by 65.99 % (P < 0.05 w.r.t. LPS). Figure 3a shows effect of olaparib on uric acid levels in serum upon LPS treatment. LPS treatment in the lungs resulted in increase of uric acid level by 391.0 % (P < 0.01 w.r.t. control) while olaparib administration restored it toward normal by 61.87 % (P < 0.01 w.r.t. LPS).
Olaparib administration ameliorate kidney function upon intratracheal administration of LPS. Mice were treated with LPS and/or olaparib as explained earlier. Blood obtained 18 h after LPS treatment were processed for serum preparation and tested for urea (a), creatinine (b), and uric acid (c). Results are depicted as mean ± SEM. **significant w.r.t. control, P < 0.01; *significant w.r.t. control P < 0.05; ##significant w.r.t. LPS, P < 0.01; #significant w.r.t. LPS, P < 0.05
Administration of olaparib restored the redox balance of lungs upon LPS treatment. Lungs were procured from different group of mice 18 h after LPS treatment and were processed for preparing total tissue homogenate. Tissue homogenate was then assessed for MDA (a) and GSH (b). Results are depicted as mean ± SEM. **significant w.r.t. control, P < 0.01; #significant w.r.t. LPS, P < 0.05
We also examined the effect of olaparib on liver function tests at a dose of 5 mg/kg b.wt. to determine if the drug causes any liver toxicity. Our data based on the analysis of alkaline phosphatase, GOT, and GPT in serum suggests that olaparib did not cause any untoward changes in liver function (data not shown).
Administration of olaparib restored the redox balance of lung and kidney tissue upon LPS treatment
Oxidative stress reflects an imbalance between the systemic manifestation of ROS and a biological system’s ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. We assessed the levels of MDA (as a marker of lipid peroxidation) and GSH as index of oxidative stress levels in tissues. Figure 3a shows the effect of olaparib on MDA levels in lungs upon intratracheal administration of LPS. The MDA level in lung tissue was increased by 138.0 % (P < 0.01 w.r.t. control) upon LPS treatment. Administration of olaparib after LPS treatment reduced the level of MDA by 43.28 % (P < 0.05 w.r.t. LPS) toward normal. The increase in the MDA level was found to be associated with decline in GSH content by 70.63 % (P < 0.01 w.r.t. control) in lungs of LPS-treated mice (Fig. 3b). Administration of olaparib after LPS treatment however restored GSH content toward normal by 161.73 % (P < 0.05 w.r.t LPS).
Next we assessed the level of MDA and GSH in kidney tissue to ascertain if oxidative stress plays an important role in causing kidney toxicity upon i.t. administration of LPS. The data in Fig. 4a show that LPS treatment increased the MDA level by 101.12 % (P < 0.01 w.r.t. control) and administration of olaparib suppressed the MDA level toward normal significantly by 42.45 % (P < 0.05 w.r.t. LPS). Figure 4b shows the effect of olaparib on GSH level in kidney upon LPS treatment. GSH content was found to be decreased by 59.65 % (P < 0.05 w.r.t. control) in kidney tissue of LPS-administered mice while olaparib administration following LPS treatment restored its level toward normal by 149.18 % (P < 0.05 w.r.t. LPS).
Administration of olaparib restored the redox balance of kidney tissue upon LPS treatment. Kidneys were procured from different groups of mice after 18 h of LPS treatment and were processed for preparing total tissue homogenate. Tissue homogenate was then assessed for MDA (a) and GSH (b). Results are depicted as mean ± SEM. **significant w.r.t. control, P < 0.01; *significant w.r.t. control, P < 0.05; #significant w.r.t. LPS P < 0.05
Olaparib administration downregulates the expression of NF-κB-dependent genes in the lungs upon LPS treatment
Our data suggests that increase in lung inflammation and ensuing kidney injury upon intratracheal administration of LPS was associated with increase in oxidative stress in both the tissues. Consistent increase in oxidative stress is known to trigger pertinent cellular signaling pathways which activate the factors responsible for promoting inflammatory process in the tissues [48, 49]. One of such factors is transcription factor NF-κB [48, 49]. Expression of numerous NF-κB-dependent genes including several cytokines, chemokines, and adhesion molecules participate in the efficient recruitment of the inflammatory cells in the tissues [50, 51]. Accordingly we determined the effect of olaparib on the expression of factors which are known to participate in the inflammatory process in the lungs upon LPS treatment. Our data on RT-PCR (Fig. 5) certainly showed that olaparib administration after LPS treatment substantially downregulates the tissue expression of several NF-κB-dependent genes such as TNF-α, IL-1β, and VCAM-1. However, there was no major difference observed in the mRNA expression of total p65-NF-κB in lung tissues derived from different groups of mice.
Olaparib administration downregulates the expression of NF-κB-dependent genes in the lungs upon LPS treatment. Lung tissue obtained 6 h after LPS treatment was processed for total RNA extraction and mRNA was transcribed to cDNA. cDNA was then analyzed by RT-PCR for expression of TNF-α, IL-1β, p65ΝF-κΒ, and VCAM-1 using specific primers. β-actin was used as loading control
Discussion
ALI in animals is characteristic of excessive neutrophil infiltration, release of pro-inflammatory, cytotoxic mediators, and loss of vascular barrier integrity [52]. Neutrophils can release damaging mediators, such as oxidants and elastase, leading to the injury of epithelial-vascular barrier [53–55]. It is a well-known fact that the intratracheal administration of LPS initiates a lung inflammatory response [33, 56–58] useful for preparing an animal model of ALI and ARDS [59, 60]. It is also known that LPS has the potential to induce symptoms of sepsis, even when administered intratracheally [59]. In our study, we found that LPS induced significant neutrophil infiltration in lungs which is in agreement with earlier reports [61–63]. Previously, we have shown that PARP inhibition blocks the lung inflammation in the context of ALI and asthma [29, 31, 32, 64–67]. Pharmacological inhibition of PARP has been investigated in various experimental conditions of ALI or other organ(s) injury mediated by systemic exposure of LPS [31, 32, 67–70]. Olaparib, which is a new generation PARP inhibitor has been reported to have anti-cancer potential [34–37] and approved by FDA for testing in humans. It is pertinent to mention that the compound has acceptable level of toxicity when tested on patients who have breast or ovarian cancer [38, 39]. Our data strongly suggest that the compound harbors strong anti-inflammatory potential against the LPS-induced ALI as well as associated kidney injury. A single injection of olaparib protected against LPS-induced infiltration of inflammatory cells in the lungs. Considering the fact that oxidative stress is known to play an important role in the pathogenesis of number of human inflammatory disorders including those inflicting lungs [71, 72], and PARP being reported to be key mediator in such a process [73–75], we analyzed the levels of MDA and GSH in the lungs as markers of oxidative stress. Indeed LPS treatment in mice was found to alter redox balance as MDA level was found to be increased and conversely GSH was found to be decreased upon LPS treatment. Excessive production of ROS has been known to cause DNA damage, which results in activation of PARP. Over activation of PARP consumes its substrate NAD+ substantially and thus depletes ATP, which results in energy crisis leading to cell death and tissue damage [24, 25]. Tissue damage causes recruitment of greater number of inflammatory cells which further leads to enhanced production of ROS and eventually culminates in the vicious inflammatory cycle [73, 76]. Number of investigators have shown that PARP inhibition, in general, blocks the inflammation and helps restore the redox status of the cell by disrupting the persistence of this vicious cycle [73–75]. Our results, that PARP inhibition by olaparib lowered lipid peroxidation and increased GSH content toward normal suggests that the drug can normalize the tissue redox status. ROS species can directly stimulate the cell signaling pathways involving activation of several pro-inflammatory transcription factors such as MAP kinases and NF-κB [48, 49]. During the course of ROS-mediated activation of NF-κB, expression of various pro-inflammatory and adhesion molecules are known to be elevated that are responsible for the complications of various inflammatory disorders including ALI [50, 51] [77]. Several reports suggest that PARP inhibition modulates several signaling pathways including NF-κB activation for their anti-inflammatory effects [26, 28] [78]. TNF-α has been suggested as one of the important early mediators of ALI [79]. Our data utilizing RT-PCR demonstrated that olaparib suppresses the expression of lung TNF-α drastically in LPS-treated mice. It has been reported that TNF-α can further alter the cellular redox status by depleting GSH [80, 81]. Given the fact that TNF-α is a NF-κB-dependent gene and PARP is known to influence NF-κB activation, we next examined the effect of olaparib on expression of total p65NF-κB and other factors dependent on the activation of transcription factor such as IL-1β and VCAM-1. Our data revealed that olaparib did not alter the expression of total p65NF-κB but its activation, as it downregulates the expression of IL-1β and VCAM-1 in addition to TNF-α in lungs of LPS-treated mice. It is possible that reduction in the levels of TNF-α and IL-1β by olaparib in lungs of LPS-treated mice would hamper the mobilization of inflammatory cells. In addition, blunted expression VCAM-1 in lungs of LPS-treated mice by olaparib potentially adds to the defect in recruitment of inflammatory cells by hindering their transmigration across the lung endothelial barrier. Taken together, it appears that defective NF-κB-dependent transcription activation resulting from PARP-1 suppression by olaparib may partly ameliorate the LPS-induced ALI.
As mentioned earlier, induction of ALI by intratracheal administration of LPS is reported to cause symptoms of sepsis. It is known that sepsis not only mediates the ALI but also other organ failure/dysfunction including kidney [12, 82]. One such study has shown the occurrence of lung–kidney crosstalk by intratracheal instillation of LPS [40]. Our results have also shown that LPS-induced ALI is associated with marked and significant increase in kidney toxicity as reflected by increase in serum levels of urea, creatinine, and uric acid. On the other hand administration of olaparib after LPS treatment restored these parameters toward normal. It is possible that olaparib protect against kidney injury by partly restoring redox status in the tissue toward normal in addition to blunting the secondary sepsis like conditions by blocking production of pro-inflammatory factors at the level of lungs. Overall, our results suggest strong anti-inflammatory potential of olaparib against LPS-induced ALI as well as secondary kidney injury. Very recently Si et al. (2013) have also shown that 3-amino benzamide (3-AB), an old generation PARP inhibitor reduces the acute lung and kidney injury caused by intratracheal administration of LPS in rats [40]. Our results are in agreement with these observations. Olaparib has been reported to be a potent inhibitor of both PARP-1 and PARP-2 [83]. Further studies are needed to determine whether the drug shows its beneficial effects on LPS-induced inflammation in lungs/kidney by blocking both of these isoforms equally or selectively in our model. Nevertheless, we conclude that olaparib as PARP inhibitor possesses a strong anti-inflammatory potential against the LPS-induced acute lung as well as kidney injury in mice. Since, the drug is approved for human testing in cancer patients, our results can pave the way for testing it on humans presented with respiratory complication because of ALI/ARDS.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349. doi:10.1056/NEJM200005043421806
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693. doi:10.1056/NEJMoa050333
Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM (2006) Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 32:1115–1124. doi:10.1007/s00134-006-0217-3
Ware LB (2006) Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27:337–349. doi:10.1055/s-2006-948288
Wheeler AP, Bernard GR (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564. doi:10.1016/S0140-6736(07)60604-7
Martinez O, Nin N, Esteban A (2009) Prone position for the treatment of acute respiratory distress syndrome: a review of current literature. Arch Bronconeumol 45:291–296. doi:10.1016/j.arbres.2008.05.010
Chang R, Wang Y, Chang J, Wen L, Jiang Z, Yang T, Yu K (2014) LPS preconditioning ameliorates intestinal injury in a rat model of hemorrhagic shock. Inflamm Res 63:675–682. doi:10.1007/s00011-014-0740-6
Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H (2001) Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med 163:762–769. doi:10.1164/ajrccm.163.3.2003065
Matute-Bello G, Winn RK, Martin TR, Liles WC (2004) Sustained lipopolysaccharide-induced lung inflammation in mice is attenuated by functional deficiency of the Fas/Fas ligand system. Clin Diagn Lab Immunol 11:358–361
Rojas M, Woods CR, Mora AL, Xu J, Brigham KL (2005) Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol 288:L333–L341. doi:10.1152/ajplung.00334.2004
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379–L399. doi:10.1152/ajplung.00010.2008
Singbartl K, Bishop JV, Wen X, Murugan R, Chandra S, Filippi MD, Kellum JA (2011) Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 80:633–644. doi:10.1038/ki.2011.201
Vieira JM Jr, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L Jr, Imanishe MH, Abdulkader RC, Deheinzelin D (2007) Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med 35:184–191. doi:10.1097/01.CCM.0000249828.81705.65
Liu KD, Matthay MA (2008) Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol 3:578–586. doi:10.2215/CJN.01630407
Sodhi RK, Singh N, Jaggi AS (2010) Poly (ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vasc Pharmacol 53:77–87. doi:10.1016/j.vph.2010.06.003
de la Lastra CA, Villegas I, Sanchez-Fidalgo S (2007) Poly (ADP-ribose) polymerase inhibitors: new pharmacological functions and potential clinical implications. Curr Pharm Des 13:933–962
Virag L, Szabo C (2002) The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429
Pacher P, Szabo C (2008) Role of the peroxynitrite-poly (ADP-ribose) polymerase pathway in human disease. Am J Pathol 173:2–13. doi:10.2353/ajpath.2008.080019
Ame JC, Spenlehauer C, de Murcia G (2004) The PARP superfamily. Bioessays 26:882–893. doi:10.1002/bies.20085
Herceg Z, Wang ZQ (2001) Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res 477:97–110
Dantzer F, Ame JC, Schreiber V, Nakamura J, Menissier-de Murcia J, de Murcia G (2006) Poly (ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol 409:493–510. doi:10.1016/S0076-6879(05)09029-4
Kraus WL, Lis JT (2003) PARP goes transcription. Cell 113:677–683
Kraus WL (2008) Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol 20:294–302. doi:10.1016/j.ceb.2008.03.006
D’Amours D, Desnoyers S, D’Silva I, Poirier GG (1999) Poly (ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J 342(Pt 2):249–268
Shall S, de Murcia G (2000) Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res 460:1–15
Hassa PO, Hottiger MO (1999) A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem 380:953–959. doi:10.1515/BC.1999.118
Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G (1999) Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J 18:4446–4454. doi:10.1093/emboj/18.16.4446
Zerfaoui M, Errami Y, Naura AS, Suzuki Y, Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, Koochekpour S, Catling A, Boulares AH (2010) Poly (ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J Immunol 185:1894–1902. doi:10.4049/jimmunol.1000646
Naura AS, Datta R, Hans CP, Zerfaoui M, Rezk BM, Errami Y, Oumouna M, Matrougui K, Boulares AH (2009) Reciprocal regulation of iNOS and PARP-1 during allergen-induced eosinophilia. Eur Respir J 33:252–262. doi:10.1183/09031936.00089008
Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, Boulares AH (2008) Nuclear translocation of p65 NF-kappaB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: differential requirement for PARP-1 expression and interaction. Cell Signal 20:186–194. doi:10.1016/j.cellsig.2007.10.007
Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C (2002) Activation of poly (ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med 165:372–377. doi:10.1164/ajrccm.165.3.2106050
Murakami K, Enkhbaatar P, Shimoda K, Cox RA, Burke AS, Hawkins HK, Traber LD, Schmalstieg FC, Salzman AL, Mabley JG, Komjati K, Pacher P, Zsengeller Z, Szabo C, Traber DL (2004) Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock 21:126–133. doi:10.1097/01.shk.0000108397.56565.4a
Vaschetto R, Kuiper JW, Chiang SR, Haitsma JJ, Juco JW, Uhlig S, Plotz FB, Della Corte F, Zhang H, Slutsky AS (2008) Inhibition of poly (adenosine diphosphate-ribose) polymerase attenuates ventilator-induced lung injury. Anesthesiology 108:261–268. doi:10.1097/01.anes.0000299434.86640.15
Lord CJ, Ashworth A (2008) Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol 8:363–369. doi:10.1016/j.coph.2008.06.016
Anders CK, Winer EP, Ford JM, Dent R, Silver DP, Sledge GW, Carey LA (2010) Poly (ADP-Ribose) polymerase inhibition: “targeted” therapy for triple-negative breast cancer. Clin Cancer Res 16:4702–4710. doi:10.1158/1078-0432.CCR-10-0939
Ihnen M, zu Eulenburg C, Kolarova T, Qi JW, Manivong K, Chalukya M, Dering J, Anderson L, Ginther C, Meuter A, Winterhoff B, Jones S, Velculescu VE, Venkatesan N, Rong HM, Dandekar S, Udar N, Janicke F, Los G, Slamon DJ, Konecny GE (2013) Therapeutic potential of the poly(ADP-ribose) polymerase inhibitor rucaparib for the treatment of sporadic human ovarian cancer. Mol Cancer Ther 12:1002–1015. doi:10.1158/1535-7163.MCT-12-0813
Zhang J (2014) Poly (ADP-ribose) polymerase inhibitor: an evolving paradigm in the treatment of prostate cancer. Asian J Androl 16:401–406. doi:10.4103/1008-682X.123684
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134. doi:10.1056/NEJMoa0900212
Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, Ranson M (2012) Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer 106:468–474. doi:10.1038/bjc.2011.555
Si MK, Mitaka C, Tulafu M, Abe S, Kitagawa M, Ikeda S, Eishi Y, Kurata S, Tomita M (2013) Inhibition of poly (adenosine diphosphate-ribose) polymerase attenuates lung-kidney crosstalk induced by intratracheal lipopolysaccharide instillation in rats. Respir Res 14:126. doi:10.1186/1465-9921-14-126
Zerfaoui M, Naura AS, Errami Y, Hans CP, Rezk BM, Park J, Elsegeiny W, Kim H, Lord K, Kim JG, Boulares AH (2009) Effects of PARP-1 deficiency on airway inflammatory cell recruitment in response to LPS or TNF: differential effects on CXCR2 ligands and duffy antigen receptor for chemokines. J Leukoc Biol 86:1385–1392. doi:10.1189/jlb.0309183
Ghonim MA, Naura AS, Rodriguez P, Al Khami A, Hernandez C, Mansy MS, Ochoa A, Boulares H (2013) Olaparib, a PARP inhibitor approved for human testing, prevents allergen-induced airway inflammation and hyper responsiveness in a mouse model of asthma and reduces proliferation of human CD3/C28-stimulated CD4+ T cells. FASEB J 27:1107.1
Kim H, Naura AS, Errami Y, Ju J, Boulares AH (2011) Cordycepin blocks lung injury-associated inflammation and promotes BRCA1-deficient breast cancer cell killing by effectively inhibiting PARP. Mol Med 17:893–900. doi:10.2119/molmed.2011.00032
Naura AS, Kalla NR, Sharma RP, Sharma R (2007) Anticarcinogenic effects of hexaamminecobalt (III) chloride in mice initiated with diethylnitrosamine. Biol Trace Elem Res 119:147–165. doi:10.1007/s12011-007-0051-7
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Kastl L, Sauer SW, Ruppert T, Beissbarth T, Becker MS, Suss D, Krammer PH, Gulow K (2014) TNF-alpha mediates mitochondrial uncoupling and enhances ROS-dependent cell migration via NF-kappaB activation in liver cells. FEBS Lett 588:175–183. doi:10.1016/j.febslet.2013.11.033
Wang LH, Li Y, Yang SN, Wang FY, Hou Y, Cui W, Chen K, Cao Q, Wang S, Zhang TY, Wang ZZ, Xiao W, Yang JY, Wu CF (2014) Gambogic acid synergistically potentiates cisplatin-induced apoptosis in non-small-cell lung cancer through suppressing NF-kappaB and MAPK/HO-1 signalling. Br J Cancer 110:341–352. doi:10.1038/bjc.2013.752
Chian CF, Chiang CH, Chuang CH, Liu SL (2014) Inhibitor of nuclear factor-kappaB, SN50, attenuates lipopolysaccharide-induced lung injury in an isolated and perfused rat lung model. Transl Res 163:211–220. doi:10.1016/j.trsl.2013.10.002
Pan H, Zhang Y, Luo Z, Li P, Liu L, Wang C, Wang H, Li H, Ma Y (2014) Autophagy mediates avian influenza H5N1 pseudotyped particle-induced lung inflammation through NF-kappaB and p38 MAPK signaling pathways. Am J Physiol Lung Cell Mol Physiol 306:L183–L195. doi:10.1152/ajplung.00147.2013
Cross LJ, Matthay MA (2011) Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin 27:355–377. doi:10.1016/j.ccc.2010.12.005
Hoth JJ, Wells JD, Hiltbold EM, McCall CE, Yoza BK (2011) Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock 35:604–609. doi:10.1097/SHK.0b013e3182144a50
Xiang M, Yin L, Li Y, Xiao G, Vodovotz Y, Billiar TR, Wilson MA, Fan J (2011) Hemorrhagic shock activates lung endothelial reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase via neutrophil NADPH oxidase. Am J Respir Cell Mol Biol 44:333–340. doi:10.1165/rcmb.2009-0408OC
Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, Tabary O, Hong SS, Boulanger P, Paulais M, Malleret L, Belaaouaj A, Edelman A, Huerre M, Chignard M, Sallenave JM (2013) Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med 187:170–179. doi:10.1164/rccm.201205-0875OC
Haitsma JJ, Lachmann B, Papadakos PJ (2009) Additives in intravenous anesthesia modulate pulmonary inflammation in a model of LPS-induced respiratory distress. Acta Anaesthesiol Scand 53:176–182. doi:10.1111/j.1399-6576.2008.01844.x
Wu CL, Lin LY, Yang JS, Chan MC, Hsueh CM (2009) Attenuation of lipopolysaccharide-induced acute lung injury by treatment with IL-10. Respirology 14:511–521. doi:10.1111/j.1440-1843.2009.01516.x
Vaschetto R, Kuiper JW, Musters RJ, Eringa EC, Della Corte F, Murthy K, Groeneveld AB, Plotz FB (2010) Renal hypoperfusion and impaired endothelium-dependent vasodilation in an animal model of VILI: the role of the peroxynitrite-PARP pathway. Crit Care 14:R45. doi:10.1186/cc8932
Reiss LK, Uhlig U, Uhlig S (2012) Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 91:590–601. doi:10.1016/j.ejcb.2011.11.004
van Helden HP, Kuijpers WC, Steenvoorden D, Go C, Bruijnzeel PL, van Eijk M, Haagsman HP (1997) Intratracheal aerosolization of endotoxin (LPS) in the rat: a comprehensive animal model to study adult (acute) respiratory distress syndrome. Exp Lung Res 23:297–316
Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB (2006) Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest 116:2333–2343. doi:10.1172/JCI27154
Huang Z, Zhao C, Chen Y, Cowell JA, Wei G, Kultti A, Huang L, Thompson CB, Rosengren S, Frost GI, Shepard HM (2014) Recombinant human hyaluronidase PH20 does not stimulate an acute inflammatory response and inhibits lipopolysaccharide-induced neutrophil recruitment in the air pouch model of inflammation. J Immunol 192:5285–5295. doi:10.4049/jimmunol.1303060
Yaxin W, Shanglong Y, Huaqing S, Hong L, Shiying Y, Xiangdong C, Ruidong L, Xiaoying W, Lina G, Yan W (2014) Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J Surg Res 188:213–221. doi:10.1016/j.jss.2013.11.1107
Naura AS, Zerfaoui M, Kim H, Abd Elmageed ZY, Rodriguez PC, Hans CP, Ju J, Errami Y, Park J, Ochoa AC, Boulares AH (2010) Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J Immunol 185:3076–3085. doi:10.4049/jimmunol.0904214
Datta R, Naura AS, Zerfaoui M, Errami Y, Oumouna M, Kim H, Ju J, Ronchi VP, Haas AL, Boulares AH (2011) PARP-1 deficiency blocks IL-5 expression through calpain-dependent degradation of STAT-6 in a murine asthma model. Allergy 66:853–861. doi:10.1111/j.1398-9995.2011.02549.x
Naura AS, Hans CP, Zerfaoui M, You D, Cormier SA, Oumouna M, Boulares AH (2008) Post-allergen challenge inhibition of poly (ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy 38:839–846. doi:10.1111/j.1365-2222.2008.02943.x
Kiefmann R, Heckel K, Doerger M, Schenkat S, Kupatt C, Stoeckelhuber M, Wesierska-Gadek J, Goetz AE (2004) Role of PARP on iNOS pathway during endotoxin-induced acute lung injury. Intensive Care Med 30:1421–1431. doi:10.1007/s00134-004-2301-x
Goldfarb RD, Marton A, Szabo E, Virag L, Salzman AL, Glock D, Akhter I, McCarthy R, Parrillo JE, Szabo C (2002) Protective effect of a novel, potent inhibitor of poly (adenosine 5′-diphosphate-ribose) synthetase in a porcine model of severe bacterial sepsis. Crit Care Med 30:974–980
Tasatargil A, Aksoy NH, Dalaklioglu S, Sadan G (2008) Poly (ADP-ribose) polymerase as a potential target for the treatment of acute renal injury caused by lipopolysaccharide. Ren Fail 30:115–120. doi:10.1080/08860220701742195
Szabo C, Cuzzocrea S, Zingarelli B, O’Connor M, Salzman AL (1997) Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest 100:723–735. doi:10.1172/JCI119585
Santus P, Corsico A, Solidoro P, Braido F, Di Marco F, Scichilone N (2014) Oxidative stress and respiratory system: pharmacological and clinical reappraisal of N-Acetylcysteine. COPD. doi:10.3109/15412555.2014.898040
Toygar M, Aydin I, Agilli M, Aydin F, Oztosun M, Gul H, Macit E, Karslioglu Y, Topal T, Uysal B, Honca M (2014) The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum Exp Toxicol. doi:10.1177/0960327114533808
Jagtap P, Szabo C (2005) Poly (ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4:421–440. doi:10.1038/nrd1718
Virag L, Salzman AL, Szabo C (1998) Poly (ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J Immunol 161:3753–3759
Szabo C, Dawson VL (1998) Role of poly (ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci 19:287–298
Peralta-Leal A, Rodriguez-Vargas JM, Aguilar-Quesada R, Rodriguez MI, Linares JL, de Almodovar MR, Oliver FJ (2009) PARP inhibitors: new partners in the therapy of cancer and inflammatory diseases. Free Radic Biol Med 47:13–26. doi:10.1016/j.freeradbiomed.2009.04.008
Atsuta J, Sterbinsky SA, Plitt J, Schwiebert LM, Bochner BS, Schleimer RP (1997) Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am J Respir Cell Mol Biol 17:571–582. doi:10.1165/ajrcmb.17.5.2685
Szabo C, Wong H, Bauer P, Kirsten E, Oconnor M, Zingarelli B, Mendeleyev J, Hasko G, Vizi E, Salzman A, Kun E (1997) Regulation of components of the inflammatory response by 5-iodo-6-amino-1,2-benzopyrone, an inhibitor of poly (ADP-ribose) synthetase and pleiotropic modifier of cellular signal pathways. Int J Oncol 10:1093–1101
Sheridan BC, McIntyre RC, Meldrum DR, Fullerton DA (1997) Pentoxifylline treatment attenuates pulmonary vasomotor dysfunction in acute lung injury. J Surg Res 71:150–154. doi:10.1006/jsre.1997.5144
Witkamp R, Monshouwer M (2000) Signal transduction in inflammatory processes, current and future therapeutic targets: a mini review. Vet Q 22:11–16. doi:10.1080/01652176.2000.9695016
Glosli H, Tronstad KJ, Wergedal H, Muller F, Svardal A, Aukrust P, Berge RK, Prydz H (2002) Human TNF-alpha in transgenic mice induces differential changes in redox status and glutathione-regulating enzymes. FASEB J 16:1450–1452. doi:10.1096/fj.01-0948fje
Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC, Anzueto A, Truwit JD, National Institutes of Health National Heart L and Blood Institute Acute Respiratory Distress Syndrome Network (2011) Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 39:2665–2671. doi:10.1097/CCM.0b013e318228234b
Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, Javaid H, Kerrigan F, Knights C, Lau A, Loh VM Jr, Matthews IT, Moore S, O’Connor MJ, Smith GC, Martin NM (2008) 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly (ADP-ribose) polymerase-1. J Med Chem 51:6581–6591. doi:10.1021/jm8001263
Acknowledgments
The grant support (BT/RLF/Re-entry/36/2012) from Department of Biotechnology, Government of India in the form of Ramalingaswami Fellowship to Dr. Amarjit S. Naura is highly acknowledged. We also acknowledge the Junior Research Fellowship to Bijayani Sahu from University Grant Commission, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapoor, K., Singla, E., Sahu, B. et al. PARP inhibitor, olaparib ameliorates acute lung and kidney injury upon intratracheal administration of LPS in mice. Mol Cell Biochem 400, 153–162 (2015). https://doi.org/10.1007/s11010-014-2271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2271-4