Zinc phosphate nanoparticles with a thickness of ~20 nm are synthesized by an ion-exchange reaction. We study the influence of the nature of reaction media and modifiers of the surface on their shapes and sizes. It is shown that acryl monomers are optimal modifiers of the surface. We investigate the anticorrosion activity of synthesized nanosized zinc phosphate in alkyd coatings on aluminum alloys by the method of electrochemical impedance spectroscopy. It is shown that nanosized zinc phosphate has better inhibiting properties than modified phosphate pigments and can be used in paint primer coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Corrosion-resistant paint coatings are extensively used for the protection of metallic structures against atmospheric corrosion. The primer layer containing inhibiting pigments plays the principal protective role in the composition of paint coatings formed on the metals. In this aspect, chromates prove to be especially efficient. However, they are extremely toxic and ecologically dangerous. At the same time, zinc phosphate is a wellknown nontoxic pigment [1, 2]. However, its anticorrosion efficiency is insufficient, especially in the initial stages of development of underfilm corrosion of the metal. This is explained by the low solubility of zinc phosphate in aqueous media. It was shown [3] that the corrosion resistance of primer paint coatings on aluminum alloys and galvanized steel increases as a result of the addition of micronized zinc phosphate instead of the phosphate pigment. This is probably caused by an increase in its specific surface area, inversely proportional to the size of particles of the pigment [4]. The anticorrosion activity of zinc phosphate in paint coatings can be increased by decreasing its particle sizes down to the nanorange. The methods used for the preparation of nanosized zinc phosphates were described in [5–7]. However, all these methods are power-consuming, technically complicated, and therefore, poorly applicable in the industry. The aim of the present work is to get nanosized zinc phosphate by using the improved procedures and study the influence of the conditions of synthesis on its size, shape, and anticorrosion properties.
Materials and Methods for Investigations
Synthesis of Nanozized Zinc Phosphate. We synthesized zinc phosphate in a glass reactor equipped with a mechanical stirrer at 20°С by an ion-exchange reaction in aqueous media. To modify the surface of obtained nanoparticles, we used acryl monomers, namely, butyl acrylate (BA) and butyl methacrylate (BMA) [8]. They have insignificant surface-active properties and are poorly soluble in water. Therefore, in the process of formation of a new phase, their molecules can be adsorbed on the surface of nanoparticles of zinc phosphate and inhibit the transfer of substances from finer to coarser particles. At the same time, in the course of drying of the obtained reaction products at elevated temperatures, the process of ion-coordination polymerization of the adsorbed monomer is possible and, hence, the formation of thin polymeric films on the surfaces of nanoparticles of zinc phosphate is also possible. To check these assumptions, we synthesized samples of zinc phosphate in the presence of BA and BMA аnd in the absence of any modifier on the surface. The shape, size, and elemental composition of zinc phosphate nanoparticles were analyzed with the help of an EVO-40XVP scanning electron microscope equipped with an INCA-Energy 350 microanalysis system.
The anticorrosion efficiency of the nanophosphate pigment in PF-170 alkyd coatings on D16Т aluminum alloy was studied by the method of electrochemical impedance spectroscopy [9]. The coating had two layers. The first of these layers contained nanosized zinc phosphate. For comparison, we modified the coating by adding Novinox-PZ02 commercial inhibiting pigment (organically modified zinc phosphate), which is more efficient in alkyd systems than standard Zn3(PO4)2. The mean thickness of the primer layer of the alkyd coating was 50 mm and the total thickness was 120 mm. In the coatings, we drilled through holes with a diameter of 1 mm in order to expose the metallic substrate to the action of corrosive media and study the influence of the introduced inhibitors on the underfilm corrosion. The impedance spectra of the samples of aluminum alloys with coatings were taken at the free corrosion potential with a Gill-AC potentiostat. We used a saturated Ag/AgCl reference electrode and a platinum auxiliary electrode. The frequency of applied current varied from 1 kHz to 0.1 Hz. The amplitude of the signal was 30 mV. A weakly acid solution with рН ~4.5 (3.18 mg/liter sulfuric acid + 4.62 mg/liter ammonium sulfate + 3.20 mg/liter sodium sulfate + 1.58 mg/liter nitric acid + 2.13 mg/liter sodium nitrate + 8.48 mg/liter sodium chloride) simulating rainfall precipitations in the industrial regions of Ukraine served as a corrosive medium.
The insulating properties of defect-free alkyd coatings were investigated by the method of determination of their capacitance and active resistance with the help of an R-5083 AC bridge in the course of holding in corrosive media. The measurements were carried out at room temperature for a current frequency of 1 kHz with the use of a platinum counter electrode. The area of the working surfaces of the samples was equal to 5 cm2.
Results and Their Discussion
According to the results of electron microscopic studies (Fig. 1), the obtained nanoparticles of zinc phosphate have lamellar shapes and a mean thickness of 20 nm for BMA (sample No. 3), about 50 nm for BA (sample No. 2), and 100–120 nm in the case of synthesis in the absence of modifier (sample No. 1). On the basis of the analysis of micrographs, we determined the mean linear sizes of the obtained nanoparticles (Fig. 2). The smaller size of nanoparticles obtained as a result of modification with BMA as compared with the nanoparticles obtained with the use of BA can probably be explained both by a much higher heat of evaporation of BMA (~75 cal/h for BMA and 46 cal/h for BA) and by a somewhat higher solubility of BA in water (~0.08% for BMA and 0.2% for BA) [10].
The electron-probe microanalysis of the obtained samples showed that the ratio of elements Zn/P is equal to 3:2, which confirms the formation of zinc phosphate. Moreover, in samples Nos. 2 and 3, we detected 1.1 and 2.3% of carbon, respectively, which corresponds to the formation of a polymeric film on the surface of nanoparticles. The higher carbon content of sample No. 3 can be explained both by the increase in the specific surface area of the pigment in the course of stabilization of nanoparticles of zinc phosphate by BMA and by the formation of a denser polymeric film than in the case of stabilization with the use of BA.
It was shown (Fig. 3) that the commercial Novinox-PZ02 inhibiting pigment and nanosized zinc phosphate modified by BMA strongly decrease the rate of underfilm corrosion on D16Т aluminum alloy with alkyd coating. This is indicated by an increase in the magnitude of the impedance of samples with inhibited paint coatings for lower frequencies of the applied alternating current.
Behaviors of the impedance Z′ (a) and phase angle φ (b) for D16T aluminum alloy with damaged alkyd coatings after holding for 48 h in weak acid media: uninhibited alkyd coatings (1), coatings inhibited by zinc nanophosphate [1 wt.% (2), 3 wt.% (3), and 5 wt.% (4)], and coatings inhibited by 5 wt.% of the Novinox-PZ02 pigment (5).
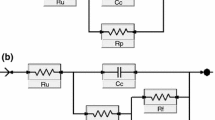
With the help of the EIS Spectrum Analyzer software [11] and a model equivalent circuit R e (Q dl R ct ) , the impedance parameters were computed for the samples of aluminum alloy in corrosive media. The highest charge-transfer resistance (R ct ) and, hence, the highest corrosion resistance were observed for the aluminum alloy with alkyd coatings containing up to 3 wt.% of nanosized zinc phosphate. The parameter R ct varies within the range 20–30 MΩ. For a concentration of nanopigment in the coating equal to 5 wt.%, the chargetransfer resistance of the samples of alloy somewhat decreases as compared with the case of application of the same amount of the Novinox-PZ02 phosphate pigment. The commercial zinc phosphate guarantees the chargetransfer resistance of the specimens on a level of 8 MΩ.
The nanosized phosphate has a larger specific surface area and, therefore, may have an elevated product of solubility and, hence, better inhibiting properties. The decrease in the resistance of corrosive solution R e operating in contact with samples of the coating inhibited by nanophosphate indirectly confirms this observation.
Since the protective properties of alkyd coatings containing 1 and 3 wt.% of nanosized zinc phosphate are much better than the protective properties of the coating containing 5 wt.% of the well-known Novinox-PZ02 phosphate pigment, it becomes possible to use zinc-phosphate inhibitors in paintwork primer materials more economically.
An important component of measuring the impedance is the phase angle φ specifying the ratio of the reactive component of impedance to its active component. At a frequency of 10 Hz, it was used in [12] for the evaluation of the corrosion resistance of organic coatings. It was shown that the behavior of this parameter directly depends on the degradation of coatings in corrosive media. It was also established that, within the range of medium frequencies of the applied current, the active resistance of the coating and the parameter φ reveal a trend to decrease as the time of holding of the specimens in corrosive media increases. For the phase angle, this effect is especially pronounced in the vicinity of a frequency of 10 Hz and can be used for the qualitative assessment of the durability of the coating [13]. The penetration of electrolytes into the paint layer initiates the decrease in the phase angle of the samples with coatings. Moreover, the attainment of its values lower than 40° corresponds to the through penetration of the electrolyte into the coating and to the electrochemical reactions running on the metallic substrate. Thus (Fig. 3b), the phase angle of the samples of the uninhibited alkyd coating on the aluminum alloy at a current frequency of 10 Hz after holding for 48 h in the medium approached 30°. This revealed the intense development of underfilm corrosion in the vicinity of a defect in the coating.
At the same time, the addition of inhibiting pigments to the alkyd composition substantially increases the phase angle of the coatings. In this case, nanophosphate has the most pronounced influence at concentrations of 1 and 3 wt.%. Thus, the parameter φ becomes as high as 80° which confirms the efficient inhibition of underfilm corrosion of the metal in the region of the defect in the alkyd coating. The increase in the concentration of nanophosphate in the alkyd compositions up to 5 wt.% makes the phase angle of the samples smaller than in the case of application of the well-known Novinox-PZ02 phosphate pigment, which is connected with the formation of defects in the coating probably due to the higher solubility of the nanoinhibitor.
The washout and dissolution of inorganic pigments by corrosive media may increase the penetration of corrosive ions into the paint layer and accelerate the development of underfilm corrosion. This is why it is important to study the influence of various concentrations of nanosized phosphate on the ionic conductivity of the alkyd coating in the absence of macrodefects. The obtained kinetic dependences Z′ (Fig. 4а) demonstrate that, for the first 20 days of holding of alkyd coatings in corrosive media, this parameter sharply decreases, probably due to the penetration of water molecules and ions into the paint layer. After this, the resistance of the samples of alkyd coatings to alternating current decreases much slower (1–1.5 МΩ∙cm2), which reveals the absence of underfilm corrosion in these coatings for the entire period of testing. In this case, the absolute values of Z′ in the coatings inhibited by nanosized phosphate are higher than for the reference alkyd coating and for the alkyd coating inhibited by the Novinox-PZ02і zinc phosphate, which is explained by a more uniform distribution of nanophosphate in the bulk of the coatings. According to the analysis performed by Sato [13], the coatings with high electrolytic resistance are characterized by low ion permeability.
In [14], the authors made a conclusion that, in numerous cases, the capacitance of a continuous defect-free organic coating in a medium can be more informative than its electrolytic resistance and correlates with the degree of absorption of the aqueous medium by the coating and with its destruction. The capacitance of more hydrophilic coatings is higher. The inhibiting pigments, as a rule, increase the degree of sorption of the medium by the paint layer, and it is important to make this increase as small as possible. It was shown (Fig. 4b) that the time dependences of the capacitance of alkyd coatings on the aluminum alloy at a frequency of 1 kHz inversely correlate with the curves of the active component of impedance Z′ (Fig. 4а). In this case, the alkyd coatings modified with 1 and 3 wt.% of nanosized zinc phosphate have the lowest capacitance after holding for 200 days, which reveals their insignificant degradation and low water absorption.
CONCLUSIONS
We synthesized nanosized zinc phosphate with mean linear sizes of 200 nm and a thickness of 20 nm by the ion-exchange reaction in a water–methanol medium. It was shown that the application of acryl monomers for the modification of the surface of nanophosphate is quite promising because nanophosphate has better protective properties than commercial zinc phosphate and can be applied as an anticorrosion pigment in primer paint coats.
References
A. C. Bastos, M. G. Ferreira, and A. M. Simões, “Corrosion inhibition by chromate and phosphate extracts for iron substrates studied by EIS and SVET,” Corros. Sci., 48, No. 6, 1500–1512 (2006).
I. M. Zin, S. B. Lyon, V. I. Pokhmurskii, and M. C. Simmonds, “Inhibition of steel and galvanized steel corrosion by zinc and calcium ions in the presence of phosphate,” Corros. Eng., Sci. Technol., 39, No. 2, 167–173 (2004).
A. Seth, W.J. van Ooij, P. Puomi, et. al., “Novel, one-step, chromate-free coatings containing anticorrosion pigments for metals—An overview and mechanistic study,” Progr. Org. Coat., 58, 136–145 (2007).
P. I. Ermilov, E. A. Indeikin, and I. A. Tolmachev, Pigments and Pigmented Paintwork Materials [in Russian], Khimiya, Moscow (1987).
B. Grzmil, B. Kic, and K. Lubkowski, “Studies on obtaining of zinc phosphate nanomaterials,” Rev. Adv. Mater. Sci., 14, 46–48 (2007).
S.-H. Jung, E. Oh, D. Shim, et. al., “Sonochemical synthesis of amorphous zinc phosphate nanospheres,” Bull. Korean Chem. Soc., 30, No. 10, 2280–2282 (2009).
J.-Y. Chane-Ching, Colloidal Dispersion of Calcium Phosphate Platelets, and Its Process of Preparation, US Patent No. 8026287 B2, Int. Cl. C09K 3/00, Рubl. on 27.09.2011.
V. I. Pokhmurs’kyi, A. R. Kytsya, I. M. Zin’, et al., A Method for the Preparation of Nanosized Zinc Phosphate [in Ukrainian], Patent of Ukraine No. 78529, С01B 25/37, Publ. 25.03.2013, Bull. No. 6.
G. W. Walter, “A review of impedance plot methods used for corrosion performance analysis of painted metals,” Corros. Sci., 26, No. 9, 681–703 (1986).
V. A. Kargin, Encyclopaedia of Polymers [in Russian], Vol. 1, Sovetskaya Éntsiklopediya, Moscow (1972).
A. S. Bondarenko and G. A. Ragoisha, “Inverse problem in potentiodynamic electrochemical impedance spectroscopy,” in: A. L. Pomerantsev (editor), Progress in Chemometrics Research, Nova Science Publishers, New York (2005), pp. 89–102.
Y. Zuo, R. Pang, W. Li, et al., “The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS,” Corros. Sci., 50 (12), 3322–3328 (2008).
Y. Sato. “Mechanism and evaluation of protective properties of paints,” Progr. Org. Coat., 9, 85–104 (1981).
J. C. Galvan, S. Feliu, and M. Morcillo, “Reproducibility of electrical impedance data for a metal/paint system,” Progr. Org. Coat., 17, 135–142 (1989).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 50, No. 5, pp. 7–12, September–October, 2014.
Rights and permissions
About this article
Cite this article
Pokhmurs’kyi, V.І., Bilyi, L.М., Zin’, Y.І. et al. Improvement of the Protective Properties of Alkyd Coatings by Nanosized Phosphate Pigments. Mater Sci 50, 627–633 (2015). https://doi.org/10.1007/s11003-015-9764-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-015-9764-5