Abstract
Introduction
Excessive maternal gestational weight gain (GWG) is strongly correlated with childhood obesity, yet how excess maternal weight gain and gestational diabetes mellitus (GDM) interact to affect early childhood obesity is poorly understood. The purpose of this study was to investigate whether overall and trimester-specific maternal GWG and GDM were associated with obesity in offspring by age 6 years.
Methods
A cohort of 10,335 maternal-child dyads was established from electronic health records. Maternal weights at conception and delivery were estimated from weight trajectory fits using functional principal components analysis. Kaplan–Meier curves and Cox regression, together with generalized raking, examined time-to-childhood-obesity.
Results
Obesity diagnosed prior to age 6 years was estimated at 19.7% (95% CI: 18.3, 21.1). Maternal weight gain during pregnancy was a strong predictor of early childhood obesity (p < 0.0001). The occurrence of early childhood obesity was lower among mothers with GDM compared with those without diabetes (adjusted hazard ratio = 0.58, p = 0.014). There was no interaction between maternal weight gain and GDM (p = 0.55). Higher weight gain during the first trimester was associated with lower risk of early childhood obesity (p = 0.0002) whereas higher weight gain during the second and third trimesters was associated with higher risk (p < 0.0001).
Discussion
Results indicated total and trimester-specific maternal weight gain was a strong predictor of early childhood obesity, though obesity risk by age 6 was lower for children of mothers with GDM. Additional research is needed to elucidate underlying mechanisms directly related to trimester-specific weight gain and GDM that impede or protect against obesity prevalence during early childhood.
Significance
Excessive maternal gestational weight gain (GWG) and gestational diabetes mellitus (GDM) have been linked to childhood obesity. Yet, research on how excessive total and trimester-specific GWG and GDM interact to affect early childhood obesity remains inconclusive. This study found that inadequate weight gain in the first trimester and excessive weight gain in the second and third trimester were associated with higher risks of childhood obesity by age 6. No significant interaction between maternal GWG and GDM was noted suggesting that these two important maternal conditions do not have a combined effect on the risk of early childhood obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of major public health concern are the dual child and adult obesity epidemics currently affecting over half of the United States (U.S.) population (Fryar et al., 2018; Hales et al., 2020). Obesity is a major risk factor for non-communicable conditions that, in children and adolescents, has been associated with early onset disease states such as hypertension (Brady, 2017) and type 2 diabetes (Pulgaron & Delamater, 2014). National health data from 2015 to 2016 indicates that an estimated 35% of U.S. children and adolescents meet criteria for overweight (16%) or obesity (19%) based on body mass index (BMI)-for-age (85-95th percentile and ≥ 95th percentile respectively) (Fryar et al., 2018; Ogden, 2004). Most recently, a report from 2017 to 2020 found that obesity prevalence in youth (2–19 years) increased to 21.5% (Hu & Staiano, 2022). While childhood obesity is a multifactorial issue, there is strong evidence linking the intrauterine environment to the metabolic health risks of offspring (Chandler-Laney et al., 2011; Tam et al., 2018). In particular, excessive maternal gestational weight gain (GWG) is strongly correlated with an increased incidence of overweight and obesity during the early childhood to adolescent years (Guo et al., 2015; Schack-Nielsen et al., 2010; Sridhar et al., 2014).
In 2009, the Institute of Medicine (IOM) published guidelines for optimal total and trimester-specific weight gain based on pre-pregnancy BMI (Institute of Medicine [IOM], 2009). However, globally and in the U.S., women often exceed these recommendations, especially mothers who have overweight or obesity prior to pregnancy (Rogozińska et al., 2019). A 2019 meta-analysis of 36 randomized controlled trials found that in a sample of 4429 mothers, 50% of women with pre- or early pregnancy overweight and 45% with pre- or early pregnancy obesity gained more weight than the IOM recommends as compared to only 19% of women with normal pre-pregnancy weight (Rogozińska et al., 2019). The study also found excessive GWG, regardless of pre- or early pregnancy weight status, was associated with an increased risk of having a large for gestational age infant. These findings (Rogozińska et al., 2019) and others (Goldstein et al., 2017) indicate that total GWG above the IOM recommendations are predictive factors for childhood obesity. Yet, newer research suggests trimester-specific GWG may also influence the risk of childhood obesity, particularly excessive weight gain in the first trimester (Margerison-Zilko et al., 2012). Studies commonly use total GWG to estimate childhood obesity outcomes (Goldstein et al., 2017), with fewer examining associations with trimester-specific GWG (Lu et al., 2019), thus highlighting a need for additional research on this topic.
Risk for childhood obesity has been well studied in the context of excessive GWG (Guo et al., 2015; Pham et al., 2013; Schack-Nielsen et al., 2010; Sridhar et al., 2014). Yet, in evaluating the associations between maternal GWG and childhood obesity, an additional concern is in utero exposure to gestational diabetes mellitus (GDM). GDM is a condition of hyperglycemia diagnosed between 24 and 28 weeks’ gestation (American Diabetes Association, 2021) and occurs in approximately 2–10% of all U.S. pregnancies annually (Centers for Disease Control and Prevention, 2021). Studies have reported that in utero exposure to GDM promotes weight-related health risks for the fetus (e.g., fetal macrosomia) and poor metabolic health outcomes (i.e., greater central adiposity and insulin secretion, low HDL-C) during early childhood (5–10 years of age) (Chandler-Laney et al., 2012; Nahavandi et al., 2019). A recent meta-analysis that included more than half a million offspring found associations with GDM and offspring overweight which progressively increased with age (Gao et al., 2022). Similarly, findings from a multinational cohort study found that offspring (mean age 11.4 years) born to mothers with GDM had significantly higher anthropometric (i.e., waist circumference, sum of skinfold thickness) and body fat measures compared to offspring born to mothers without GDM (Lowe et al., 2018). Yet, there remains significant heterogeneity in the literature between study design, diagnostic criteria for GDM/BMI, range of maternal age and BMI, and age at follow-up limiting between study comparisons (Gao et al., 2022). Also, pre-pregnancy BMI or peri-pregnancy BMI collected at single time-points are often used to examine the relationship between maternal weight and GDM on childhood obesity (Lowe et al., 2018; Patro Golab et al., 2018). Fewer studies examine how total GWG and GDM interact to affect childhood obesity.
Thus, the purpose of this study is to examine the associations between total and trimester-specific maternal GWG during pregnancy on time-to-obesity in a cohort of offspring 2–6 years of age. Additionally, this study will test whether the associations observed for total maternal GWG on time-to-obesity in offspring differ by the mother’s GDM status confirmed during the pregnancy.
Methods
Study Design and Setting
A retrospective cohort study of mother–child dyads was conducted. Participant data were de-identified and collected from inpatient and outpatient electronic health records (EHR) (Epic Systems, Madison, WI) from Vanderbilt University Medical Center, a major academic medical center in the Southeastern United States. This study was approved June, 26th, 2019 by the Institutional Review Board at Vanderbilt University Medical Center under a waiver of informed consent. This study followed the STROBE guidelines for reporting in cohort studies and the SAMPL guidelines for statistical reporting.
Participants and Study Procedures
Data were electronically extracted from the health records of mothers who gave birth to a child (linked to the mother’s EHR data) between December 2005 and August 2019. Mother–child dyad data consisted of only EHR records that included: (1) index child with at least one pair of height–weight measurements after 2 years of age, (2) mother with at least one reported height measurement and one weight measurement during the year preceding the pregnancy up to the date of delivery. The first delivered child was included for mothers who delivered more than one child in separate pregnancies. In the case of multiple births from a single pregnancy, one child was randomly selected for inclusion. Mothers with extreme weights (> 180 kg) or weight changes during pregnancy (> 70 kg) were excluded due to data quality concerns.
Out of 20,684 mothers and 25,284 linked children, a total of 10,335 mother–child dyads met inclusion criteria and were included in this study.
Definitions
Child BMI (kg/m2) was calculated using same day height and weight measurements. If same day measurements were missing for child height, data were imputed using the nearest height measurement within ± 3, ± 7, ± 14, or ± 30 days if the child’s age was < 90 days, 90–119 days, 120–729 days, and ≥ 730 days respectively; approximately 35% of child heights required imputation in this manner. Child BMI measures were not included if there were no corresponding weight or height measures within the acceptable window.
Childhood obesity was defined as a BMI percentile ≥ 95th for age and sex using the U.S. Centers for Disease Control and Prevention (CDC) growth curves for ages 2 to 5 years (up to 6th birthday) (Flegal & Cole, 2013). A child (≥ 2 years) was considered to have “obesity” on the first date they met the CDC growth curve endpoints for obesity.
To estimate maternal weight before pregnancy and weight changes during pregnancy, a functional principal components analysis (FPCA) was implemented (Yao et al., 2005). The FPCA used all maternal weight data during pregnancy and the year preceding pregnancy, where the number and timing of weight measurements differed across mothers, to extract weight change patterns across the duration of the pregnancy for each mother. The use of FPCA allowed for the fit of a smooth, individual weight curve to each mother’s weight data and obtain the estimated weight for each woman at the dates of conception and delivery in a setting where weight measurements at these time points were not always available. For women with unknown estimated length of pregnancy, maternal weight at conception and at delivery were extracted from the fitted FPCA curves at dates 1 day and 273 days before delivery, respectively, from which weight change during pregnancy was computed. For those with validated data, the maternal weight at conception was extracted from the fitted FPCA curves at dates matching the estimated length of pregnancy. Trimester-specific weight changes were similarly extracted from the fitted FPCA curves.
Maternal BMI was calculated using the extracted weight at conception and the mother’s median height based on all her height measurements in the EHR. Extreme heights (≤ 50 cm or ≥ 200 cm) and measurements recorded prior to 15 years of age were excluded. Maternal diagnosis of GDM was based on corresponding ICD-9 and ICD-10 codes collected from EHR data (Wu et al., 2019).
Data Validation
Data were manually validated by a research nurse on a subsample of 996 mother–child dyads. Records for validation were selected using a probabilistic (random) sample that attempted to minimize bias from error-prone EHR data and optimize the efficiency of the estimate of the association between maternal weight gain during pregnancy and childhood obesity. To optimize efficiency, mother–child dyads with children who developed obesity and mothers with particularly large or small weight gains during pregnancy were over-sampled. The registered nurse (author SP), a research nurse specialist employed at Vanderbilt University Medical Center, validated all study variables by thoroughly reviewing the EHR, including medications, free text notes, and other data in the EHR that were not readily extractable by programmers who extracted the original dataset. For example, the estimated length of pregnancy was not part of the original dataset, but was manually extracted from the EHR by the research nurse for all validated mother–child dyads. The EHR data available on all 10,335 mother–child dyads is referred to as the phase 1 data and the validated data on the sub-sample of 996 mother–child dyads as the phase 2 data. Additional details on the validation sampling strategy and data validation procedures are described elsewhere (Shepherd et al., 2023).
Statistical Analysis
Analyses were performed in R statistical software (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria) using the ‘survey’ package (Lumley, 2011). All analyses, including all data presented in tables and figures, were based on validated phase 2 data for the 996 mother–child dyads but were weighted to be representative of the larger phase 1 study population of 10,335 mother–child dyads. Weights were based on the inverse probability of being sampled for validation. Demographic characteristics were summarized using median and interquartile range (IQR) for continuous variables and proportions for categorical variables. Variables of interest included: maternal age, maternal race/ethnicity, marital status, insurance status (private vs. not private), maternal weight gain during pregnancy (kg/week), maternal pre-pregnancy BMI (kg/m2), smoking status during pregnancy (smoker vs. non-smoker), delivery type (vaginal vs. cesarean), number of prior live births, singleton (yes vs. no), maternal depression, and maternal diabetes diagnosis (none, gestational, vs. type 1/2), child sex, obesity status, and gestational age at birth (weeks).
The primary outcome was time-to-childhood-obesity. The cumulative probability of obesity from ages 2 to 6 was estimated using Kaplan–Meier curves and covariate associations were modeled using Cox proportional hazards regression. The hazard is the probability of having an event in a short period of time given that one has not yet had the event; it is sometimes referred to as the instantaneous event rate. Hazard ratios were used to present associations between predictors and the time to childhood obesity. Kaplan–Meier estimates and Cox models were weighted using inverse probability of validation weights that were augmented using generalized raking techniques to include information from the unvalidated phase 1 data to improve efficiency (Oh et al., 2021). The resulting augmented inverse probability weighted (AIPW) estimators have the benefits of being based on the carefully validated data (i.e., minimal bias because based on validated data) but have improved efficiency (i.e., narrower confidence intervals) over standard inverse probability weighted estimators because they are calibrated with unvalidated data (which tended to be highly correlated with the validated data) from the larger phase 1 cohort to improve precision. More details about the implementation of these AIPW are described elsewhere (Shepherd et al., 2023). In the primary Cox model, the risk associated with weight change during pregnancy was estimated using natural splines (3 knots) to allow for a potential non-linear relationship between this variable and the log-hazard of childhood obesity. The non-linearity of the association was assessed using a likelihood ratio test. An additional likelihood ratio test was performed to assess the association between maternal weight change and time-to-childhood-obesity by comparing the full model (with all spline terms) versus a model that did not include maternal weight change. Additional models of childhood obesity investigated the interaction between maternal BMI and maternal weight gain during pregnancy, and the interaction between GDM and maternal weight gain during pregnancy. A model was also fitted that separately included weight gain during the first trimester and weight gain during the second and third trimesters. The model adjusted for all other variables except for weight change over the course of the pregnancy.
Results
Of the 10,335 eligible mother–child dyads with EHR data, 996 were selected for chart review. Table 1 shows estimated characteristics of mother–child dyads in the larger study population (n = 10,335) based on the validated, chart review data. Estimated self-reported maternal race was 59% white, 26% Black, 5% Asian, and 9% other; 14% self-reported as of Hispanic ethnicity. Median age at delivery was 27 years. An estimated 12.5% of mothers used tobacco during pregnancy, 9.5% had a history of depression, 65% had no private insurance, and 54% were married. An estimated 2.3% of mothers had Type 1 or 2 diabetes whereas 8% had GDM. The median number of prior live births was 1, and 36% of deliveries were via cesarean. The median estimated gestational age at delivery was 39 weeks. The vast majority (98%) of children were singletons and 52% were male.
Estimated median maternal BMI at conception was 26.7 kg/m2 (IQR 22.6, 31.2); 61% of mothers were estimated to have overweight (BMI > 25 kg/m2) and 31% to have obesity (BMI > 30 kg/m2). The estimated median weight gained per week during pregnancy was 0.28 kg (IQR 0.24, 0.35), corresponding to approximately 11 kg gained during pregnancy (IQR 9.2, 13.8). The estimated median weight gains in trimesters 1, 2, and 3 were 0.67 kg (IQR 0.32, 1.20), 4.47 kg (IQR 3.90, 5.24), and 5.77 kg (4.90, 6.97), respectively. The estimated percentage of children diagnosed with obesity prior to their 6th birthday was 19.7% (95% confidence interval [CI] 18.3, 21.1).
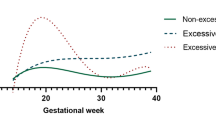
Figure 1 shows the estimated incidence of childhood obesity during ages 2–6 by maternal weight gain, based on IOM classifications of inadequate, adequate, and excessive weight gain. At age 6 years, 26.5% and 25.3% of children from women with excessive (53.4% of mothers) or adequate (19.8% of mothers) weight gain during pregnancy, respectively, were estimated to have obesity, whereas 18.9% of children from mothers with inadequate pregnancy weight gain (26.9% of mothers) were estimated to have obesity (p < 0.001).
Cumulative incidence of childhood obesity between 2 and 6 years of age. Separate cumulative incidence curves are shown based on whether mothers had inadequate gestational weight gain, adequate gestational weight gain, or excessive gestational weight gain based on the 2009 Institute of Medicine Guidelines, accounting for pre-pregnancy BMI
Table 2 shows the associations between potential predictors and childhood obesity in the primary analysis model. Holding all other factors constant, including estimated maternal BMI at conception, maternal weight gain during pregnancy was a strong predictor of childhood obesity (likelihood ratio p-value < 0.0001), with solid evidence of a non-linear relationship (likelihood ratio test for non-linearity, p = 0.007). The occurrence of childhood obesity was similar for women who gained less than an average of 0.3 kg/wk (corresponding to 11–12 kg weight gain during pregnancy [time between estimated conception and birth], approximately the median in this study’s cohort), but was higher for larger maternal weight gains. For example, compared to a woman who gained 0.2 kg/wk during pregnancy, the estimated hazard of childhood obesity was similar for a woman who did not gain any weight during pregnancy (adjusted hazard ratio [aHR] = 1.12, 95% CI 0.88–1.43), 20% higher for a woman who gained 0.4 kg/wk (aHR = 1.20, 95% CI 1.03–1.39), and 66% higher for a woman who gained 0.6 kg/wk (aHR = 1.66, 95% CI 1.32–2.09). Figure 2 shows the predicted probability of childhood obesity as a function of the average maternal weight gain during pregnancy. Holding all other covariates at their medians/modes, the children of women who gained on average 0.25 kg/wk over the course of their pregnancy (approximately the 33rd percentile in the study’s cohort) had a predicted probability of developing obesity prior to age 6 of 0.125 (95% CI 0.10, 0.15) compared with 0.167 (95% CI 0.13, 0.20) for someone who gained on average 0.5 kg/wk (approximately the 95th percentile in the study’s cohort).
Estimated maternal BMI at conception was also highly predictive of childhood obesity. Holding all other factors constant, the child from a mother with a 5 kg/m2 greater BMI at conception had a 36% higher hazard of developing obesity (aHR = 1.36; 95% CI 1.28, 1.44; p < 0.0001). In a separate model, an interaction term was included to see if the association between maternal weight change during pregnancy and risk of childhood obesity varied according to the mother’s estimated BMI at conception; the interaction was not statistically significant (p = 0.23), so results reported in Table 2; Fig. 2 assume additive effects.
The estimated hazard of childhood obesity was 42% lower for mothers with GDM compared with those without diabetes (aHR = 0.58; 95% CI 0.38, 0.89; p = 0.014). There was no evidence of an interaction between maternal weight gain and GDM (p = 0.55). There was also no evidence of an association between Type 1 or 2 diabetes and childhood obesity (aHR = 0.84; 95% CI 0.50, 1.40; p = 0.51).
Other factors associated with an increased risk of childhood obesity included Hispanic ethnicity, tobacco use during pregnancy, no private insurance, and being married. Other factors not associated with an increased risk of childhood obesity included maternal history of depression, prior number of live births, mode of delivery (cesarean), gestational age, male gender at birth, or having a singleton pregnancy (Table 2).
In the model evaluating trimester-specific maternal GWG, higher weight gain during the first trimester was associated with a lower risk of childhood obesity (aHR = 0.77 for a 1 kg difference; 95% CI = 0.68, 0.88; p = 0.0002) whereas higher weight gain during the combined second and third trimesters was associated with a higher risk of childhood obesity (aHR = 1.53 for a 5 kg difference; 95% CI = 1.24, 1.89; p < 0.0001). There was no evidence of an interaction between these two variables (p = 0.66). There was evidence of interactions between maternal BMI at conception and trimester-specific weight gains on the occurrence of childhood obesity (p = 0.001 for interaction with first trimester weight gain, p = 0.023 for interaction with combined second and third trimesters). Comparing children from two women with pre-pregnancy BMI of 20 kg/m2 and identical covariates, the child from a woman who gained 1 kg more during the first trimester was estimated to have a 2% increased hazard of childhood obesity. If these two women had pre-pregnancy BMI of 30 kg/m2, then the child from a woman who gained 1 kg more during the first trimester would be estimated to have a 15% decreased hazard of obesity. A 5 kg increased weight gain during the combined second and third trimesters was associated with 16% and 39% increased hazards of childhood obesity for mothers with BMI of 20 and 30 kg/m2, respectively.
Discussion
This retrospective cohort of 10,335 mother–child dyads, of whom 996 had their charts validated, demonstrated that a higher rate of total maternal GWG was associated with a higher risk of childhood obesity by age 6, after controlling for important covariates like pre-pregnancy BMI. The data on trimester-specific weight gain showed that inadequate weight gain in the first trimester and excessive weight gain in the second and third trimester were associated with higher risks of childhood obesity by age 6. Mothers with GDM had a 42% lower hazard for childhood obesity compared to mothers without GDM, controlling for a wide range of potential confounders. Finally, there was not a statistically significant interaction between maternal GWG and GDM. Taken together, these data indicate that maternal weight gain, including trimester-specific weight gain, remains an important factor for predicting childhood obesity and should be monitored and discussed throughout the course of a pregnancy.
The results of this study are consistent with previous research on excessive weight gain during pregnancy and increased risk for childhood obesity. In a recent meta-analysis of 37 international pregnancy and birth cohort studies, the proportion of childhood overweight/obesity that was attributed to excessive GWG in mothers ranged from 11.4% in early childhood (2–5 years), 15.4% in mid-childhood (5–10 years), and 19.2% in late childhood (10–18 years) (Voerman et al., 2019). The study examined varying child age ranges and found that overweight/obesity prevalence increased with age with the highest prevalence observed in late childhood between 10 and 18 years (OR 1.72 [95% CI: 1.56, 1.91]) (Voerman et al., 2019).
The data from this current analysis adds a more nuanced understanding of the relationship between maternal GWG and early childhood obesity. First, this study used a cohort design to evaluate incident obesity, which strengthens causal inference compared to previous cross-sectional analyses. In addition, the analytic approach and sufficient sample size allowed for the detection of a non-linear association between total maternal GWG and the risk of childhood obesity. Finally, the specific focus on trimester-specific weight gain and childhood obesity is an important contribution to this body of work, particularly given the current IOM recommendations for GWG based on pre-pregnancy BMI. In cases where pregnant mothers are overweight or obese at the start of pregnancy, the IOM recommends no more than 25 pounds and 20 pounds of weight gain respectively (IOM, 2009). In this study, the average maternal BMI at conception was 26.7 kg/m2 (i.e., overweight) which is unsurprising given that roughly 48% of U.S. women have either overweight or obesity at the start of their pregnancies (Dudenhausen et al., 2015). This study found that having a higher maternal BMI at the start of pregnancy was highly predictive of offspring developing childhood obesity. This highlights a major public health challenge linked to both increased economic costs (Moran et al., 2020) and adverse maternal and offspring outcomes (Goldstein et al., 2017; Rogozińska et al., 2019) that begin in the pre-pregnancy period. Currently, the American College of Obstetrics and Gynecology recommends strategies for weight control counseling prior to pregnancy (e.g., motivational interviewing to promote dietary modifications and exercise for weight loss) (Sagi-Dain, 2021). However, there remains a lack of high-quality evidence to support an optimal lifestyle intervention, resulting in clinicians having little guidance on what strategies are most appropriate to recommend to reduce pre-pregnancy weight (Stephenson et al., 2018). Moreover, existing public health programs (e.g., Supplemental Nutrition Program for Women, Infants, and Children) that provide nutrition support only offer assistance during the perinatal or postnatal period and are not focused on modifying diet to promote a healthy weight prior to pregnancy (United States Department of Agriculture, 2020). Thus, understanding the specific timing and magnitude of how maternal weight gain affects early childhood obesity could help shape the timing and content of future interventions and public health policies designed to support healthy weight gain for mothers and children.
In this study, GDM was inversely associated with the incidence of childhood obesity and maternal weight gain did not appear to influence the relationship between GDM and childhood obesity. These findings are consistent with a similar retrospective cohort study of EHR data from a large hospital system in the U.S. by Pham and colleagues (2013) which reported no significant differences in BMI percentiles for 2–4 year olds born to mothers with GDM compared to those without GDM. In contrast, other studies have noted a significant relationship between maternal GDM and childhood obesity risk. Obesity risk has been shown to increase sequentially with age, more so during mid-childhood (Gao et al., 2022). In two large cohort studies, GDM was associated with childhood obesity roughly within the first decade of life (Hillier et al., 2016; Lowe et al., 2018). However, in this study, excess maternal GWG and fetal exposure to GDM did not significantly influence the risk for childhood obesity by age 6 years. One plausible explanation is that pre-pregnancy factors may alternatively influence the risk for early childhood obesity. For example, a recent cohort study found that having either pre-pregnancy obesity or pre-pregnancy obesity and GDM strongly predicted early childhood obesity (up to age 5 years) (Choi et al., 2022). Yet, other cohort studies have reported either no association or an attenuated association between GDM and childhood obesity risk when controlling for pre-pregnancy BMI (Ehrenthal et al., 2013; Pham et al., 2013). Collectively, these findings suggest that pre-pregnancy BMI may exert a greater influence on later childhood obesity risk compared with perinatal factors alone such as GDM. Given the lack of scientific consensus on this topic and the continued heterogeneity within the literature, future mechanistic studies are required to clarify what factors may be responsible for childhood obesity in the presence of fetal GDM exposure.
This study had several strengths. It was performed in a large population of linked mothers and children in the Southeastern U.S., where the obesity epidemic is particularly burdensome (Conway et al., 2018). Data were extensively validated on a targeted, but probabilistically selected, subsample of nearly one thousand mother–child dyads. Cutting-edge analysis techniques were applied to combine the validated data with data from the larger cohort to improve the power and accuracy of the study estimates.
There were also several study limitations. First, the study is subject to natural limitations that come from using routinely collected data from the EHR. For example, the timing of maternal weight measurements was not necessarily consistent across subjects so weight gain during pregnancy had to be estimated using statistical techniques. Similarly, to calculate child BMI, 35% of child heights had to be imputed using a height taken on a nearby, but not the same, date as their weight measurement. The corrective analysis decisions are reasonable and based on sound statistical procedures but may yield different results than would have been obtained had data been prospectively collected in a systematic manner. Second, it is possible that child weight fluctuations occurred throughout the timeline of possible obesity diagnosis (≥ 2 years based on CDC BMI criteria) and children who were initially considered to have obesity may have reverted to a normal weight category. Thus, it is possible that childhood obesity may have been overestimated. Third, data were not collected on the use of medications to treat GDM (e.g., insulin, metformin). Metformin crosses the placenta and has been associated with lower birth weight, but higher BMI in mid-childhood (5–9 years) compared to treatment with insulin (Tarry-Adkins et al., 2019, 2020). Therefore, it could not be determined how medication use and GDM interact to affect childhood obesity. Finally, as with all cohort studies, particularly those using passive data collection through EHR, the potential for residual confounding variables exists, which may have biased the results in an unpredictable manner.
Conclusion
Using a large EHR data set of 10,335 mother–child dyads, of which 996 had their records validated, maternal weight gain during pregnancy was found to be a strong predictor of childhood obesity during the first 6 years of life. This study also found that both excessive maternal weight gain in the second and third trimesters and inadequate weight gain during the first trimester were predictive of childhood obesity. Lastly, GDM was associated with lower risk of childhood obesity in this study. As researchers continue to understand more about which gestational factors influence childhood obesity, additional research is needed to elucidate underlying mechanisms directly related to trimester-specific weight gain and GDM that may impede or protect against obesity prevalence in children.
Data Availability
Not applicable.
Code Availability
Analysis code is available at https://biostat.app.vumc.org/ArchivedAnalyses.
References
American Diabetes Association. (2021). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care,44(Supplement 1), S15. https://doi.org/10.2337/dc21-S002
Brady, T. M. (2017). Obesity-related hypertension in children [Review]. Frontiers in Pediatrics. https://doi.org/10.3389/fped.2017.00197
Centers for Disease Control and Prevention (2021). Gestational diabetes. Centers for Disease Control and Prevention. Retrieved September 20, 2022 from https://www.cdc.gov/diabetes/basics/gestational.html.
Chandler-Laney, P. C., Bush, N. C., Rouse, D. J., Mancuso, M. S., & Gower, B. A. (2011). Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care,34(3), 741–745.
Chandler-Laney, P. C., Bush, N. C., Granger, W. M., Rouse, D. J., Mancuso, M. S., & Gower, B. A. (2012). Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatric Obesity,7(1), 44–52.
Choi, M. J., Yu, J., & Choi, J. (2022). Maternal pre-pregnancy obesity and gestational diabetes mellitus increase the risk of childhood obesity. Children,9(7), 928.
Conway, B. N., Han, X., Munro, H. M., Gross, A. L., Shu, X. O., Hargreaves, M. K., Zheng, W., Powers, A. C., & Blot, W. J. (2018). The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PLoS One, 13(1), e0190993. https://doi.org/10.1371/journal.pone.0190993
Dudenhausen, J. W., Grünebaum, A., & Kirschner, W. (2015). Prepregnancy body weight and gestational weight gain—recommendations and reality in the USA and in Germany. American Journal of Obstetrics and Gynecology,213(4), 591–592.
Ehrenthal, D. B., Maiden, K., Rao, A., West, D. W., Gidding, S. S., Bartoshesky, L., Carterette, B., Ross, J., & Strobino, D. (2013). Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstetrics and Gynecology,121(1), 115–121. https://doi.org/10.1097/AOG.0b013e318278f56a
Flegal, K. M., & Cole, T. J. (2013). Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. US Department of Health and Human Services, Centers for Disease Control and Prevention National Center for Health Statistics.
Fryar, C. D., Carroll, M. D., & Ogden, C. L. (2018). Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. Centers for Disease Control and Prevention. Retrieved May 19, 2022 from https://stacks.cdc.gov/view/cdc/58669
Gao, M., Cao, S., Li, N., Liu, J., Lyu, Y., Li, J., & Yang, X. (2022). Risks of overweight in the offspring of women with gestational diabetes at different developmental stages: A meta-analysis with more than half a million offspring. Obesity Reviews,23(3), e13395. https://doi.org/10.1111/obr.13395
Goldstein, R. F., Abell, S. K., Ranasinha, S., Misso, M., Boyle, J. A., Black, M. H., Li, N., Hu, G., Corrado, F., & Rode, L. (2017). Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. Journal of the American Medical Association,317(21), 2207–2225.
Guo, L., Liu, J., Ye, R., Liu, J., Zhuang, Z., & Ren, A. (2015). Gestational weight gain and overweight in children aged 3–6 years. Journal of Epidemiology. https://doi.org/10.2188/jea.JE20140149
Hales, C. M., Carroll, M. D., Fryar, C. D., & Ogden, C. L. (2020). Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Centers for Disease Control and Prevention. Retrieved May 19, 2022 from https://www.cdc.gov/nchs/products/databriefs/db360.htm
Hillier, T. A., Pedula, K. L., Vesco, K. K., Oshiro, C. E., & Ogasawara, K. K. (2016). Impact of maternal glucose and gestational weight gain on child obesity over the first decade of life in normal birth weight infants. Maternal and Child Health Journal,20(8), 1559–1568. https://doi.org/10.1007/s10995-016-1955-7
Hu, K., & Staiano, A. E. (2022). Trends in obesity prevalence among children and adolescents aged 2 to 19 years in the US from 2011 to 2020. JAMA Pediatrics,176(10), 1037–1039. https://doi.org/10.1001/jamapediatrics.2022.2052
Institute of Medicine (2009). Weight gain during pregnancy: Reexamining the guidelines. The National Academies Collection: Reports Funded by National Institutes of Health, 2.
Lowe, W. L., Scholtens, D. M., Lowe, L. P., Kuang, A., Nodzenski, M., Talbot, O., Catalano, P. M., Linder, B., Brickman, W. J., & Clayton, P. (2018). Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. Journal of the American Medical Association,320(10), 1005–1016.
Lu, W., Zhang, X., Wu, J., Mao, X., Shen, X., Chen, Q., Zhang, J., Huang, L., & Tang, Q. (2019). Association between trimester-specific gestational weight gain and childhood obesity at 5 years of age: Results from Shanghai obesity cohort. BMC Pediatrics,19(1), 1–9.
Lumley, T. (2011). Complex surveys: A guide to analysis using R (Vol. 565). Wiley.
Margerison-Zilko, C. E., Shrimali, B. P., Eskenazi, B., Lahiff, M., Lindquist, A. R., & Abrams, B. F. (2012). Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Maternal and child health journal,16(6), 1215–1223.
Moran, P. S., Wuytack, F., Turner, M., Normand, C., Brown, S., Begley, C., & Daly, D. (2020). Economic burden of maternal morbidity – a systematic review of cost-of-illness studies. PLoS One,15(1), e0227377. https://doi.org/10.1371/journal.pone.0227377
Nahavandi, S., Price, S., Sumithran, P., & Ekinci, E. I. (2019). Exploration of the shared pathophysiological mechanisms of gestational diabetes and large for gestational age offspring. World Journal of Diabetes,10(6), 333–340. https://doi.org/10.4239/wjd.v10.i6.333
Ogden, C. L. (2004). Defining overweight in children using growth charts. Maryland Medicine,5(3), 19–21.
Oh, E. J., Shepherd, B. E., Lumley, T., & Shaw, P. A. (2021). Raking and regression calibration: methods to address bias from correlated covariate and time-to‐event error. Statistics in Medicine,40(3), 631–649.
Patro Golab, B., Santos, S., Voerman, E., Lawlor, D. A., Jaddoe, V. W. V., & Gaillard, R. (2018). Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: An individual participant data meta-analysis. Lancet Child Adolesc Health,2(11), 812–821. https://doi.org/10.1016/s2352-4642(18)30273-6
Pham, M. T., Brubaker, K., Pruett, K., & Caughey, A. B. (2013). Risk of childhood obesity in the toddler offspring of mothers with gestational Diabetes. Obstetrics and Gynecology,121(5), 976–982.
Pulgaron, E. R., & Delamater, A. M. (2014). Obesity and type 2 diabetes in children: Epidemiology and treatment. Current Diabetes Reports,14(8), 508.
Rogozińska, E., Zamora, J., Marlin, N., Betrán, A. P., Astrup, A., Bogaerts, A., Cecatti, J. G., Dodd, J. M., Facchinetti, F., Geiker, N. R. W., Haakstad, L. A. H., Hauner, H., Jensen, D. M., Kinnunen, T. I., Mol, B. W. J., Owens, J., Phelan, S., Renault, K. M., & Salvesen, K. (2019). Å., for the international weight management in pregnancy collaborative, G. gestational weight gain outside the institute of medicine recommendations and adverse pregnancy outcomes: analysis using individual participant data from randomised trials. BMC Pregnancy and Childbirth,19(1), 322. https://doi.org/10.1186/s12884-019-2472-7
Sagi-Dain, L. (2021). Obesity in pregnancy: ACOG Practice Bulletin, Number 230. Obstetrics and Gynecology,138(3), 489. https://doi.org/10.1097/aog.0000000000004527
Schack-Nielsen, L., Michaelsen, K., Gamborg, M., Mortensen, E., & Sørensen, T. (2010). Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. International Journal of Obesity,34(1), 67–74.
Shepherd, B. E., Han, K., Chen, T., Bian, A., Pugh, S., Duda, S. N., Lumley, T., Heerman, W. J., & Shaw, P. A. (2023). Multi-wave validation sampling for error-prone electronic health records. Biometrics,79, 2649–2663.
Sridhar, S. B., Darbinian, J., Ehrlich, S. F., Markman, M. A., Gunderson, E. P., Ferrara, A., & Hedderson, M. M. (2014). Maternal gestational weight gain and offspring risk for childhood overweight or obesity. American Journal of Obstetrics and Gynecology,211(3), 259.
Stephenson, J., Heslehurst, N., Hall, J., Schoenaker, D., Hutchinson, J., Cade, J. E., Poston, L., Barrett, G., Crozier, S. R., Barker, M., Kumaran, K., Yajnik, C. S., Baird, J., & Mishra, G. D. (2018). Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet,391(10132), 1830–1841. https://doi.org/10.1016/s0140-6736(18)30311-8
Tam, C. H., Ma, R. C., Yuen, L. Y., Ozaki, R., Li, A. M., Hou, Y., Chan, M. H., Ho, C. S., Yang, X., & Chan, J. C. (2018). The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia,61(12), 2539–2548.
Tarry-Adkins, J. L., Aiken, C. E., & Ozanne, S. E. (2019). Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Medicine,16(8), e1002848. https://doi.org/10.1371/journal.pmed.1002848
Tarry-Adkins, J. L., Aiken, C. E., & Ozanne, S. E. (2020). Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLoS Medicine,17(5), e1003126. https://doi.org/10.1371/journal.pmed.1003126
United States Department of Agriculture (2020). WIC 2017 Eligibility and Coverage Rates. United States Department of Agriculture, Food and Nutrition Services. Retrieved December 19, 2022 from https://www.fns.usda.gov/wic-2017-eligibility-and-coverage-rates
Voerman, E., Santos, S., Patro Golab, B., Amiano, P., Ballester, F., Barros, H., Bergström, A., Charles, M. A., Chatzi, L., & Chevrier, C. (2019). Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Medicine,16(2), e1002744.
Wu, P., Gifford, A., Meng, X., Li, X., Campbell, H., Varley, T., Zhao, J., Carroll, R., Bastarache, L., & Denny, J. C. (2019). Mapping ICD-10 and ICD-10-CM codes to phecodes: Workflow development and initial evaluation. JMIR Medical Informatics,7(4), e14325.
Yao, F., Müller, H. G., & Wang, J. L. (2005). Functional data analysis for sparse longitudinal data. Journal of the American Statistical Association,100(470), 577–590.
Funding
This work was supported by funding from the Patient Centered Outcomes Research Institute, the National Institute of Allergy and Infectious Disease, and a T32 training grant through the Agency for Healthcare Research and Quality. This content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality, Patient Centered Outcomes Research Institute, or the National Institute of Allergy and Infectious Disease.
Author information
Authors and Affiliations
Contributions
NMS, WJH, and BES contributed to the study conception and design. Material preparation and data collection were performed by SD and SP. Data analysis and interpretation were performed by KH, TC, AB, PS, TL, WJH, BES, and NMS. The first draft of the manuscript was written by NMS with substantial contributions from WJH, and BES. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical Approval
This study was approved by the Institutional Review Board at Vanderbilt University Medical Center under a waiver of informed consent.
Consent to Participant
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sneed, N.M., Heerman, W.J., Shaw, P.A. et al. Associations Between Gestational Weight Gain, Gestational Diabetes, and Childhood Obesity Incidence. Matern Child Health J 28, 372–381 (2024). https://doi.org/10.1007/s10995-023-03853-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-023-03853-8