Abstract
House dust mites are one of the most important allergen sources worldwide and affect approximately 50% of asthmatic patients. Allergen-specific immunotherapy (AIT) is the only disease-modifying treatment for allergic diseases. However, clinical applications of allergen extract-based AIT were greatly restricted due to the potential adverse reactions. In order to improve the efficacy and reduce adverse effects, modified hypoallergens have been proposed for molecular forms of AIT. Therefore, in the present study, we converted the major house dust mite allergen Der f 34 into a B cell epitope-based hypoallergenic vaccine by the immunoinformatics and peptide-carrier fusion approaches. Initially, the physiochemical and structural properties of Der f 34 were analyzed. Accordingly, the linear and conformational B cell epitopes, as well as the helper T lymphocytes epitopes, were computed based on the properties of Der f 34. Three different fragments (residues 12–18, 83–89, and 98–116) of major allergen Der f 34 that containing candidate B cell epitope and that without T cell epitopes were linked at the N terminal and C terminal of the PreS carrier. The three-dimensional structure of the final vaccine was then predicted and the interaction with immune receptors (toll-like receptor-3) was evaluated by ligand-receptor docking. The immunogenic profiles and immune response of the final vaccine were in silico assessed after immunization, which represented the vaccine could induce an effective immune response. In addition, the codon sequences of the vaccine were cloned and expressed in E.coli, the vaccine was purified and exhibited a lower IgE-binding ability. Our results indicated that the Der f 34 hypoallergen could be a potential vaccine candidate for molecular forms of AIT in the house dust mite allergy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
House dust mites (HDM) are the most predominant source of indoor allergens and a causative factor for allergic diseases, such as allergic rhinitis, asthma, conjunctivitis and atopic dermatitis. It was found that 1% to 2% of the world’s population were affected by house dust mite allergy and up to 50% of asthmatic patients were sensitized to it (Calderón et al. 2015b).
Currently, the clinical management of mite allergic diseases consists of allergen avoidance, pharmacotherapy and allergen immunotherapy (AIT) (Calderón et al. 2015a). Although the reducing exposure to dust mite allergens and pharmacotherapies are indeed valid, both of them are not disease-modifying therapies which still remain at the layer of symptom control in patients (Valenta et al. 2017). AIT is the only way that can change the course of allergic diseases (Niederberger and Valenta 2006), and mite allergen extracts have been successfully applied in the management of patients with HDM allergies. However, such crude extracts are a mixture of varying amounts of relevant and irrelevant allergens and undefined impurities which would cause some unpredictable immune responses (Niederberger and Valenta 2006; Valenta et al. 2018). Moreover, the components and allergen contents in these crude extracts are not completely controlled and it depends on various factors like protein degradation, heterogeneity of allergen source, and even contamination (Niederberger and Valenta 2006). All the above characteristics limit the clinical therapeutic efficacy of HDM AIT and may induce severe immediate and late phase side effects (Zieglmayer et al. 2016; Valenta et al. 2017). Therefore, molecular therapy that uses well-defined allergen derivatives is a promising method for treatment of mite allergy (Valenta et al. 2017).
Currently, thirty-nine groups of allergens from HDM have been identified and recorded in the Allergen Nomenclature Database (http://www.allergen.org). Among them, the group 1, group 2 and group 23 allergens have long been recognized as the major allergens in HDM (Thomas 2015; Cao and Liu 2020). Hence, many studies have focused on the three groups of major allergens and designed a series of hypoallergenic vaccines for the individualized treatment of mite allergy (Asturias et al. 2009; Walgraffe et al. 2009; Chen et al. 2012; Banerjee et al. 2014). Der f 34, a new major HDM allergen, was identified in recent years which would be an important cross-reactive allergen among various allergen sources (ElRamlawy et al. 2016). However, the design of hypoallergenic vaccine for Der f 34 has not been described to date.
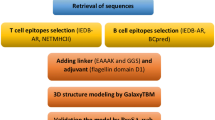
In this study, we firstly analyzed the structural features of Der f 34 as well as the B and T cell epitopes. Accordingly, a B cell epitope-carrier fusion protein was designed to convert Der f 34 into a hypoallergenic vaccine with reduced IgE (Immunoglobulin E)- and T cell-mediated side effects. The physicochemical and structural properties of the constructed vaccine were in silico characterized. In addition, the immune response of the final vaccine as well as its interaction with an immune receptor were evaluated by immunoinformatics approaches.
Material and Methods
Sequence Retrieval and Physiochemical Analysis of Der f 34
The amino acid sequence of Der f 34 was retrieved from NCBI Protein Database (https://www.ncbi.nlm.nih.gov/protein/) with the accession number of BAV90601. Physicochemical analysis including molecular weight, theoretical isoelectric point (pI), the number of negatively and positively charged residues, instability index, and grand average of hydropathicity (GRAVY) of Der f 34 was performed by using ProtParam tool (http://web.expasy.org/protparam/). The signal peptide of Der f 34 was predicted by SignaIP5.0 server (http://www.cbs.dtu.dk/services/SignalP/).
Homology Modeling and Refinement of the Tertiary Structure
The 3-dimensional (3D) structure of Der f 34 was constructed by homology modeling. Briefly, the amino acid sequence of Der f 34 was submitted to the SWISS-MODEL server (https://swissmodel.expasy.org/). The homologous template was selected based on the combined parameters of Global model quality estimation (GMQE), quaternary structure quality estimation (QSQE) and Sequence Identity, then the model was built in the server.
The quality of the obtained model was assessed in SAVES v6.0 serve (https://saves.mbi.ucla.edu/). The stereochemical quality, the compatibility of an atomic model with its own amino acid sequence, and the statistics of non-bonded interactions between different atom types were analyzed by PROCHECK (Laskowski et al. 1993), Verify3D (Lüthy et al. 1992) and ERRAT (Colovos and Yeates, 1993), respectively. The protein model was refined by using GalaxyRefine server (http://galaxy.seoklab.org/) (Heo et al. 2013).The refined structural model was validated using the above-mentioned methods. Structural visualization and analysis was performed in PyMOL software (https://pymol.org/2/).
Prediction of the Linear and Conformational B Cell Epitopes
Three different types of immunoinformatic approaches including DNAStar Protean software, Bepipred 2.0 server, and ElliPro server were used for predicting linear B cell epitopes of Der f 34. In DNAStar Protean program (https://www.dnastar.com/), four physiochemical properties of the amino acid sequence of Der f 34 were analyzed, and the peptides with good hydrophilicity, flexibility, accessibility, and high antigenicity were selected. Bepipred 2.0, a random forest algorithm trained on epitopes and non-epitope amino acids (http://tools.iedb.org/bcell/) (Jespersen et al. 2017), was used to predict B cell epitopes with a default threshold of 0.5. Moreover, the refined 3D protein model of Der f 34 was loaded into the ElliPro server (http://tools.iedb.org/ellipro/) (Ponomarenko et al. 2008) to predict B cell epitopes with score > 0.5. The consensus peptides that obtained by more than two prediction methods were selected as the final linear B cell epitopes.
In addition, the discontinuous B cell epitopes play an important role in IgE-binding (Pomés, 2010). Therefore, DiscoTope 2.0 server was employed to predict the residues that contributed to the discontinuous epitopes (http://tools.iedb.org/discotope/) (Kringelum et al. 2012). Based on the 3D structure of Der f 34, the final score was calculated by combining the residual-propensity score in spatial proximity and the contact numbers. The residues which had DiscoTope score above -3.7 were considered as discontinuous B-Cell epitope residues.
Prediction of T-Cell Epitopes
MHC-peptide binding is the most important determinant of T-cell epitopes. The accurate prediction of these binders is crucial for efficient vaccine design due to the importance of MHC binders for the activation of T-cells of the immune system. The MHC-II binding prediction was performed by TepiTool in IEDB database (http://tools.iedb.org/tepitool/) using IEDB recommended method (Paul et al. 2016). The 26 most common human class II alleles from HLA-DR, HLA-DQ, and HLA-DP were selected to predict CD4 + helper T-lymphocytic epitopes with percentile rank ≤ 10. The resulted epitopes with activity that binding with more than 2 alleles were selected as final MHC-II epitopes.
Design of B Cell Epitope-Based Hypoallergenic Der f 34 Vaccine
Hepatitis B virus-derived PreS domain has been used as a good carrier for fusion of B cell epitope-containing part of major house dust mite allergen Der p 23, which exhibited good prospects for application to immunotherapy (Banerjee et al. 2014). Likewise, in this study, the different fragments of major allergen Der f 34 that containing candidate B cell epitope and that without MHC-II epitopes were linked at the N terminal and C terminal of the PreS carrier by the KK linker. The native folding of Der f 34 and its conformational IgE binding epitopes were destroyed in the hypoallergenic vaccine, leading to the reduction of IgE reactivity (Banerjee et al. 2014). The nonallergenic carrier protein that provides T cell help upon immunization and could increasing the immunogenicity of allergy vaccines (Banerjee et al. 2014). The KK linkers between each fragment could play vital roles in producing an extended domain for protein folding and separation of functional epitopes, and make the protein structure more stable (Dong et al. 2020).
Cloning and Characterization of the Physicochemical Properties of Constructed Vaccine
For expression and purification of the constructed vaccine in Escherichia coli (E. coli), codon adaptation of the carrier-bound B cell epitope vaccine was performed by The Java Codon Adaptation tool (http://www.jcat.de/). The optimized codon sequences were cloned into the pET-28a vector between the Nco I and Xho I site and fused with the C-terminal 6*His tag by SnapGene software (https://www.snapgene.com/).
The sequence of hypoallergenic Der f 34 vaccine was constructed and transformed into E. coli strain BL21(DE3). The Der f 34 and hypoallergenic Der f 34 were expressed by adding a final concentration of 1.0 mM isopropyl -β-d-thiogalactoside (IPTG). The E. coli cells were collected by centrifugation and disrupted by sonication at 70 kHz on ice (9 s pulse on, 5 s pulse off) for 20 min. Cell lysates were centrifuged at 4 °C at 12,000 rpm for 10 min and the soluble supernatant was purified by HisTrap™ HP affinity column and HiTrap™ Q HP anion exchange column (GE Healthcare, Uppsala, Sweden).
A set of physicochemical parameters of carrier-bound multi-B cell epitope vaccine including molecular weight, theoretical isoelectric point (pI), the number of negatively and positively charged residues, instability index, and grand average of hydropathicity (GRAVY) were evaluated by the ProtParam tool (http://web.expasy.org/protparam/).
Allergenicity Analysis of the Constructed Der f 34 Vaccine
The Der f 34 and hypoallergenic Der f 34 (1 µg) were loaded into a 96-well polystyrene plate (Corning, NY, USA) and incubated overnight, respectively. After washing with PBS-0.05% Tween 20 (PBST) for 3 times, 1% BSA was blocked at 37 °C for 1 h. 100 μL of serum from 10 patients and 1 healthy doner were added into the well, incubate at 37 °C for 2 h. After washing, the IgE binding was detected with 100 μL HRP-labeled goat anti-human IgE (1:2500 dilution) (KPL, MD, USA) and reacted with 3, 3', 5, 5'-tetramethylbenzidine substrate (Beyotime, Shanghai, China). The reaction was then terminated by adding 50 μL 2 M H2SO4 and the absorbance was read at 450 nm using Multiskan GO (Thermo Fisher Scientific, MA, USA). All ELISAs were done in triplicates, and the data ware analyzed by Wilcoxon matched-pairs signed rank test. Ethics approval for this study was granted by the Ethics Committee in the First Affiliated Hospital of Nanjing Medical University.
Immune Simulation of the Constructed Der f 34 Vaccine
The immunogenicity and immune response profile after immunization with Der f 34 hypoallergenic vaccine were analyzed by the C-ImmSim server (http://kraken.iac.rm.cnr.it/C-IMMSIM/). The C-ImmSim is a simulator of an agent-based model, which combines techniques of systems biology with information provided by data-driven methods to predict immune interactions (Rapin et al. 2010; Sanches et al. 2021). According to the previously reported parameters (Gharbavi et al. 2021; Sanches et al. 2021), three injections were administrated at intervals of 4 weeks with random seed at 12,345, the simulation steps was set as 1000 with time steps set at 1, 84, and 168, respectively. The other simulation parameters were kept defaults.
Structural Modeling of Der f 34 Vaccine and Molecular Docking with Toll-like Receptor 3
The 3D protein structure of B cell epitope based hypoallergenic vaccine was constructed by using the Iterative Threading ASSEmbly Refinement (I-TASSER) server (https://zhanglab.ccmb.med.umich.edu/I-TASSER/) (Yang and Zhang 2015). The server generates a full-length 3D model of a protein sequence based on the sequence-to-structure-to-function paradigm. The amino acid sequences of constructed vaccine were loaded into I-TASSER server, and the resulted protein model that had the highest C-score was selected as the final model.
In order to evaluate the protein–protein interaction between the constructed vaccine and the innate immune receptors Toll-like receptor 3 (TLR3), we retrieved the TLR3 receptor structure from the RCSB PDB database (PDB ID: 2A0Z) and selected the model of constructed vaccine as a ligand. The molecular docking between the above two protein models was performed in the ClusPro server (https://cluspro.bu.edu/login.php?redir/queue.php). The obtained protein–protein complex was further loaded into LigPlot software (Laskowski and Swindells 2011) to visualize the hydrogen bonds and hydrophobic interactions between constructed vaccine and immune receptor (TLR3).
Results
Sequence Retrival and Physiochemical Analysis
The ProtParam results showed that the complete amino acid sequence of Der f 34 comprises 128 amino acids and has a molecular weight of 14 kDa and theoretical pI of 6.25. The number of negatively charged residues (Asp + Glu) and positively charged residues (Arg + Lys) were 12 and 12, respectively. The aliphatic index of Der f 34 was 87.50, and grand average of hydropathicity (GRAVY) of − 0.018. The instability index (II) is computed to be 54.95 which classifies the protein as unstable. The SignaIP 5.0 result indicated that there was no signal peptide in Der f 34 protein.
Prediction and Evaluation of the Tertiary Structure of Der f 34
The amino acid sequence of Der f 34 protein was 43.20% identical to that of YabJ protein (code: 5y6u.1.A) which was further used as a high-quality template with a coverage of 98% to build homologous model of Der f 34 (Fig. 1A). The resulted model had a high quality as the GMQE score and QMEANDisCo global score of Der f 34 model was 0.76 and 0.77 ± 0.05, respectively. The Ramachandran plot of tertiary structure showed that 93.5% of the amino acid residues of Der f 34 were in the most favored regions, 6.5% of the residues were in the additional allowed region, none was in disallowed regions (Fig. 1C). The application of the ERRAT program showed that the overall quality factor is 93.103 (Fig. 1E). However, the Der f 34 model was failed to pass VERIFY 3D program in which 76% (fewer than 80%) of the residues have averaged 3D-1D score ≥ 0.2. After refinement, 97.2% amino acid residues of Der f 34 were located in the most favored regions, 2.8% of residues were in the additional allowed region (Fig. 1D), the overall quality factor increased to 99.138 (Fig. 1F), and 84% of the residues have averaged 3D-1D score ≥ 0.2 which indicated that the refined model had passed the VERIFY 3D program. All of the above results indicated that the final Der f 34 model had good quality and high resolution. The overall structure of Der f 34 was found to consist of 3 α-helices (residues 49–67, 71–73, 84–97) and 6 β-sheets (residues 5–7, 21–25, 28–31, 75–81, 104–110, 119–126) (Fig. 1B).
Three-dimensional (3D) structure of Der f 34 allergen. A Structural prediction and B refined protein model of Der f 34. The α-helices and β-sheets were labeled in the structure. C Validation of the initial protein structure and D refined Der f 34 model by Ramachandran plot. Residues in most favored regions (red); residues in allowed regions (yellow); residues in generally allowed regions (light yellow); residues in disallowed regions (white). E Validation of initial protein structure and F refined Der f 34 model by the ERRAT program (color figure online)
Prediction of Linear and Conformational B Cell Epitopes
We used DNAStar, Bepipred 2.0, and ElliPro servers to predict the linear B cell epitopes. According to the sequence properties analyzed by DNAStar Protean, three epitopes (residues 48–54, 101–105, 114–117) were obtained (Fig. 2, Table 1). Four epitopes (residues 12–18, 39–48, 84–89, 95–99) were predicted by Bepipred 2.0 server (Table 1). Based on the 3-D protein structure of Der f 34, four linear epitopes located at residues 4–19, 37–51, 82–92, 98–113 were predicted by the ElliPro server (Table 1). In order to improve the accuracy of the predicted epitopes, the overlapping peptide regions from two or three of the predicted methods were selected as the final linear B cell epitope. Therefore, four linear B cell epitopes (residues 12–18, 39–51, 84–89, 98–105) were chosen for the development of the vaccine (Table 1).
The discontinuous B cell epitopes were predicted by DiscoTope 2.0. A total of 25 residues that contributed to the formation of discontinuous B cell epitopes were predicted. The residue number, amino acid, contact number, propensity score and DiscoTope score of the predicted epitopes were listed in Table 2. Among the residues, most of them were located in the linear epitopes except of residues 83, 111, 112, 115, 116. The distribution of the linear and discontinuous B cell epitopes were displayed in the 3D protein model and all of the B cell epitopes were located on the surface of Der f 34 (Fig. 3).
The distribution of final B cell epitopes on the three-dimensional structure of Der f 34. The linear B cell epitopes were displayed with different colors. the residues that contributed to formation of discontinuous B cell epitopes were labeled on the surface of the Der f 34 model. LB E1-E4 linear B cell epitope 1-epitope 4
Computational Prediction of Helper T Cell Epitopes
The strength of peptide binding to MHC-II molecular was used in prediction of helper T cell epitopes. The 26 most frequent human class II alleles from DP, DQ and DR loci were selected for peptides binding prediction by TepiTool. A total of 11 15-mer peptides were identified with percentile rank ≤ 10 in which 6 were selected as candidate T cell epitopes that bound to at least 2 MHC-II alleles. The region of final 6 helper T cell epitopes were 3–17, 54–68, 71–85, 76–90, 91–105 and 114–128. The detailed amino acid sequence of each epitope and binding alleles were listed in Table 3.
Construction and Characterization of the Der f 34 Vaccine
In order to convert Der f 34 into a hypoallergenic vaccine, a B cell epitope-carrier fusion protein was designed according to the previously reported patterns. The fragments containing B cell epitopes of the Der f 34 except of the T cell epitope regions were divided into three parts that are Fragment 1 (residues 12–51), Fragment 2 (residues 83–89) and Fragment 3 (98–116) (Fig. 4A). The three Fragments of Der f 34 were linked to the PreS carrier by the KK linker as illustrated in Fig. 4B. The tertiary structure of the constructed B cell epitope vaccine of Der f 34 was predicted by I-TASSER serve. The resulted model with the highest C-score of -3.99 was selected as the tertiary structure of the vaccine. The three Fragments of Der f 34 allergen were all located on the surface of the constructed vaccine (Fig. 4C).
Design and construction of B-cell epitope based Der f 34 vaccine. A Distribution of the B cell epitopes, helper T cell epitopes and the three peptide fragments in the amino acid sequence of Der f 34. The linear B cell epitopes were boxed with solid lines and discontinuous B cell epitopes were highlighted with red residues. The helper T cell epitopes were underlined. Three fragments that containing candidate B cell epitope and that without helper T epitopes were boxed with dashed lines. LB E1-E4 linear B cell epitope 1-epitope 4, T E1-E6 helper T cell epitope 1–6, Frag-1 to Frag-3 three different fragments used in vaccine design. B Schematic representation of finally designed vaccine. Frag-1 to Frag-3 three different fragments used in vaccine design. C The distribution of three fragments on the three-dimensional structure of the hypoallergenic vaccine
From the results in ProtParam tool, the B cell epitope-bound carrier vaccine had 317 amino acids with a molecular weight of 33.83 kDa and the theoretical pI was calculated as 10.15. The total numbers of negatively and positively charged residues were 16 and 30, respectively. The estimated half-life of vaccine was 30 h in mammalian reticulocytes (in vitro), > 20 h in yeast (in vivo) and > 10 h in E. coli (in vivo). The instability index (II) of the constructed vaccine was calculated as 43.10 and the GRAVY value was calculated as − 0.569 which indicated that the vaccine in hydrophilic and soluble in nature.
The Cloning and Allergenicity of the Der f 34 Vaccine
The codon conversion of the B cell epitope vaccine of Der f 34 was performed by the Java Codon Adaptation Tool. An optimized codon sequence containing 951 base pairs encoding the vaccine was generated. The Codon Adaptation Index of the optimized sequences was 1.0, and the GC content was 50.73%, which remained in the optimal range (30–70%) that ensured the expression of Der f 34 hypoallergen in the E. coli. In addition, the codon sequence was in silico cloned into the expression vector pET-28a ( +) between Nco I and Xho I site and fused with a His-tag at the C terminal of adapted codon sequence (Fig. 5). The IgE binding ability of purified hypoallergenic Der f 34 were tested by ELISA and compared with that of Der f 34 with sera from House Dust Mite (HDM) allergic patients. The hypoallergenic Der f 34 exhibited a significantly lower absorbance value of IgE binding than that of Der f 34 in the same group of HDM patients (P < 0.01) (Figure S1).
The in silico cloning and the codon sequence of the hypoallergenic Der f 34 vaccine. A Schematic representation of the sequence cloning into the pET28a ( +) vector between the Nco I and Xho I sites. Red areas represent the codon sequences of the designed vaccine, while the black areas represent the sequence of the expression vector. B The cloned nucleotide sequence and amino acid sequence of the B-cell epitope based Der f 34 vaccine from the pET28a ( +) vector (color figure online)
Immune Simulation of the Der f 34 Vaccine
The immunogenic profiles and immune responses of the Der f 34 hypoallergen were assessed by C-ImmSim server. Elevated levels of IgM, IgM + IgG, IgG1 + IgG2, IgG1 were observed after the primary vaccination and an increased level of IgG2 was occurred after the followed vaccination, with reduction in the level of antigens (Fig. 6A). The increases of B-cell populations were also observed at each exposure as well as that in T cell populations (Fig. 6B–D). A significant increase in the level of IFN-γ, IL-2 and TGF-β were highly evident after vaccination (Fig. 6E). These results indicated that the Der f 34 hypoallergen vaccine generated a robust and inducing long-lasting immune response.
Immune Simulation results by C-ImmSim. A The levels of various subtypes of immunoglobulin after immunization were represented as colored peaks. B The changes of B-cell population after the administration of Der f 34 vaccine. C The population of Th cells and D the population of TC cells after the administration of vaccine. E The profiles of cytokine and interleukins upon administration of vaccine (color figure online)
Docking Analysis of the Der f 34 Vaccine with Immune Receptors TLR3
Molecular docking was used to evaluate the binding affinity of the constructed vaccine to the antigen receptor TLR3, which was performed by ClusPro server. A total of 29 candidate models of ligand-receptor complex were generated, and one model with the lowest energy score of − 953.3 was chosen to analyze the ways of protein–protein interactions (Fig. 7A). LigPlot software was used to analyze the interaction between the ligand (constructed vaccine) and the receptor (TLR3). The complex exhibited 36 hydrogen bonds, 25 residues of ligand involved in hydrophobic contacts and 27 residues from the receptor, as shown in (Fig. 7B).
Ligand-receptor docking analysis. A Molecular docking structure generated by ClusPro server; TLR 3 (receptor) was shown in gray and the designed hypoallergenic Der f 34 vaccine (ligand) in orange. B Ligand-receptor interaction showed by Ligplot server. The green line represented hydrogen bonds, the pink and red semicircle from the vaccine and TLR3 receptor represented the residues involved in hydrophobic contacts (color figure online)
Discussion
House dust mites are an important source of inhalant and contact allergens that are closely associated with allergic diseases such as asthma, atopic dermatitis, and rhinitis (Jeong et al. 2012). Currently, allergen immunotherapy is only disease-modifying treatment that is able to alter the natural course of allergic disease (Niederberger and Valenta 2006). The current vaccines used for mite immunotherapy are mainly based on natural allergen extracts which are complex in composition since the extracts contain not only patient-sensitive allergen components, but also some patient-insensitive allergen proteins as well as nonallergenic materials and contaminations from other sources (Valenta et al. 2018; Curin et al. 2021). Moreover, the qualities of the extracts-based vaccines are probably influenced by multiple factors, such as protein degradation, different batches or sources of crude materials and methods of extracts production (Valenta et al. 2018). These factors together with potential immediate and late phase side effects greatly limit the clinical use of mite desensitization vaccines (Zieglmayer et al. 2016; Tulaeva et al. 2020). The new forms of allergen-specific immunotherapy that based on disease-causing allergens can overcome the disadvantages of the traditional allergen extract-based immunotherapy.
In this study, we firstly analyzed the physiochemical properties of Der f 34. The GRAVY value and of Der f 34 was − 0.018 and instability index was 54.95, which indicated the Der f 34 is a hydrophilic but unstable molecule. Subsequently, the tertiary structure of Der f 34 allergen was built in SWISS-MODEL and evaluated in SAVES server. Although the model was initially showed that 93.5% of residues in most favored regions and the overall quality factor was 93.103, it was failed to pass the VERIFY 3D program. Therefore, the model was further refined and the improved model shows that a total of 97.2% of residues in the most favored regions of Ramachandran plot indicated it had a high quality. The overall quality factor of the refined model was 99.138 suggested it was a high-resolution structure. Moreover, the refined model passed the validation by VERIFY 3D, which meant the atomic model is compatible with its own its own amino acid sequence. According to the refined model of Der f 34, 3 α-helices and 6 β-sheets were found. Analysis of the molecular structure could facilitate the understanding of the immune recognition of B cell-antigenic determinants since they mostly located at β turns and random coils on the protein surface (Pomés, 2010; Rahman et al. 2016).
During allergen sensitization in allergic diseases, IgE antibodies are produced against specific epitopes by B cells, the recognition of these epitopes of allergen by receptor bound IgE antibodies on basophils and/or mast cells is essential for activation of the effector cells and the subsequent allergic reaction (Pomés 2010). Knowledge of the B cell epitopes of allergen is important for the design of hypoallergen vaccine. Based on the intrinsic amino acid properties and the 3D protein model, four linear B cell epitopes (residues 12–18, 39–51, 84–89, 98–105) were obtained by the combined approaches of DNAStar, Bepipred 2.0 and ElliPro. Due to the important role of discontinuous B cell epitopes in the allergenicity of inhaled allergens, the 3D structure of Der f 34 was submitted to DiscoTope serve and a total of 25 residues that contributed to the formation of discontinuous B cell epitopes were obtained. It was found that most of the residues were overlapped with linear epitopes. Therefore, linearized peptide fragments derived from the discontinuous B cell epitopes changed the native 3D structure of the allergen, which could reduce the IgE reactivity and allergenicity but reserve antigenicity for production of blocking IgG. Six T cell epitopes were predicted as they had binding activity to MHC-II molecules, which were avoided during the design of B-cell epitope based allergen vaccine in order to reduce the later phase reactions and T-cell mediated side effects (Valenta et al. 2016, 2017). PreS, a nonallergenic protein that provide T cell help upon immunization, had been used as carrier for various B-cell epitopes based vaccine, such as the major mite allergen (Der p 23) (Banerjee et al. 2014), the major cat allergen (Fel d 1) (Niespodziana et al. 2011) and the major grass pollen allergens (Phl p 1, Phl p 2, Phl p 5 and Phl p 6) (Zieglmayer et al. 2016), all represent promising safe vaccines for immunotherapy. In this study, the PreS was also used as a carrier protein to fuse with the B cell epitope containing peptide fragments of Der f 34 in order to convert it into a hypoallergenic vaccine. Three peptide fragments containing the B cell epitopes of Der f 34 were coupled to the PreS. The instability index of B cell epitope based Der f 34 vaccine was calculated as 43.10 which was improved from that of Der f 34, this indicated the hypoallergen had a more stable property and would be suitable in desensitization treatment. The optimized codon sequence was 951 base pairs in length and had a good score of codon optimization index and the average GC content, which indicated that the vaccine could be highly expressed in E.coli. The codon sequence was cloned in pET28a ( +) vector with a His-tag at the C-terminal for convenient purification. Moreover, both of the plasmids of hypoallergenic Der f 34 vaccine and Der f 34 were synthesized and the proteins were expressed in E. coli. Both of them were purified by Ni2+ affinity and ion-exchange chromatography. The IgE binding ability of purified hypoallergenic Der f 34 were tested by ELISA and compared with that of Der f 34 with sera from HDM allergic patients. The hypoallergenic Der f 34 exhibited a significantly lower absorbance value of IgE binding than that of Der f 34 in the same group of HDM patients.
The immune simulation confirmed the immunogenic nature of the B-cell epitope vaccine, which was consistent with typical immune responses. A high secretion of relevant antibodies (IgM, IgM + IgG, IgG1 + IgG2, IgG1) was observed after repeated injection of the Der f 34 vaccine without the extra adjuvant LPS. Moreover, both of the memory B cells and T cells was developed. Through ligand-receptor docking, the Der f 34 hypoallergenic vaccine had a high binding affinity towards TLR3 including hydrogen bonds and hydrophobic contacts. These data confirmed the constructed Der f 34 vaccine had the ability to induce an effective immune response.
Our study mainly focused on immunoinformatics design and construction of hypoallergenic Der f 34 vaccine, and its hypoallergencity was confirmed by IgE-ELISA using sera form HDM allergic patients, which indicated its feasibility in desensitization treatment. Further studies need to focus on verifying the efficacy of the desensitization therapy with B-cell epitope based Der f 34 hypoallergen in consideration of the long-time and repeated injection of the hypoallergen, the optimized formula of hypoallergen with adjuvants (such as aluminum hydroxide, microcrystalline tyrosine, calcium phosphate, or the TH1 adjuvant monophosphoryl lipid A) that prolong tissue deposition and promote uptake by antigen presenting cells, and/or provide a beneficial immunomodulatory action on animal model of HDM allergy (Pali-Schöll et al. 2020; Jensen-Jarolim et al. 2021).
In summary, we firstly characterized the physiochemical and structural properties of the major house dust mite allergen Der f 34. Both of the B cell and T cell epitopes of Der f 34 were comprehensively analyzed. According to the information of epitopes, a new hypoallergen of Der f 34 was designed as a carrier-bound B cell epitope vaccine which represents a promising candidate for molecule-based HDM immunotherapy.
Data Availability
The programs/software/datasets used and/or analyzed during the present study are all available at the websites in the Methods section.
References
Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, Viñuela JE, Sanz ML, Andreu C, Martínez A (2009) Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy 39:1088–1098
Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, Valenta R, Vrtala S (2014) Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol 192:4867–4875
Calderón MA, Kleine-Tebbe J, Linneberg A, De Blay F, De Rojas DHF, Virchow JC, Demoly P (2015a) House dust mite respiratory allergy: an overview of current therapeutic strategies. J Allergy Clin Immunol Pract 3:843–855
Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, De Rojas DHF, Virchow JC, Demoly P (2015b) Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol 136:38–48
Cao H, Liu Z (2020) Clinical significance of dust mite allergens. Mol Biol Rep 47:6239–6246
Chen KW, Blatt K, Thomas WR, Swoboda I, Valent P, Valenta R, Vrtala S (2012) Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J Allergy Clin Immunol 130:435-443.e434
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519
Curin M, Huang HJ, Garmatiuk T, Gutfreund S, Resch-Marat Y, Chen KW, Fauland K, Keller W, Zieglmayer P, Zieglmayer R, Lemell P, Horak F, Hemmer W, Focke-Tejkl M, Flicker S, Vrtala S, Valenta R (2021) IgE epitopes of the house dust mite allergen Der p 7 are mainly discontinuous and conformational. Front Immunol 12:687294
Dong R, Chu Z, Yu F, Zha Y (2020) Contriving multi-epitope subunit of vaccine for COVID-19: immunoinformatics approaches. Front Immunol 11:1784
Elramlawy KG, Fujimura T, Baba K, Kim JW, Kawamoto C, Isobe T, Abe T, Hodge-Hanson K, Downs DM, Refaat IH, Beshr Al-Azhary D, Aki T, Asaoku Y, Hayashi T, Katsutani T, Tsuboi S, Ono K, Kawamoto S (2016) Der f 34, a novel major house dust mite allergen belonging to a highly conserved Rid/YjgF/YER057c/UK114 family of imine deaminases. J Biol Chem 291:21607–21615
Gharbavi M, Danafar H, Amani J, Sharafi A (2021) Immuno-informatics analysis and expression of a novel multi-domain antigen as a vaccine candidate against glioblastoma. Int Immunopharmacol 91:107265
Heo L, Park H, Seok C (2013) GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res 41:W384-388
Jensen-Jarolim E, Roth-Walter F, Jordakieva G, Pali-Schöll I (2021) Allergens and adjuvants in allergen immunotherapy for immune activation, tolerance, and resilience. J Allergy Clin Immunol Pract 9:1780–1789
Jeong KY, Park JW, Hong CS (2012) House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res 4:313–325
Jespersen MC, Peters B, Nielsen M, Marcatili P (2017) BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res 45:W24-w29
Kringelum JV, Lundegaard C, Lund O, Nielsen M (2012) Reliable B cell epitope predictions: impacts of method development and improved benchmarking. PLoS Comput Biol 8:e1002829
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–85
Niederberger V, Valenta R (2006) Molecular approaches for new vaccines against allergy. Expert Rev Vaccines 5:103–110
Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, Van Hage M, Grönlund H, Valenta R (2011) A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol 127:1562–1570
Pali-Schöll I, Deboer DJ, Alessandri C, Seida AA, Mueller RS, Jensen-Jarolim E (2020) Formulations for allergen immunotherapy in human and veterinary patients: new candidates on the horizon. Front Immunol 11:1697
Paul S, Sidney J, Sette A, Peters B (2016) TepiTool: a pipeline for computational prediction of T cell epitope candidates. Curr Protoc Immunol 114:18–19
Pomés A (2010) Relevant B cell epitopes in allergic disease. Int Arch Allergy Immunol 152:1–11
Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, Peters B (2008) ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinform 9:514
Rahman KHS, Chowdhury EU, Sachse K, Kaltenboeck B (2016) Inadequate reference datasets biased toward short non-epitopes confound B-cell epitope prediction. J Biol Chem 291:14585–14599
Rapin N, Lund O, Bernaschi M, Castiglione F (2010) Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 5:e9862
Sanches RCO, Tiwari S, Ferreira LCG, Oliveira FM, Lopes MD, Passos MJF, Maia EHB, Taranto AG, Kato R, Azevedo VAC, Lopes DO (2021) Immunoinformatics design of multi-epitope peptide-based vaccine against Schistosoma mansoni using transmembrane proteins as a target. Front Immunol 12:621706
Thomas WR (2015) Hierarchy and molecular properties of house dust mite allergens. Allergol Int 64:304–311
Tulaeva I, Kratzer B, Campana R, Curin M, Van Hage M, Karsonova A, Riabova K, Karaulov A, Khaitov M, Pickl WF, Valenta R (2020) Preventive allergen-specific vaccination against allergy: mission possible? Front Immunol 11:1368
Valenta R, Campana R, Focke-Tejkl M, Niederberger V (2016) Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol 137:351–357
Valenta R, Campana R, Niederberger V (2017) Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol Lett 189:19–26
Valenta R, Karaulov A, Niederberger V, Zhernov Y, Elisyutina O, Campana R, Focke-Tejkl M, Curin M, Namazova-Baranova L, Wang JY, Pawankar R, Khaitov M (2018) Allergen extracts for in vivo diagnosis and treatment of allergy: is there a future? J Allergy Clin Immunol Pract 6:1845-1855.e1842
Walgraffe D, Mattéotti C, El Bakkoury M, Garcia L, Marchand C, Bullens D, Vandenbranden M, Jacquet A (2009) A hypoallergenic variant of Der p 1 as a candidate for mite allergy vaccines. J Allergy Clin Immunol 123:1150–1156
Yang J, Zhang Y (2015) I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 43:W174-181
Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, Kiss R, Blatt K, Valent P, Stolz F, Huber H, Neubauer A, Knoll A, Horak F, Henning R, Valenta R (2016) Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine 11:43–57
Funding
This work was partially supported by the National Natural Science Foundation of China (81960301); the Natural Science Foundation of Jiangxi Province (20202BABL206043); Science and Technology Planning Project of Jiangxi Health Commission (20204495); the Key Project of Gannan Medical University (ZD201908); the Key Research and Development Project of Ganzhou.
Author information
Authors and Affiliations
Contributions
XWG, CL and ZQX designed and supervised the study. PYY, YZ and LXT carried out the analysis and wrote the manuscript. PYY and ZQX revised and edited the manuscript. All of the authors approved the final version of this article.
Corresponding authors
Ethics declarations
Conflicts of interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, PY., Zhu, Y., Tan, LX. et al. Immunoinformatics Construction of B Cell Epitope-Based Hypoallergenic Der f 34 Vaccine for Immunotherapy of House Dust Mite Allergy. Int J Pept Res Ther 28, 17 (2022). https://doi.org/10.1007/s10989-021-10337-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-021-10337-2