Abstract
Biofilm forming pathogens are among the major causes of hospital-acquired infections and are not much affected by antibiotic treatment. Consequently, novel agents and therapeutics are required urgently that possess antibacterial and antibiofilm activities. This study analyzed two bacteriocins from Lactobacillus plantarum subsp. argentoratensis SJ33 strain for their antibacterial and antibiofilm activity as well as cytotoxic properties. BacF1 and BacF2 showed broad spectrum activity against both Gram-positive (Listeria monocytogenes, Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa, Escherichia coli) bacteria. Significant bactericidal action was also observed on S. aureus cells by pore formation. Additionally, bacteriocins disrupted biofilms formed by S. aureus and P. aeruginosa which were shown by crystal violet staining assay and visualized by fluorescence as well as scanning electron microscopy. Quantitative Real-Time PCR study revealed changes in gene expression of biofilm formation in S. aureus (ica) and P. aeruginosa (pelA, psl, rhlA). Cytotoxicity of bacteriocins was further analyzed on normal mammalian cells and Caenorhabditis elegans. Notably, bacteriocins showed no major effect on HEK-293 cell line and enhanced the survival of S. aureus infected HEK-293 cells. Similarly, no cytotoxic effect was visible on C. elegans even after treatment with higher concentration than MIC at different time intervals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofilms are microbial communities embedded in a self-secreting extracellular matrix attached on an abiotic or biotic surface. Organisms inside the biofilms can escape the external environment and limit the entry of antimicrobial agents thus developing more resistance towards antibiotics (Tan et al. 2018). Bacterial biofilms are mainly composed of extracellular polymeric substances (EPS) such as polysaccharides, proteins, metabolites and extracellular DNA (Okuda et al. 2013). Most of bacterial infections are caused by biofilms which are formed by various pathogens and are reasons of serious concern to human health. Bacterial adhesion and formation of biofilms can amplify the risk of biofilm-associated infections. According to the present data, biofilm plays a vital role in nearly 80% of human infections (Cirkovic et al. 2016).
Staphylococcus includes Gram-positive pathogens, specifically Staphylococcus aureus, an opportunistic pathogen which forms biofilms by secreting exopolysaccharides and proteins. Polysaccharide intercellular adhesin (PIA), the major component of S. aureus biofilms is regulated by icaADBC operon (Xu et al. 2017). S. aureus is one of the major causes of community-acquired and nosocomial infections. Pseudomonas aeruginosa, Gram-negative bacteria are another important opportunistic pathogen that causes several infections in clinical settings and are among the common causes of hospital-acquired and medical device-related infections (Gerits et al. 2016). Conventional antibiotics that are active against planktonic bacteria are often ineffective against biofilms formed by pathogens (Vahedi Shahandashti et al. 2016). Hence, biofilm-associated infections have become a major healthcare challenge in the current times and has urged for the requirement of alternatives and new therapies to develop potent antibiofilm agents (Konai and Haldar 2016).
Bacteriocins are small peptides and proteinaceous substances possessing antibacterial activity against various pathogenic microorganisms. Bacteriocins can be considered as alternatives to traditional antibiotics with already existing antimicrobial compounds either alone or in combination to target biofilms (Mathur et al. 2018). Lactobacillus species belongs to lactic acid bacteria (LAB) group that constitutes a major part of the gut microbiota. LABstrains are known to produce numerous bacteriocins and antimicrobial peptides that interfere with infections by inhibiting adherence of pathogenic bacteria (Ahn et al. 2018). Nisin is the most studied bacteriocin of LAB which was approved by FDA (Food and Drug Administration) and produced industrially but could mostly inhibit Gram-positive pathogens (Miao et al. 2014). Hence, the search for other bacteriocins with a wide antibacterial spectrum and better stability at broader temperature and pH range has been in constant focus and is gaining more attention (Zhang et al. 2018; Ibrahim et al. 2019). Different procedures have been used to analyze bacteriocins and the most common method being in vitro assays. It is difficult to perform in vivo cytotoxicity assays for bacteriocins involving direct mammalian models. So, to overcome these limitations, a simple in vivo screening method using Caenorhabditis elegans animal model was developed. C. elegans is a small nematode that feeds on E. coli and has been extensively used as an experimental model in biological studies due to its simplicity and appropriateness for genetic analysis (Son et al. 2016). Though C. elegans is one of the common models in laboratory setups, only few researches have focused on monitoring the effect of bacteriocins on growth of the worms under natural conditions.
Previously, we have shown the production of two low molecular weight bacteriocins by Lactobacillus plantarum subsp. argentoratensis SJ33 strain in our earlier study (Ray Mohapatra and Jeevaratnam 2019) and demonstrated its antibacterial and antibiofilm activities as well as therapeutic potential of bacteriocins as antibacterial coating on urinary catheters, thereby reducing the occurrence of medical device-associated infections (Ray Mohapatra and Jeevaratnam 2019). The present study focused to further characterize and analyze the antibacterial potential of individually purified bacteriocins, BacF1 and BacF2 on planktonic as well as biofilm forming pathogens. The study also evaluates the cytotoxic effect of bacteriocins on HEK-293 cells and C. elegans.
Materials and Methods
Bacterial Strains and Growth Media
All pathogenic bacterial strains used in the study (Table 1) were obtained from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTech), Chandigarh, India. The pathogenic strains were grown using Tryptic Soya broth (TSB) and agar media (TSA) (HiMedia, India). Lactobacillus plantarum subsp. argentoratensis SJ33 was grown and maintained in Man Rogosa Sharpe (MRS) medium. Pure bacterial cultures were maintained with 50% glycerol and stored at −80 °C.
Growth and Maintenance of Caenorhabditis elegans Strain
The wild type N2 C. elegans and the bacteria E. coli OP50 was procured from Caenorhabditis Genetics Center, USA. The wild type strains of C. elegans were grown and maintained at 20 ºC in Petri plates containing Nematode Growth Medium (NGM) and were cultivated with alive E. coli OP50 (Gusarov et al. 2013). The eggs were acquired from the grown adult worms after being treated with M9 buffer containing NaOCl -KOH solution. After hatching, the L1 stage worms were transferred onto the NGM agar plates containing E. coli OP50 and incubated on agar plates till it obtains the L4 stage (Fabian and Johnson 1994).
Purification and Characterization of Bacteriocins
The partially purified bacteriocin from Lactobacillus plantarum SJ33 obtained in our previous study (Ray Mohapatra and Jeevaratnam 2019) was further purified by semi-preparative RP-HPLC and subjected to analytical RP-HPLC for analyzing the purity of bacteriocins.
Molecular mass of purified bacteriocins was identified by discontinuous Tris-tricine SDS-PAGE method (Hermann Schägger 2006). The electrophoresis was performed by using molecular weight marker of ultralow range from 1.06 to 26.6 kDa (Sigma-Aldrich, India) as standard with the purified bacteriocins. The gel was stained with Coomassie Brilliant Blue G-250 dye for 2–4 h and destained to visualize the bands. The molecular mass of bacteriocins was confirmed in our previous study by Q-TOF (Q-TOF SYNAPT G2) ESI mass spectrometer (Ray Mohapatra and Jeevaratnam 2019).
Antibacterial Activity
Bacteriocin activity was tested by agar well diffusion assay after serial dilutions (Hernández et al. 2005). An overnight culture of indicator strains (Table 1) was grown in TSB medium and seeded by pour plate method and purified bacteriocins were placed on solidified agar in 6 mm diameter wells and the plates were incubated at 37 °C for 24 h. Spectrum of bacteriocins activity was determined against various Gram-positive and Gram-negative pathogens (Table 1) by well diffusion method.
Effect of Protease Enzyme on Bacteriocins
Efficacy of protease enzyme on bacteriocins activity was determined by treating purified bacteriocins with 1 mg/mL of protease enzyme. Bacteriocins treated with protease enzyme (1 mg/mL) were incubated at 37 ºC for overnight 10 mM citrate buffer (pH 3.0). Bacteriocins fractions were then adjusted to pH 6.0 and the antibacterial activity was tested against S. aureus MTCC 96 by agar well diffusion assay (Lin and Pan 2019) and compared with control which was processed by same method but not treated with the enzyme (Agaliya and Jeevaratnam 2013).
Determination of Minimum Inhibitory Concentration
Minimum inhibitory concentration (MIC) of purified bacteriocins was tested against S. aureus MTCC 96 using broth dilution assay. Different concentrations of bacteriocins (3.9 to 250 µM), 100 µL per well was added to the microtiter plate and Nisin was taken as standard for comparison. The indicator strain (106 CFU/mL) was instilled in each well and incubated at 37 °C for 24 h. The MIC values were considered as the lowermost concentration at which the bacteriocins visibly inhibited the bacterial growth. Broth media with bacterial culture was taken as negative control whereas medium containing only bacteriocins without indicator strain was considered as positive control; both the controls were maintained with the test samples.
Time-Kill Assay
Overnight culture of S. aureus MTCC 96 (106 CFU/mL) was diluted (1:100) with TSB media and incubated for 2 h at 37 ºC was used for growth kinetic assay. After incubation, the bacteriocins (MIC) were added to the indicator strain. Both untreated and treated cultures were incubated up to 12 h at 37 ºC (Sahoo et al. 2015). The viable cell count of pathogen was measured after appropriate dilutions of both untreated and treated bacteriocins (MIC), then these cultures were used for plating on agar medium at 4th, 8th and 12th h (Kindoli et al. 2012). The number of colonies formed after bacteriocin treatment was determined and compared with untreated bacterial cultures after incubation at 37 °C for 24 h. S. aureus MTCC 96 culture without any treatment of bacteriocins was used as control (Amortegui et al. 2014).
Scanning Electron Microscopy
Culture of S. aureus MTCC 96 was grown overnight, diluted (1:100) with the growth medium and added with bacteriocins at MIC. The samples were then incubated for 6 h to view under microscope for morphological changes as compared to untreated cells. The untreated and treated cells were thoroughly washed twice with PBS and fixed by 2.5% glutaraldehyde. After fixation, cells were further treated with 1% osmium tetroxide and finally dehydrated with a gradient series of ethanol (25, 50, 75, 90, 95 and 100%). The dehydrated cells were coated with gold and examined under HR SEM(FEI Quanta FEG 200, USA) for analysis of cell morphology.
Biofilm Assays
Congo Red Assay
Congo red stain with agar medium was prepared with 37 g/L brain heart infusion (BHI) broth, 50 g/L sucrose, 10 g/L agar and 8 g/L Congo red dye was added with 5% sucrose. Congo red stain was sterilized at 121 °C for 15 min independently from the other medium constituents. Then it was supplemented to the sterilized BHI agar with sucrose at 55 °C. Congo red agar (CRA) plates were inoculated with indicator organisms and incubated for 24 h at 37 °C aerobically (Mathur et al. 2006; Hassan et al. 2011).
Antibiofilm Activity
Biofilm formation by S. aureus MTCC 96 and Pseudomonas aeruginosa MTCC 3541 was determined qualitatively and quantitatively by crystal violet (CV) assay. Bacteriocins effect was examined on the biofilm forming pathogens with different concentrations and the microtitre plate was incubated for 24 h at 37 °C. After incubation, the unadhered bacteria were gently washed with Phosphate Buffer Saline (PBS) and the biofilms were stained with 0.1% crystal violet stain for 10 min. Wells with excess stain was gently rinsed with PBS and the attached stain was dissolved with 33% acetic acid (Overhage et al. 2008). The optical density was measured in microtitre plate reader at 590 nm. The biofilms of indicator strains without bacteriocins were considered as untreated control.
Visualization of Biofim Formation and Inhibition under Microscope
Biofilm formation and inhibition was observed under microscope by using 12-well polystyrene plates containing cover slips. The cover slips were treated with and without bacteriocins at sub-MIC (31.25 µM), which were placed in the 12-well plate containing S. aureus MTCC 96 and P. aeruginosa MTCC 3541 cultures (1:100 dilution with TSB) with OD600nm ~ 0.01 and incubated for 24 h at 37 ºC (Wu et al. 2016). The cover slips were rinsed with PBS and stained with 0.01% of acridine orange (w/v) for 5 min. After staining, the cover slips were again rinsed with PBS to remove additional stain and finally viewed under fluorescence microscope (Olympus, Japan).
The inhibition of biofilm of S. aureus MTCC 96 and P. aeruginosa MTCC 3541 by bacteriocins was also viewed under SEM. Biofilms formed by both the pathogens were developed on cover slips and the cover slips were added with bacteriocins at sub-MIC (31.25 µM) and incubated at 37 ºC for 24 h. The cover slips were rinsed, fixed by 2.5% glutaraldehyde and then dehydrated with a gradient ethanol series (25–100%). The dehydrated cover slips were then gold coated for observation under HR SEM (FEI Quanta FEG 200, USA).
Quantitative Real-Time PCR
RNA was isolated from both bacteriocins treated and untreated biofilms and the RNA isolation was carried out using HiPurA Total RNA isolation kit (HiMedia, India). Dnase treatment was given to remove genomic DNA contamination. The quantity and quality of RNA were assessed by nanodrop and agarose gel electrophoresis analysis. One microgram of RNA was used to prepare cDNA by Reverse Transcriptase kit (Genei, Bangalore, India). Comparative differential gene expression of icaC and icaD in S. aureus MTCC 96 as well as pelA, psl, rhlA genes in P. aeruginosa MTCC 3541 were analyzed by quantitative reverse transcription PCR (Lakshmanan et al. 2019). The primer sequences used in this analysis are mentioned in the Supplementary material Table S2. Reactions were carried out using KAPA SYBR master mix (Sigma, India) and the reaction condition includes, an initial denaturation at 95 °C for 2 min, then 40 cycles of three step amplification of denaturation at 95 °C for 3 min, annealing at 55 °C for 30 s and extension at 72 °C for 30 s run in Roche Light Cycler 96. The results were analyzed by 2−ΔΔCt method (Livak and Schmittgen 2001). The gene expression levels were standardized against gyrA and rpoD constitutive genes in S. aureus MTCC 96 and P. aeruginosa MTCC 3541, respectively.
Cytotoxicity Assays
Effect of Bacteriocins on Cell Viability
Human embryonic kidney (HEK-293) cell line was obtained from the NCCS, Pune and was grown and maintained in 90% Dulbecco Modified Eagle Medium (DMEM) containing HEPES buffer and 10% FBS with 0.1% of Penicillin and Streptomycin. The effect of bacteriocins on HEK-293 cell viability was analyzed using MTT assay (Sakurazawa and Ohkusa 2005). Two-fold serial dilutions of bacteriocins (ranging from 15.6 to 500 µM) were prepared with culture media which was added to the cells across the microtiter plate and kept in 5% CO2 incubator at 37 °C for 24 h. The plate supplemented with MTT (0.25 mg/mL) was incubated for 4 h, 200 µL DMSO was added and the absorbance was finally recorded at 570 nm. Results were expressed as percentage of viable cells.
Cell Adherence Assay
HEK-293 cells at density of 104 cells were seeded and added to each well of microtitre plate for 24 h. The cells were treated with bacteriocins at MIC and sub-MIC (31.25 µM) whereas untreated control wells did not receive any bacteriocin treatment prior to infection with the pathogen. After incubation, overnight culture of S. aureus MTCC 96 (OD ̴ 0.01) was used to infect HEK-293 cell line and then cells were incubated for 4 h at 37 °C. After incubation, the cells were rinsed with PBS and gentamicin (100 µg/mL) was added to remove unattached bacteria. The cells were again rinsed with PBS and processed for MTT assay.
For fluorescence microscopy analysis, the cells processed as above were fixed using 2.5% glutaraldehyde, stained using Live/Dead staining and viewed under fluorescence microscope. For determining the viability count of adhered bacteria on the cell line, 0.1% of TritonX 100 was added to lyse the cells in the microtitre plate. Hundred microliter of suspensions of untreated infected cells and different concentrations of bacteriocin treated infected cells were serially diluted with TSB medium, poured on agar plates and incubated for 24 h. After incubation, number of colonies was counted to compare with untreated samples.
Effect of Bacteriocins on C. elegans
Effect of bacteriocins was observed on C. elegans by using triplicate batches of 10 C. elegans for each bacteriocin concentration in a 24-well plate. Wells with S-medium containing bacteriocins but without worms and wells containing worms without bacteriocins were taken as positive and negative control, respectively (Bakkiyaraj and Pandian 2010). Synchronized L4 stage C. elegans were added to the 24-well plate and inoculated with bacteriocins in S-medium with E. coli OP50. C. elegans were treated with bacteriocins at 0.5XMIC, MIC and 2XMIC concentrations and incubated at 20 °C. Subsequently, the worms were scored for live or dead at 24 h and 48 h. A worm was regarded as dead when straight rigid structures appear and no body movement was observed. C. elegans from each bacteriocin treated and untreated wells was taken, washed thrice with M9 buffer, treated with 1% sodium azide and visualized under phase contrast microscopy (IX71 OLYMPUS, Singapore) for morphological changes in C. elegans.
Statistical Analysis
All experiments were performed independently in triplicates and repeated minimum three times. The individual means and mean ± standard error (SE) were calculated using Microsoft Excel 2010. The results were analysed using Student’s t test and p < 0.05 value was considered as statistically significant.
Results
Purification and Characterization of Bacteriocins
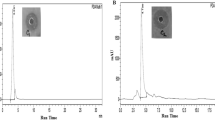
Purified bacteriocin fractions BacF1 and BacF2 revealed total activity of 1280 and 640 AU/mL, respectively (Table S1) and showed single peaks at retention time 3.43 and 4.45 min, respectively in analytical RP-HPLC profile at A280 nm and purified bacteriocins BacF1 and BacF2 showed antibacterial activity against S. aureus MTCC 96 (Fig. 1c and d). BacF1 showed 16% bacteriocin recovery as compared to BacF2 with only 8% of bacteriocin yield.
Tricine SDS-PAGE of bacteriocins. M is the ultra-low range molecular weight protein marker. Gel bands shown by purified bacteriocins a BacF1 and b BacF2 Chromatographic analytical RP HPLC profile of purified bacteriocins monitored at 280 nm, c BacF1 and d BacF2 and their antibacterial activity against S. aureus MTCC 96
Tris-tricine SDS-PAGE showed bands of purified bacteriocins obtained from semi-preparative RP-HPLC (Fig. 1). Dense separate gel bands were visible in Fig. 1a and b and compared with the molecular weight marker. Figure 1a demonstrated clear band above molecular marker 3.5 kDa, whereas Fig. 1b showed a dense band between 1.06 and 3.5 kDa molecular weight. The molecular weight of bacteriocins, BacF1 and BacF2 were reported as 4.039 kDa and 1.6 kDa by Q-TOF ESI mass spectrometry in our previous study (Ray Mohapatra and Jeevaratnam 2019).
Antibacterial Activity
Antibacterial efficacy of purified bacteriocins BacF1 and BacF2 were determined against Gram-positive and Gram-negative pathogens, which were found to be potent against Listeria monocytogenes, S. aureus, E. coli, Aeromonas hydrophila bacterial strains (Table 1). BacF1 showed comparatively lower potential against P. aeruginosa strains, whereas BacF2 were less effective against Gram-negative bacteria such as P. aeruginosa, E. coli and K. pneumoniae strains (Table 1) compared to Gram-positive pathogens.
Effect of Protease Enzyme on Purified Bacteriocin
The purified bacteriocins BacF1 and BacF2 showed complete loss of activity when treated with protease enzyme and zone of inhibition was not observed against S. aureus MTCC 96 after protease enzyme treatment (Fig. S1), confirming their proteinaceous nature. Also, bacteriocins without enzyme treatment were taken as control which retained their antibacterial efficacy.
Determination of Minimum Inhibitory Concentration
Bacteriocins BacF1 and BacF2 showed > 90% inhibition of S. aureus MTCC 96 at 62.5 µM and 125 µM, respectively (Fig. 2). Nisin also showed 90–95% inhibition at 62.5 µM and 125 µM compared to BacF1 activity. The results suggested bacteriocins to be effective against S. aureus MTCC 96 and no significant difference between inhibitory concentration of bacteriocins, specifically BacF1 and Nisin was observed (Fig. 2). However, BacF2 showed higher inhibitory concentration against S. aureus MTCC 96 compared to BacF1.
Time-Kill Assay
Bactericidal activity of BacF1 and BacF2 was studied by killing assay on exponential growth of S. aureus MTCC 96 after treatment with bacteriocins and compared with untreated bacteria. Both BacF1 and BacF2 treated culture showed significant reduction in growth of the pathogen after 6 h of the treatment and gradually decreased till 12 h as compared to the control. Cell viability of treated samples was determined after 2 h of incubation but not much difference in viability was observed in comparison to the control sample (5 Log CFU/mL). Considerable reduction in CFU count of S. aureus MTCC 96 was found after 6 h and 8 h of treatment, nearly 50% of reduction in the cell viability was observed by BacF1 and BacF2 (Fig. 3). After 12 h of BacF1 and BacF2 treatment, the viability count was decreased to 2.5 and 3 Log CFU/mL, respectively from 10 Log CFU/mL of S. aureus MTCC 96 untreated culture (Fig. 3). However, no colonies survived the bacteriocin treatment after 12 h.
Effect of purified bacteriocins BacF1 and BacF2 on growth and survival of S. aureus. BacF1 and BacF2 showed effect on S. aureus and calculated as colony forming units (CFU) after equal time intervals of incubation. Results expressed as mean ± standard error calculated from three independent experiments repeated in triplicates
Scanning Electron Microscopy
The SEM results clearly displayed disrupted cell morphology in bacteriocin treated S. aureus MTCC 96 cells compared to the untreated ones. Untreated cells were intact, turgid and separated from one another as viewed under SEM (Fig. 4a), whereas cells treated with purified bacteriocins BacF1 and BacF2 at MIC (62.5 µM and 125 µM) were deformed and had prominent pores on the cell membrane (Fig. 4b and c). Scanning electron microscopic images clearly showed pore formations and leakage of cellular contents which confirmed the bactericidal nature of the bacteriocins.
Biofilm Assays
Congo Red Assay
Congo red agar (CRA) method showed S. aureus MTCC 96 and P. aeruginosa MTCC 3541 strains that displayed black colonies on Congo red medium as distinctive biofilm producing strains but no dry crystalline morphology was observed (Fig. S2). However, S. aureus MTCC 737 and P. aeruginosa MTCC 2295 isolates showed red or pink colonies (Fig. S2). Only a few bacterial strains screened by CRA assay displayed red (pink) colonies at 37 °C after 24 to 48 h of incubation.
Antibiofilm Activity of Bacteriocins
S. aureus MTCC 96 and P. aeruginosa MTCC 3541 showed strong biofilm forming ability as analyzed by crystal violet staining method. Purified bacteriocins BacF1 and BacF2 significantly reduced biofilm formation by pathogens shown by qualitative (Fig. 5a and b) as well as quantitative crystal violet assay (Fig. 5c and d). BacF1 and BacF2 treated at sub-MIC (31.25 µM) inhibited 70% and 65% of biofilms formed by S. aureus MTCC 96, respectively while 55% (Fig. 5c) and 50% of biofilm disruption was found in P. aeruginosa MTCC 3541 (Fig. 5d). Bacteriocins were more effective against S. aureus biofilms than on biofilms formed by P. aeruginosa. BacF1 was found to be more potent antibiofilm agent as compared to BacF2.
Antibiofilm activity of purified bacteriocins BacF1 and BacF2 on biofilm formed by S. aureus and P. aeruginosa. Qualitative analysis of biofilm formation and inhibition by crystal violet stain against a S. aureus and b P. aeruginosa. Quantitative results of biofilm inhibition of c S. aureus and d P. aeruginosa biofilms using crystal violet assay. Results of experiments performed thrice in triplicates expressed as mean ± standard error
Bacteriocins significantly inhibited biofilm formation which was also evidently observed under fluorescence microscope and confirmed by SEM images (Figs. 6 and 7). These results were consistent with our findings of crystal violet assay.
Biofilms formed by S. aureus and P. aeruginosa and disruption of biofilms after treatment with purified bacteriocins as viewed under fluorescence microscope. a and d Untreated S. aureus and P. aeruginosa biofilms. b and c Bacteriocins treated biofilms of S. aureus and e and f P. aeruginosa biofilms treated with bacteriocins (31.25 µM)
Quantitative Real-Time PCR
Biofilm forming genes present in S. aureus MTCC 96 and P. aeruginosa MTCC 3541 were suppressed by bacteriocins at sub-MIC (31.25 µM). The genes analyzed in this study demonstrated reduced expression in the treated groups as compared with the untreated conditions. The intercellular adhesion genes are virulence factors accountable for formation of biofilm in S. aureus. Similarly, pelA, psl, rhlA genes were found to induce polysaccharide and rhamnolipid production in P. aeruginosa shown in Fig. 8c and d. The genes icaC, icaD and pelA, psl, rhlA showed down-regulation of expression compared to untreated control upon treatment with bacteriocins. BacF1 was found to be more effective in suppressing the expression of icaC and icaD gene as compared to BacF2 after bacteriocin treatment of S. aureus biofilms (Fig. 8a and b). Relative quantitative expression of pelA gene was found to be more reduced than gene expression of psl and rhlA. psl and rhlA genes, which showed a suppressed expression but comparatively less than pelA gene. These results revealed that BacF1 and BacF2 treated conditions were considerably effective in down-regulating the expression of biofilm forming genes in S. aureus MTCC 96 and P. aeruginosa MTCC 3541, also confirmed the antibiofilm activity of bacteriocins.
Effect of BacF1 and BacF2 on the expression of biofilm forming genes in S. aureus and P. aeruginosa using real time PCR. Quantitative relative gene expression of biofilm adhesion in S. aureus and P. aeruginosa when supplemented with BacF1and BacF2 at sub-MIC (31.25 µM) compared with untreated control showed significant down-regulation of gene expression by both bacteriocins a and b S. aureus and c and d P. aeruginosa. The values expressed as mean ± standard error were calculated from experiments performed three times independently in triplicates
Cytotoxicity Assays
Effect of Bacteriocins on Cell Viability
Bacteriocins BacF1 and BacF2 were analyzed for cytotoxicity on HEK-293 cells by MTT assay. The viability increases as concentration decreases and at lower concentration i.e. sub-MIC (31.25 µM) both BacF1 and BacF2 showed > 90% of cell viability. Bacteriocins at 62.5 µM showed more than 80% of viability and very less cytotoxicity was observed. Only at higher concentration (500 µM) of BacF1 and BacF2, nearly 60% of cell viability was found as compared to untreated cells (Fig. S3). No significant cytotoxic effect was found on HEK-293 cells by bacteriocins BacF1 and BacF2.
Cell Adherence Assay
Cytotoxicity assay of bacteriocins on HEK-293 cell line by MTT assay showed very minimal cytotoxicity but only at higher concentrations. The effect of the bacteriocins on cytotoxicity induced by S. aureus MTCC 96 adhered to HEK-293 cells was analyzed by viable plate count and microscopic observation by Live/Dead staining under fluorescence microscopy. S. aureus MTCC 96 infected cells treated with BacF1 and BacF2 revealed improved survival rate and cell viability as compared to untreated infected control (Fig. 9a and b). Cells treated with BacF1 and BacF2 at MIC (62.5 µM) showed better survival and lower bacterial adherence than untreated infected control (Fig. 9b). However, BacF1 was found to be more effective even at lower concentrations in prevention of S. aureus MTCC 96 adherence to HEK-293 cells. The morphological changes in S. aureus MTCC 96 adhered and unadhered HEK-293 cells as well as bacteriocins treated bacteria adhered cells can be clearly seen by microscopic images (Fig. 9c). The difference in cell morphology of treated cells was also clearly visible compared to the adhered cells without bacteriocins treatment (Fig. 9c).
Effect of purified bacteriocins BacF1 and BacF2 on S. aureus adhered mammalian cell line HEK-293. a Survival percentage of S. aureus infected HEK-293 cells treated with BacF1 and BacF2 by MTT assay taking infected control. b Viability assay by CFU count shows percentage of bacterial adherence to HEK-293 treated with BacF1 and BacF2 compared with infected control. c Fluorescence microscopic images showing morphological changes of (a) uninfected HEK-293 and (b) S. aureus infected cells without treatment (c) S. aureus infected cells with BacF1 and (d) BacF2 treatment at 62.5 µM
Effect of Bacteriocins on C. elegans
Effect of purified bacteriocins on wild type C. elegans was investigated until all the worms were found dead. Our results showed that C. elegans treated with bacteriocins BacF1 and BacF2 were alive with longer survival time. The worms were viable for more than 48 h after the bacteriocins treatment. After incubation, no significant cytotoxicity was found on C. elegans and also showed undamaged morphology at different time intervals (Fig. S4). The microscopic images clearly demonstrated live worms which maintained sinusoidal position and showed body movements after treatment with BacF1 and BacF2 at 24 h and 48 h as compared with untreated worms which were only provided with live E. coli OP50 bacteria without any bacteriocins (Fig. S4). Moreover, the treated C. elegans also showed survival in different concentrations of bacteriocins at specific time periods.
Discussion
This study mainly focuses on characterization and evaluation of two bacteriocins produced by Lactobacillus plantarum SJ33 strain. The purified bacteriocins BacF1 and BacF2 were recovered from semi-preparative RP-HPLC. Our result was also supported by previous reports, demonstrating bacteriocins from L. plantarum, L. acidophilus and L. fermentum (Saranya and Hemashenpagam 2013; Zhao et al. 2015). Tris-Tricine SDS-PAGE analysis showed the presence of two separate gel bands for bacteriocins BacF1 and BacF2 which were also demonstrated in our previous study (Ray Mohapatra and Jeevaratnam 2019). These results were in agreement with earlier studies on bacteriocins produced from LR/14 strain (Tiwari and Srivastava 2008) and bacteriocins produced by various LAB strains such as Lactobacillus sakei, Leuconostoc mesenteroides, Carnobacterium piscicola and Enterococcus faecium which reported production of more than one bacteriocin (Sawa et al. 2013). Here, BacF1 exhibited a broad range of antibacterial activity on Gram-positive and Gram-negative pathogens, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Bacillus cereus, Aeromonas hydrophila and Pseudomonas aeruginosa, whereas BacF2 was more effective against Gram-positive pathogens as compared to Gram-negative bacteria. The partially purified bacteriocin preparation obtained previously also exhibited a wide array of antibacterial activity against various Gram-positive and Gram-negative bacteria. The bacteriocin preparation demonstrated combinatorial activity of BacF1 and BacF2 which were present in a ratio of 2:3 as revealed by RP-HPLC data in our earlier study (Ray Mohapatra and Jeevaratnam 2019). Few bacteriocins from Lactobacillus spp. were reported earlier showing better antibacterial activity on Gram-negative bacteria (Todorov and Dicks 2005), whereas other bacteriocins could not inhibit Gram-negative bacteria (Jiang et al. 2012; Miao et al. 2014). In this study, no activity was observed after the treatment of protease enzyme (1 mg/mL) on purified bacteriocins and complete loss of antibacterial activity was observed that confirmed proteinaceous nature of bacteriocins. Previous study also suggested loss of activity by bacteriocin-like compound from L. plantarum TF711 strain with proteases (Hernández et al. 2005). Similar activity was also observed in bacteriocins produced by Pediococcus acidilactici and other bacteriocins from various Lactobacilli strains which were reported to produce proteinaceous antimicrobial compounds (Leroy and De Vuyst 2010; Pal et al. 2010; Vidhyasagar and Jeevaratnam 2013) and antimicrobial substances those remained unaffected by treatment with lipase and α-amylase (Todorov and Dicks 2009; Moh et al. 2015). Whereas, some bacteriocins such as BAC-IB17 were reported as protease resistant in nature and showed stability against various proteolytic enzymes (Ansari et al. 2018).
Purified bacteriocin, BacF1 inhibited the growth of S. aureus MTCC 96 at 62.5 µM, whereas BacF2 showed inhibition at 125 µM, proving BacF1 to be more effective than BacF2. An earlier study reported Plantaricin K25 from L. plantarum showing MIC of 125 and 250 µg/mL against different pathogenic strains (Wen et al. 2016) and other study on bacteriocin, caseicin from L. casei showed higher MIC of 5 mg/mL and 2.5 mg/mL on E. coli and S. aureus pathogens (Lü et al. 2014). According to these previous reports bacteriocins of our study were found to be more potent against various pathogens than the previously characterized and identified bacteriocins. Most of the bacteriocins such as Plantaricin 423 and Amylovorin L471 showed bactericidal effect, whereas other bacteriocins such as plantaricins and bacteriocin-like substances were found to be bacteriostatic in nature (Reenen et al. 1998; Callewaert et al. 1999). Bacteriocins with bactericidal activity showed rapid effect on pathogens after treatment. However, time-dependent assay demonstrated effect of bacteriocins within 6 h of treatment and also showed decrease in viable CFU count in S. aureus after 4–6 h of bacteriocins treatment. Bacteriocin KBC11 produced by Lactobacillus plantarum strain from a previous study also showed reduction of pathogen growth after 6 h of the treatment, whereas no colonies were found after 12 h of incubation (Sadishkumar and Jeevaratnam 2018) which supported our results.
BacF1 and BacF2 showed membrane disruption and pore formation on S. aureus cells indicating leakage of cellular contents. Scanning electron micrographs clearly showed deformation and damage of cell morphology, thus confirming the bactericidal nature of purified bacteriocins. Previous studies on bacteriocins, such as BAC-IB17 and Bac-IB45 demonstrated bactericidal action on the indicator strains that may be due to increase in membrane permeability that leads to pore formation and subsequently causes cell death (Ansari et al. 2018; Ibrahim et al. 2020). The above data supported the results of our study. The bactericidal effect of each bacteriocin from various LAB strains may vary against different pathogenic strains i.e., bacteriocins can be bacteriostatic or bactericidal in nature (Dimitrov et al. 2010). Few bacteriocins were reported to be bacteriostatic against specific pathogenic strain while other bacteriocins showed bactericidal activity against other pathogens (Tiwari and Srivastava 2008). The most common mechanism of action of bacteriocin was recognized as pore formation leading to leakage of cellular materials from target cells (Héchard and Sahl 2002; Hammami et al. 2011).
Biofilms have become a major cause of health related problems, thus new antibiotics are urgently required to reduce the growing hazard from biofilm forming pathogens that are resistant to commercial antibiotics (Roe et al. 2018). This study evaluated the biofilm formation in pathogens by Congo red assay which screened the bacterial strains and was simple to perform. The CRA analysis was commonly based on color of bacterial colonies formed on the agar plates, ranging from pink or red for non-biofilm forming bacteria to black colonies for biofilm formers (Kaiser et al. 2013). The study revealed potential antibiofilm activity of purified bacteriocins against pathogens, S. aureus MTCC 96 and P. aeruginosa MTCC 3541 which were classified as strong biofilm formers based on crystal violet assay. BacF1 and BacF2 showed potential antibiofilm activity against S. aureus than P. aeruginosa strain at sub-MIC (31.25 µM). A novel bacteriocin, Sonorensin was reported earlier that showed inhibition of biofilms formed by S. aureus strain (Chopra et al. 2015). As per previous studies bacteriocins such as lacticin Q and nisin A also exhibited antibiofilm potential against methicillin-resistant Staphylococcus aureus (Okuda et al. 2013).
The quantitative expression studies demonstrated suppression of biofilm adhesion genes icaC and icaD in S. aureus by purified bacteriocins whereas only pelA gene expression was down-regulated considerably than psl and rhlA genes when compared to control in case of P. aeruginosa. Although previous studies have supported the presence of intercellular adhesion genes and icaADBC dependent pathway in S. aureus (Rachid et al. 2000; Ong et al. 2019) but ica-operon independent pathways were also found in some of the Staphylococcus species. Moreover, few studies on polyphenols were reported to have inhibitory potential to disrupt biofilms formed by S. aureus isolates at sub-minimal concentration (Blanco et al. 2005). A previous study also reported the effect on extracellular polysaccharide production in P. aeruginosa by using plant extracts and antibiotics (Lakshmanan et al. 2019). Additionally, polyphenols were also known as anti-virulence compounds that attenuated and down-regulated the production of EPS at sub-MIC, thus possessing the ability to control and modulate the quorum-sensing pathway in P. aeruginosa (Yin et al. 2015).
The cytotoxic study of purified bacteriocins showed no adverse effect on HEK-293 cell line even at higher concentration (4XMIC) and only showed mild cytotoxicity at higher concentration (500 µM). The study showed no significant cytotoxicity on HEK-293 cells by BacF1 and BacF2. Previous study on bacteriocins from Bacillus spp. also showed 91% cell viability on HEK-293 cells and Plantaricin A from L. plantarum showed toxicity at 10–100 µM concentration range where no toxic effect was seen on normal as well as cancer cells below 10 µM concentration (Sand et al. 2010). Another recent report on bacteriocin BAC-IB17 showed anticancer activity on HeLa cells but no cytotoxicity was found against normal cells and showed 90% of cell viability (Ansari et al. 2020). Similarly, BacIB45 produced by Lactobacillus plantarum revealed 85% cell viability at inhibitory concentration after bacteriocin treatment (Ibrahim et al. 2020), which supported this study. Few earlier studies also reported further characterized bacteriocins with less potential activity than bacteriocins of our present interest. Nisin and pediocin demonstrated lower cytotoxic effect on normal Vero cell lines (Vaucher et al. 2010). Additionally, Nisin and other lantibiotics showed mild cytotoxic effect on various eukaryotic cell lines at higher than lethal concentration (Griffiths et al. 2011). Bioactive metabolites produced by L. plantarum showed low cytotoxicity on normal mammalian cells (Chuah et al. 2019). Bacteriocins have been reported for their variable effect on mammalian cell lines due to different unknown factors and the actual mechanism involved in cytotoxicity is yet to be fully explained. Purified bacteriocins reported in this study also improved the survival of S. aureus adhered HEK-293 cells. The adhered cells treated with BacF1 and BacF2 showed better survival at inhibitory concentration that killed the bacteria. Morphological changes of bacteria adhered cells treated with bacteriocins were clearly visible under fluorescence microscope using Live/Dead staining. Previous reports suggested Lactobacillus strains to reduce cytotoxicity as well as attachment of bacteria to the normal epithelial cells and human intestinal epithelial cell line was treated with Plantaricin P1053 which was found to induce cell viability of CCD841, a normal cell line (Giani et al. 2019) which supported our results. Very few studies were found to be reported on human embryonic cells and the effect of bacteriocins on pathogen interaction and bacterial adherence to the normal mammalian cells.
Further, our findings have clearly displayed that C. elegans treated with bacteriocins exhibited better survival with intact morphology and no significant cytotoxicity. Results of a previous study showed that C. elegans infection liquid assay assists in better identification of potential bacteriocins than in vitro diffusion assays (Son et al. 2016). In earlier studies, C. elegans was only used as a toxicity indicator for screening heavy metals, organic solvents, environmental pollutants and toxins (Moy et al. 2006). Though C. elegans is one of the common model used for biological experiments but only few studies have focused on the effect of antimicrobial substances on survival and growth of the nematode (Niu et al. 2016).
The above study characterized and analyzed two bacteriocins which showed significant antibiofilm potential and broad spectrum activity. The bacteriocins effectively inhibited bacterial adherence on normal mammalian cell line and enhanced the cell viability without adversely affecting the mammalian cells.
This work reported purification of two bacteriocins from Lactobacillus plantarum SJ33, which were bactericidal, antibiofilm and non-cytotoxic. The yields of bacteriocins were low and were sensitive to protease. The combination therapies of bacteriocins with traditional antibiotics can be used as a better antimicrobial and antibiofilm agent with potential activity even at lower doses. The complete characterization of bacteriocins, their mechanism of action and evaluation of the mechanism of action associated with survival of C. elegans upon treatment of antimicrobial agents are the limitations of this study. The increased knowledge in these areas can improve the effectiveness of bacteriocins in pharmaceutical applications.
Conclusions
This study characterized two purified low molecular weight bacteriocins produced by Lactobacillus plantarum SJ33 that possess significant properties such as broad spectrum antibacterial and antibiofilm activity against biofilm forming pathogens. Purified bacteriocins showed antibacterial activity against various pathogens and bactericidal effect on S. aureus cells was confirmed by SEM analysis that revealed membrane pore formation. Antibiofilm potential of purified bacteriocins on S. aureus and P. aeruginosa was confirmed through fluorescence and scanning electron microscopic images. Bacteriocins remarkably down-regulated the expression of ica genes in S. aureus and pelA, psl, rhlA genes in P. aeruginosa, respectively. Bacteriocins improved viability of S. aureus adhered normal mammalian cells with no considerable cytotoxicity at inhibitory concentration. C. elegans was used as a direct tool for assessing the potential of bacteriocins and can also facilitate the analysis of more prospective bacteriocins in future. The study highlights the possible medical applications of bacteriocins that opened new avenues in healthcare industry.
Data Availability
All the data analyzed during this study are included in this article and its supplementary material.
References
Agaliya PJ, Jeevaratnam K (2013) Molecular characterization of lactobacilli isolated from fermented idli batter. Brazilian J Microbiol 44:1199–1206. https://doi.org/10.1590/S1517-83822013000400025
Ahn KB, Baik JE, Park OJ et al (2018) Lactobacillus plantarum lipoteichoic acid inhibits biofilm formation of Streptococcus mutans. PLoS ONE 13:1–16. https://doi.org/10.1371/journal.pone.0192694
Amortegui J, Rodríguez-López A, Rodríguez D et al (2014) Characterization of a new bacteriocin from Lactobacillus plantarum LE5 and LE27 isolated from ensiled corn. Appl Biochem Biotechnol 172:3374–3389. https://doi.org/10.1007/s12010-014-0757-x
Ansari A, Ibrahim F, Pervez S, Aman A (2020) Inhibitory mechanism of BAC-IB17 against β-lactamase mediated resistance in methicillin-resistant Staphylococcus aureus and application as an oncolytic agent. Microb Pathog 149:104499
Ansari A, Zohra RR, Tarar OM et al (2018) Screening, purification and characterization of thermostable, protease resistant Bacteriocin active against methicillin resistant Staphylococcus aureus (MRSA). BMC Microbiol 18:1–10. https://doi.org/10.1186/s12866-018-1337-y
Bakkiyaraj D, Pandian STK (2010) In vitro and in vivo antibiofilm activity of a coral associated actinomycete against drug resistant Staphylococcus aureus biofilms. Biofouling 26:711–717. https://doi.org/10.1080/08927014.2010.511200
Blanco AR, Sudano-Roccaro A, Spoto GC et al (2005) Epigallocatechin gallate inhibits biofilm formation by ocular Staphylococcal isolates. Antimicrob Agents Chemother 49:4339–4343. https://doi.org/10.1128/AAC.49.10.4339-4343.2005
Callewaert R, Holo H, Devreese B et al (1999) Characterization and production of amylovorin L471, a bacteriocin purified from Lactobacillus amylovorus DCE 471 by a novel three-step method. Microbiology 145:2559–2568. https://doi.org/10.1099/00221287-145-9-2559
Chopra L, Singh G, Kumar Jena K, Sahoo DK (2015) Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep 5:1–13. https://doi.org/10.1038/srep13412
Chuah LO, Foo HL, Loh TC et al (2019) Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern Med 19:1–12. https://doi.org/10.1186/s12906-019-2528-2
Cirkovic I, Bozic DD, Draganic V et al (2016) Licheniocin 50.2 and bacteriocins from Lactococcus lactis subsp. lactis biovar. Diacetylactis BGBU1-4 inhibit biofilms of coagulase negative Staphylococci and Listeria monocytogenes clinical isolates. PLoS ONE 11:1–12. https://doi.org/10.1371/journal.pone.0167995
De Giani A, Bovio F, Forcella M et al (2019) Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 9(1):88
Dimitrov S, Wachsman M, Tomé E et al (2010) Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol 27:869–879. https://doi.org/10.1016/j.fm.2010.05.001
Fabian TJ, Johnson TE (1994) Production of age-synchronous mass cultures of Caenorhabditis elegans. J Gerontol 49:B145–B156. https://doi.org/10.1093/geronj/49.4.B145
Gerits E, Blommaert E, Lippell A et al (2016) Elucidation of the mode of action of a new antibacterial compound active against Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE 11:1–17. https://doi.org/10.1371/journal.pone.0155139
Griffiths S, Maclean M, MacGregor SJ et al (2011) Decontamination of collagen biomatrices with combined pulsed electric field and nisin treatment. J Biomed Mater Res Part B Appl Biomater 96:287–293
Gusarov I, Gautier L, Smolentseva O et al (2013) Bacterial nitric oxide extends the lifespan of C. elegans. Cell 152:818–830. https://doi.org/10.1016/j.cell.2012.12.043
Hammami I, Triki MA, Rebai A (2011) Purification and characterization of the novel bacteriocin Bac IH7 with antifungal and antibacterial properties. J Plant Pathol 93:443–454
Hassan A, Usman J, Kaleem F et al (2011) Evaluation of different detection methods of biofilm formation in the clinical isolates. Brazilian J Infect Dis 15:305–311. https://doi.org/10.1590/S1413-86702011000400002
Héchard Y, Sahl HG (2002) Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545–557
Hernández D, Cardell E, Zárate V (2005) Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: Initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J Appl Microbiol 99:77–84. https://doi.org/10.1111/j.1365-2672.2005.02576.x
Ibrahim F, Siddiqui NN, Aman A et al (2020) Characterization, cytotoxic analysis and action mechanism of antilisterial bacteriocin produced by Lactobacillus plantarum isolated from cheddar cheese. Int J Pept Res Ther 26:1751–1764. https://doi.org/10.1007/s10989-019-09982-5
Ibrahim F, Zafar SB, Aman A et al (2019) Improvement of Lactobacillus plantarum for the enhanced production of bacteriocin like inhibitory substance using combinatorial approach. Biocatal Agric Biotechnol 22:101386. https://doi.org/10.1016/j.bcab.2019.101386
Jiang J, Shi B, Zhu D et al (2012) Characterization of a novel bacteriocin produced by Lactobacillus sakei LSJ618 isolated from traditional Chinese fermented radish. Food Control 23:338–344. https://doi.org/10.1016/j.foodcont.2011.07.027
Kaiser TDL, Pereira EM, dos Santos KRN et al (2013) Modification of the Congo red agar method to detect biofilm production by Staphylococcus epidermidis. Diagn Microbiol Infect Dis 75:235–239. https://doi.org/10.1016/j.diagmicrobio.2012.11.014
Kindoli S, Lee HA, Kim JH (2012) Properties of Bac W42, a bacteriocin produced by Bacillus subtilis W42 isolated from Cheonggukjang. J Microbiol Biotechnol 22:1092–1100. https://doi.org/10.1007/s10068-012-0232-9
Konai MM, Haldar J (2016) Lysine-based small molecules that disrupt biofilms and kill both actively growing planktonic and nondividing stationary phase bacteria. ACS Infect Dis 1:469–478. https://doi.org/10.1021/acsinfecdis.5b00056
Lakshmanan D, Harikrishnan A, Vishnupriya S, Jeevaratnam K (2019) Swarming inhibitory potential of cinnamtannin B1 from Cinnamomum tamala T. Nees and Eberm on Pseudomonas aeruginosa. ACS Omega 4:16994–16998. https://doi.org/10.1021/acsomega.9b02471
Leroy F, De Vuyst L (2010) Bacteriocins of lactic acid bacteria to combat undesirable bacteria in dairy products. Aust J Dairy Technol 65:143
Lin TH, Pan TM (2019) Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J Microbiol Immunol Infect 52:409–417. https://doi.org/10.1016/j.jmii.2017.08.003
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lü X, Hu P, Dang Y, Liu B (2014) Purification and partial characterization of a novel bacteriocin produced by Lactobacillus casei TN-2 isolated from fermented camel milk (Shubat) of Xinjiang Uygur Autonomous region, China. Food Control 43:276–283. https://doi.org/10.1016/j.foodcont.2014.03.020
Mathur H, Field D, Rea MC et al (2018) Fighting biofilms with lantibiotics and other groups of bacteriocins. npj Biofilms Microbiomes. https://doi.org/10.1038/s41522-018-0053-6
Mathur T, Singhal S, Khan S et al (2006) Detection of biofilm formation among the clinical isolates of Staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol 24:25–29. https://doi.org/10.4103/0255-0857.19890
Miao J, Guo H, Ou Y et al (2014) Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet, China. Food Control 42:48–53. https://doi.org/10.1016/j.foodcont.2014.01.041
Moh C, Engelhardt T, Albano H et al (2015) Antilisterial activity of bacteriocinogenic Pediococcus acidilactici HA6111-2 and Lactobacillus plantarum ESB 202 grown under pH and osmotic stress conditions. Food Microbiol 48:109–115. https://doi.org/10.1016/j.fm.2014.11.015
Moy TI, Ball AR, Anklesaria Z et al (2006) Identification of novel antimicrobials using a live-animal infection model. PNAS 103:10414–10419
Niu Q, Zhang L, Zhang K et al (2016) Changes in intestinal microflora of Caenorhabditis elegans following Bacillus nematocida B16 infection. Nat Publ Gr. https://doi.org/10.1038/srep20178
Okuda KI, Zendo T, Sugimoto S et al (2013) Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob Agents Chemother 57:5572–5579. https://doi.org/10.1128/AAC.00888-13
Ong TH, Chitra E, Ramamurthy S et al (2019) Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm formation by modulating gene expression and exhibit synergism with antibiotics. PLoS ONE 14:1–13. https://doi.org/10.1371/journal.pone.0213079
Overhage J, Campisano A, Bains M et al (2008) Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–4182
Pal V, Pal A, Patil M et al (2010) Isolation, biochemical properties and application of bacteriocins from Pediococcus pentosaceous isolates. J Food Process Preserv 34:1064–1079
Rachid S, Ohlsen K, Witte W et al (2000) Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother 44:3357–3363. https://doi.org/10.1128/AAC.44.12.3357-3363.2000
Ray Mohapatra A, Jeevaratnam K (2019) Inhibiting bacterial colonization on catheters: antibacterial and antibiofilm activities of bacteriocins from Lactobacillus plantarum SJ33. J Glob Antimicrob Resist 19:85–92. https://doi.org/10.1016/j.jgar.2019.02.021
Reenen CA Van, Dicks LMT, Chikindas ML (1998) Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum
Roe D, Karandikar B, Bonn-savage N et al (2018) Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother 61:869–876. https://doi.org/10.1093/jac/dkn034
Sadishkumar V, Jeevaratnam K (2018) Purification and partial characterization of antilisterial bacteriocin produced by Pediococcus pentosaceus KJBC11 from Idli batter fermented with Piper betle leaves. J Food Biochem 42:1–9
Sahoo TK, Jena PK, Patel AK, Seshadri S (2015) Purification and molecular characterization of the novel highly potent bacteriocin TSU4 produced by Lactobacillus animalis TSU4. Appl Biochem Biotechnol 177:90–104. https://doi.org/10.1007/s12010-015-1730-z
Sakurazawa T, Ohkusa T (2005) Cytotoxicity of organic acids produced by anaerobic intestinal bacteria on cultured epithelial cells. J Gastroenterol 40:600–609
Sand SL, Oppegård C, Ohara S et al (2010) Plantaricin A, a peptide pheromone produced by Lactobacillus plantarum, permeabilizes the cell membrane of both normal and cancerous lymphocytes and neuronal cells. Peptides 31:1237–1244. https://doi.org/10.1016/j.peptides.2010.04.010
Saranya S, Hemashenpagam N (2013) Purification and characterization of bacteriocin Produced by different Lactobacillus species isolated from fermented foods. Int J Microbiol Res 5:341–348. https://doi.org/10.9735/0975-5276.5.1.341-348
Sawa N, Koga S, Okamura K et al (2013) Identification and characterization of novel multiple bacteriocins produced by Lactobacillus sakei D98. J Appl Microbiol 115:61–69. https://doi.org/10.1111/jam.12226
Schägger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22. https://doi.org/10.1007/978-1-4939-8793-1_15
Son SJ, Park MR, Ryu SD et al (2016) Short communication: in vivo screening platform for bacteriocins using Caenorhabditis elegans to control mastitis-causing pathogens. J Dairy Sci 99:8614–8621. https://doi.org/10.3168/jds.2016-11330
Tan Y, Leonhard M, Ma S et al (2018) Efficacy of carboxymethyl chitosan against Candida tropicalis and Staphylococcus epidermidis monomicrobial and polymicrobial biofilms. Int J Biol Macromol 110:150–156. https://doi.org/10.1016/j.ijbiomac.2017.08.094
Tiwari SK, Srivastava S (2008) Purification and characterization of plantaricin LR14: a novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl Microbiol Biotechnol 79:759–767. https://doi.org/10.1007/s00253-008-1482-6
Todorov SD, Dicks LMT (2005) Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme Microb Technol 36:318–326. https://doi.org/10.1016/j.enzmictec.2004.09.009
Todorov SD, Dicks LMT (2009) Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). Int J Food Microbiol 132:117–126
Vahedi Shahandashti R, Kasra Kermanshahi R, Ghadam P (2016) The inhibitory effect of bacteriocin produced by Lactobacillus acidophilus ATCC 4356 and Lactobacillus plantarum ATCC 8014 on planktonic cells and biofilms of Serratia marcescens. Turkish J Med Sci 46:1188–1196. https://doi.org/10.3906/sag-1505-51
Vaucher RA, Teixeira ML, Brandelli A (2010) Investigation of the cytotoxicity of antimicrobial peptide P40 on eukaryotic cells. Curr Microbiol 60:1–5. https://doi.org/10.1007/s00284-009-9490-z
Vidhyasagar V, Jeevaratnam K (2013) Bacteriocin activity against various pathogens produced by Pediococcus pentosaceus VJ13 isolated from Idly batter. Biomed Chromatogr 27:1497–1502. https://doi.org/10.1002/bmc.2948
Wen LS, Philip K, Ajam N (2016) Purification, characterization and mode of action of plantaricin K25 produced by Lactobacillus plantarum. Food Control 60:430–439. https://doi.org/10.1016/j.foodcont.2015.08.010
Wu S, Liu G, Jin W et al (2016) Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front Microbiol 7:1–15. https://doi.org/10.3389/fmicb.2016.00102
Xu T, Wu Y, Lin Z et al (2017) Identification of genes controlled by the essential YycFG two-component system reveals a role for biofilm modulation in Staphylococcus epidermidis. Front Microbiol 8:1–17. https://doi.org/10.3389/fmicb.2017.00724
Yin H, Deng Y, Wang H et al (2015) Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Sci Rep 5:1–12. https://doi.org/10.1038/srep16158
Zhang J, Yang Y, Yang H et al (2018) Purification and partial characterization of Bacteriocin Lac-B23, a novel bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Front Microbiol 9:1–7. https://doi.org/10.3389/fmicb.2018.02165
Zhao R, Duan G, Yang T et al (2015) Purification, characterization and antibacterial mechanism of bacteriocin from lactobacillus acidophilus XH1. Trop J Pharm Res 14:989–995
Acknowledgements
Authors are thankful to DST-FIST, UGC-SAP for providing funds for the Department and IIT Madras, India for SEM analysis. Authors are also grateful to the University Grant Commission (UGC), New Delhi for the financial support.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This manuscript does not contain any research involving any human and/or animal participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ray Mohapatra, A., Lakshmanan, D., Mahesh, R. et al. Characterization and Cytotoxic Evaluation of Bacteriocins Possessing Antibiofilm Activity Produced by Lactobacillus plantarum SJ33. Int J Pept Res Ther 27, 1783–1797 (2021). https://doi.org/10.1007/s10989-021-10210-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10210-2