Abstract
Herein, we report design and synthesis of series of adamantane derivatives containing modified peptides with thiazol moiety. New compounds were synthesized in solution using TBTU (2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate) as coupling agent. All derivatives were obtained with good yields. The antiviral activity of newly synthesized compounds against influenza virus H1N1 is studied. Cytotoxicity assay for determination of CC50 were done and IC50 values were calculated. The rimantadine analogue with thiazole ring Gly-Thz-rimantadine showed good activity against influenza virus A/Hongkong/68 with IC50 = 0.11 μg/mL and CC50 = 50 μg/mL. In addition, antimicrobial and antifungal activity against model strains of gram positive (Bacillus cereus), gram negative (Escherichia coli) microorganisms and fungi strain Yarrowia lipolytica 3344 were investigated. The compounds Gly-Thz-rimantadine with good antiviral activity also showed very good antifungal activity in two different concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
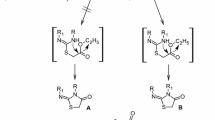
Inhibitors of M2 ion channel amantadine and rimantadine (Fig. 1) are the first antiviral drugs developed in the 70s of the last century (Bennett et al. 2015; Bender et al. 1999).
By structure-activity relationship studies adamantane containing organic compounds with antiviral, antimicrobial, anticancerogenic, anticataleptic, immunotropic, neuropsychotropic activities are distinguished (Spilovska et al. 2016). Nowadays, they are widely used in medicinal practice for treatment of patients with different diseases (Chen and Swope 2007; Stouffer et al. 2008; Brotchie 2010; Bozdaganyan et al. 2014; Long et al. 2015).
Antibiotics are very important for decreasing the incidence of bacterial infections globally, but with the increasing number of resistant pathogens, the effectiveness of antibiotics has been reduced. In addition, many viruses already shown resistance against adamantine derivatives amantadine and rimantadine. Many research groups have focused their efforts on finding alternative antimicrobial agents, which leads to re-evaluation of the therapeutic use of drugs (Gaiday et al. 2008; Karpenko et al. 2010; Smirnova et al. 2011; Shibnev et al. 2012; Kolocouris et al. 2013; Rey-Carrizo et al. 2013; Wang et al. 2013; Moorthy et al. 2014). Adamantanes containing amino acids or peptide moieties are perspective alternative of applied antiviral drugs. The aim of the presented work is the synthesis of adamantane analogues (rimantadine and amantadine) containing peptide mimetics with thiazole ring(s) and monitoring of their antiviral activity against influenza virus A/Hong Kong strain H1N1 as well as their antimicrobial activity against model G+, G− microorganisms and fungi.
Experimental Part
Materials and Methods
Chemicals
Rimantadine and amantadine were purchased from Sigma. DMAP is from Merck and TBTU from Iris Biotech (Germany). Aluminum TLC plates, silica gel coated with flourescent indicator F254 (Merck) were used for TLC analysis and spots were monitored using UV detection at 254 nm. As an elution system a mixture of 80:30:5 CHCl3:CH3OH:H2O and 95:5 CHCl3:CH3OH were used.
Methods
NMR spectra were recorded on Bruker Avance DRX-500 spectrometer at 500 MHz for 1H NMR and 100 MHz for 13C NMR spectra in DMSO-d6 and CDCl3, δ is in ppm.
Electrospray mass spectrometry (ESI-MS) experiments were acquired on Bruker Compact QTOF-MS (Bruker Daltonics, Germany) and controlled by the Compass 1.9 Control software. The data analysis was performed and the mono-isotopic mass values were calculated using Data analysis software v 4.4 (Bruker Daltonics, Germany). The analyses were conducted in the positive ion mode at a scan range from m/z 50 to 1000 and nitrogen was used as nebulizer gas at a pressure of 4 psi and flow of 3 L min−1 for the dry gas. The capillary voltage and temperature were set at 4500 V and 220 °C, respectively. An external calibration for mass accuracy was carried out by using sodium formate as calibration solution. The precursor ion of each compound was selected, and ESI–MS/MS analysis was performed by collision-induced dissociation (CID); nitrogen was the collision gas, and the collision energy varied from 5 to 40 eV. MSn experiments were conducted on an ion trap instrument Esquire 3000 (Bruker Daltonics, Germany) and controlled by the Esquire Control 5.3.11 software. ESI-MS data were collected in positive-ion mode at a scan range from m/z 50 to 500. In all ESI-MS measurements, the nebulizer gas pressure was 124.1 kPa at a flow rate of 5 L min−1; the desolvation temperature was 300 °C and capillary voltage was adjusted to 4000 V. The sample solutions were delivered to nebulizer by a syringe pump (Cole Parmer, USA) at a flow rate 3 μL min−1.
Synthetic procedures
A mixture of amantadine.HCl or rimantadine.HCl (80 mmol) and TBTU (80 mmol) in tetrahydrofurane (THF) was stired and a solution of Boc-Gly-Thz-OH or Fmoc-Gly-Thz-Thz-OH (80 mmol) and 4-(N,N-dimethylamino)-pyridine (DMAP) (80 mmol) were added to the reaction mixture and stirred for 3 h. Then THF was evaporated in vacuo and the residue was chromatographed on silica gel, using hexane:ethyl acetate (4:5) (Knorr et al. 1989). The compounds containing Boc group (4a–4b) were dissolved in 20 mL of TFA and stirred at 0 °C for 1 h to remove the Boc group. Further TFA is removed in vacuo. Then 20 mL of aqueous ammonia was added to the pH 7. The solution was then added drop-wise to the cold diethyl ether. After filtration, compounds were collected as white solid.
4a. Boc-Gly-Thz-amantadine
1H NMR ([D6]-DMSO): δ = 1.40 (s,9H,3xCH3), 1.57. (d, J = 12,8 Hz, 3H, amantadine), 1.61 (d, J = 12,8 Hz, 3H, amantadine), 1.83 (s, 6H, amantadine), 1.99 (s, 3H, amantadine), 4.39 (d, 2H, CH2), 7.56 (s, 1H, NH), 7.81 (s, 1H, NH), 8.12 (s, 1H, CHThz). 13C NMR ([D6]-DMSO): δ = 28.6 (CH, amantadine), 35.7 (CH2, amantadine), 38.1 (CH2), 40.6(CH2, amantadine), 42.0 (CH2), 50.9 (Cquat, amantadine), 80.3 (Cq, tBu), 156.2 (CO-Boc), 168.8 (CO); 128.1 (C 5Thz ), 149.1 (C 4Thz ), 151.1 (C-4), 162.6 (C 2Thz ). ESI-MS: Molecular formula: C20H29N3O3S; Mexact: 391,1930, Mfound [M+H]+ 392,1220. Yield: 65%.
Gly-Thz-amantadine
1H NMR: ([D6]-DMSO): δ = 1.61 (d, J = 13,2 Hz, 3H, amantadine), 1.45–2.0 (15H,cycl. system), 2.12 (m,1H), 4.51 (d, 2H, CH2), 7.25 (s, 1H, NH), 8.01 (br t 1H, NH), 8.18 (s, 1H, CHThz). 13C NMR ([D6]-DMSO): δ = 28.6 (CH, amantadine), 35.7 (CH2, amantadine), 38.1 (CH2), 40.6(CH2, amantadine), 42.0 (CH2), 50.9 (Cquat, amantadine), 168.8 (CO); 128.1 (C 5Thz ), 149.1 (C 4Thz ), 151.1 (C-4), 162.6 (C 2Thz ). ESI-MS: Molecular formula: C15H21N3OS; Mexact: 291,4117, Mfound [M+H]+ 292,5520.
4b. Boc-Gly-Thz-rimantadine
1H-NMR: (CDCl3): δ = 1.15 (dt, 1H, J = 12.4 Hz, J = 1.5 Hz),1.19 (dt, 1H, J = 12.4 Hzm J = 2.4 Hz), 1.31 (d, 2H, J = 12.2 Hz), 1.39 (dt, 2H, J = 12.4 Hz, J = 2.5 Hz), 1.47 (s, 9H), 1.63 (d, 2H, J = 12.0 Hz), 1.67 (m, 2H), 1.84 (m, 2H), 2.16 (m, 1H), 3.71 (s, 2H), 4.17 (d, 2H, CH2), 7.87 (br, 1H, NH), 8.02 (s, 1H, NH), 8.36 (s, 1H, CHThz). 13C-NMR ([D6]-DMSO): δ = 14.5 (CH3, C-2′), 28.2 (CH3, C-3*), 28.3 (C(CH3)3*), 35.7 (C-1′), 36.9 (CH2, C-4), 38.3 (CH2, C-2), 44.9 (CH2), 53.1 (CH, C-3′), 80.3 (Cq, tBu), 128.6 (C 5Thz ), 147.9 (C 4Thz ), 156.2 (CO-Boc), 162.6 (C 2Thz ), 168.8 (CO). ESI-MS: Molecular formula: C22H33N3O3S; Mexact: 419,2243, Mfound [M+H]+ 420,1255. Yield: 63.5%.
Gly-Thz-rimantadine
1H (CDCl3): δ = 1.12 (m, 2H), 1.26 (d, 2H, J = 12.6 Hz), 1.32 (dd, 2H, J = 2.8 Hz, J = 12.5 Hz), 1.55 (d, 2H, J = 11.9 Hz), 1.60 (d, 2H, J = 11.6 Hz), 1.77 (d, 2H, J = 2 Hz) 2.09 (m, 1H), 3.45 (s, 2H), 4.39 (d, 2H, CH2), 7.25 (s, 1H, NH), 8.04 (br t, 1H, NH), 8.95 (s, 1H, CHThz). 13C (DMSO-d6) d (ppm): 171.9 (CO-amide), 156.2 (C-Thz), 150.6 (C-Thz), 123.6 (CH-Thz), 79.9 (C), 50.6 (CH2), 47.4 (CH2), 42.7 (CH2), 42.4 (CH2), 29.6 (CH), 35.7 (CH2), 38.1 (CH2), 29.6 (CH). ESI-MS: Molecular formula: C17H25N3OS; Mexact: 319,1718, Mfound [M+H]+ 320,2550.
5a. Fmoc-Gly-Thz-Thz-amantadine
1H (DMSO-d6) d (ppm): 0.84 (s, 6H, CH3), 1.16 (m, 2H), 1.29 (d, 2H, J = 12.2 Hz), 1.37 (d, 2H, J = 12.4 Hz), 1.68 (m, d, 2H, J = 11.6 Hz), 1.79 (m, d, 2H, J = 11.6 Hz), 1.93 (m, 2H), 2.12 (m, 1H), 4.26 (t, 1H, J = 6.5 Hz), 4.42 (d, 2H, J = 6.2 Hz), 4.53 (d, 2H, J = 6.7 Hz), 7.34 (t, J = 7.5 Hz, 2H), 7.43 (t, J = 7.5 Hz, 2H), 7.45 (s, 1H), 7.72 (d, J = 7.7 Hz, 2H), 7.91 (d, J = 7.7 Hz, 2H), 8.20 (s, 1H), 8.35 (s, 1H). 13C NMR: ([D6]-DMSO): δ = 14.2 (CH3, C-2′, D1), 14.4 (CH3, C-2′, D2), 28.2* (AmC-3, D2), 28.2** (Am C-3, D1), 36.9 (CH2, C-4, D2), 36.9 (CH2, C-4, D1), 38.0 (CH2, D2), 38.1 (CH2, C-2, D1), 38.2 (Am-CH2, C-2, D1), 47 (Fmoc-C-9), 53.0 (CH, C-1′, D1), 53.1(CH, C-1′, D2), 56.2 (Am-CH, D2+D1), 65.4 (Fmoc-CH2), 116.0–146.94 (C-aromatic) 131.5 (C 5Thz ), 146.9 (C 4Thz ), 156.03 (Fmoc-CO), 162.1 (C 2Thz ). ESI-MS: Molecular formula: C33H32N4O3S2; Mexact: 596,1916, Mfound [M+H]+ 597,2223. Yield: 55%.
5b. Fmoc-Gly-Thz-Thz-rimantadine
1H-NMR: 0.79 (s, 6H, CH3), 1.18 (m, 2H), 1.31 (d, 2H, J = 13.2 Hz), 1.39 (d, 2H, J = 12.9 Hz), 1.81 (m, d, 2H, J = 12.2 Hz), 1.71 (m, d, 2H, J = 10.6 Hz), 1.99 (m, 2H), 2.16 (m, 1H), 4.26 (t, 1H, J = 6.5 Hz), 4.42 (d, 2H, J = 6.2 Hz), 4.53 (d, 2H, J = 6.7 Hz), 7.34 (t, J = 7.5 Hz, 2H), 7.43 (t, J = 7.5 Hz, 2H), 7.45 (s, 1H), 7.72 (d, J = 7.7 Hz, 2H), 7.91 (d, J = 7.7 Hz, 2H), 8.26 (s, 1H), 8.37 (s, 1H). 13C NMR: ([D6]-DMSO): δ = 15.2 (CH3, C-2′, D1), 15.4 (CH3, C-2′, D2), 28.4 (Rim C-3, D2), 28.6 (Rim C-3, D1), 28.1 (C(CH3)3, D2), D1), 37.1 (CH2, C-4, D2), 37.9 (CH2, C-4, D1), 38.1 (CH2, D2), 38.2 (Rim-CH2, C-2, D1), 47.3 (Fmoc-C-9), 53.3 (CH, C-1′, D1), 53.5 (CH, C-1′, D2), 56.4 (Rim-CH, D2+D1), 65.7 (Fmoc-CH2), 132.5 (C 5Thz ), 147.9 (C 4Thz ), 157.03 (Fmoc-CO), 163.1 (C 2Thz ). ESI-MS: Molecular formula: C35H36N4O3S2; Mexact: 624,2229, Mfound [M+H]+ 625,3259. Yield: 59%.
Biological Activities
Cytotoxicity assay for determination of the 50% cytotoxic concentrations (CC50) of test compounds in MDCK (Madin Darby canine kidney cell monolayers) were realized. Influenza virus A/Hongkong/68 is used for current experiments.
Bacillus cereus, Escherichia coli and fungi Yarrowia lipolytica were supplied by National Bank for Industrial Microorganisms and Cell Cultures at the University of Chemical Technology and Metallurgy, Sofia.
Results and Discussion
Some authors developed new molecules based on the known anti-influenza medical drugs introducing different modifications in the parent molecule (Smith et al. 1997; Zoidis et al. 2010). One of the successful modification leading to increasing of anti-influenza activity is incorporation of different heterocycles (Zoidis et al. 2006; Zarubaev et al. 2010). Taking into account this successful experience we synthesized derivatives of adamantane—rimantadine and amantadine modified with Gly-thiazol and Gly-thiazol-thiazol moieties in order to investigate their antiviral and antimicrobial potential. The new adamantane derivatives with peptidomimetics including thiazol moieties were synthesized according to Scheme 1.
Boc-Gly-Thz-amantadine (4a), Boc-Gly-Thz-rimantadine (4b) were dissolved in 20 mL of TFA and stirred at 0 °C for 1 h to remove the Boc protecting group. Deprotection procedure is followed by neutralization with aqueous ammonia to obtain the final products Gly-Thz-amantadine and Gly-Thz-rimantadine (Fig. 2).
Antiviral Activity
The replication of influenza viruses in MDCK cells induces the complete destruction of host cells, a distinct cytopathic effect (CPE). The virus induced CPE can be inhibited by addition of antiviral compounds (100 μl/well; 2 parallels/concentration, dilution factor 2). Untreated (virus control) and compound treated confluent monolayers of test cells were infected with a multiplicity of infection that induces a complete CPE in virus control 24 h after virus addition. Thereafter, adherent cells were fixed and stained with a crystal violet/formalin solution. After elution of the stain, inhibition of virusinduced CPE was quantified by optical density (OD) determination in a Dynatech microplate reader. The percentage of antiviral activities of tests compounds was calculated. Based on the mean dose response curve of at least two assays, the 50% CPE inhibitory concentration (IC50) was calculated (Stankova et al. 1999).
Initially the new analogues were evaluated for antiviral activity towards influenza virus A/Hongkong/68. According to Shibnev et al. (2012) introducing of bulky protection group in some amino acid and peptide analogues of rimantadine leads to obtaining of very active at low concentrations (5 μg/mL) compounds. Taking into account this conclusion we decided to investigate the influence of spatially bulky Fmoc-group on antiviral and antifungal activity. Results of the antiviral screening of the new adamantane containing thiazole and thiazolyl-thiazole rings are summarized in Table 1.
Unfortunately, in our case the tests reveal that the presence of a spatially bulky group does not lead to the improvement of the antiviral effect. Moreover, the protected at αNH2-function compounds do not show any antiviral activity. The analogue of rimantadine with free αNH2-function including thiazole moiety showed moderate activity against influenza virus A/Hongkong. In contrary, the analogue of amantadine with free αNH2-function including thiazole moiety do not also show antiviral effect. The results show that neither the thiazole cycle nor the free amino function are crucial for antiviral activity.
Antimicrobial Activity
B. cereus are facultative anaerobes, and as other members of the genus Bacillus, can produce protective endospores.
The bacteria is Gram-positive, motile, beta hemolytic bacterium commonly found in soiland food. Some strains are harmful to humans and cause foodborne illness, while other strains can be beneficial as probiotics for animals (Ryan and Ray 2004; Charalampopoulos and Rastall 2009). Escherichia coli, also known as E. Coli is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms (endotherms) (Tenaillon et al. 2010; Singleton 1999). Most E. Coli strains are harmless, but some serotypes can cause serious food poisoning in their hosts, and are occasionally responsible for product recalls due to food contamination (Escherichia coli 2019; Vogt and Dippold 2005). Yarrowia lipolytica is a yeast species belonging to the Ascomycota fungi phylum (like the well-known baker’s and brewer’s yeasts, both from Saccharomyces cerevisiae species). This innocuous yeast can be found in a large range of ecosystems (soils, marine waters, mycorrhizae—symbiotic associations of fungi with roots of plants) and in a variety of foods (particularly in dairy products, including numerous cheeses, and in meat products) (Madzak 2015).
The bacterial cells were cultivate on a solid agar medium containing glucose, meat extract, peptone and NaCl at 30 °C for 24 h. After that, the colonies were picked and suspended in a liquid nutrient medium containing LB and 10% glucose for 24 h in bath shaker at 30 °C, pH 7. Further the cells were suspended in fresh liquid medium, containing LB and 10% glucose and the biomass was used for preparation of disk diffusion method of antibacterial monitoring of tested compounds. Yeast’s cells were cultivated in YPD agar medium, containing peptone, glucose, yeast extract at 25 °C for 24 h. Further the incubation colonies were picked and suspended in a liquid nutrient medium containing the same nutrient composition, without agar and the biomass was used for preparation of disk diffusion method of antifungal monitoring of tested compounds.
The disk diffusion method was used to determine antimicrobial activity of the tested compounds. The principle of the method is based on the use of a disc contains the antibiotic with constant concentration as a control. Antibiotic sensitivity of studied microorganisms is determined by the presence and the width of the clear zone of bacterial growth around the antibiotic disc. The zone diameter is proportional to the sensitivity of the tested microorganism.
The day before the implementation disc diffusion test, the isolated colonies were picked and moved in fresh nutrient medium to grow overnight or to stationary phase. The day of the test, the cultures were diluted to back and grow to mid-log phase, around an OD of 0.5 (Angelova et al. 2012).
Precultures of microbial strains were prepared on a liquid nutrium medium as was described in the methodological section above. Test microorganisms (Bacillus cereus, Escherichia coli and Yarrowia lipolytica) were inoculated in sterile Petri-dishes with agar medium. Quantity of 0.1 mL preculture were taken and placed in the Petri-dish to wet agar surface. The excess of culture was pipetted. Further, the Petri-dishes were dried, using an incubator for 20 min at 30 °C. The filter disks were impregnated with the appropriate antimicrobial agent, using sterile forceps, and placed gently pressed onto the agar surface. After that, the plates were kept for 20 min at room temperature, and then were cultured at 30 °C for 24 h.
The study of antimicrobial activity is realized using above described strains E.Coli (as model G-negative microorganism), Bacillus cerreus 1085 (as model G-positive microorganism) and Yarrowia lipolytica 3344 (as model fungi).
Disk diffusion method was used for determination of antimicrobial activity. Ampicillin (10 mM), Gentamicin (10 mM), fluconazole (25 mM) and l-rimantadine as a standard (10 mM, 30 mM and 60 mM) were used as refers. Newly synthesized compounds were tested for antimicrobial activity at different concentrations: The αNH-protected analogues Fmoc-Gly-Thz-Thz-OH-amantadine 5a and Fmoc-Gly-Thz-Thz-OH-rimantadine 5b at 10 mM and 30 mM and the αNH-deprotected analogues Gly-Thz-amantadine 4c and Gly-Thz-rimantadine 4d at 10 mM, 30 mM and 60 mM.
All tested compounds do not show any activity against model G+ (Bacillus cerreus 1085) and G− (E.Coli) microorganisims. Compound Gly-Thz-rimantadine shows very strong antifungal activity against Yarrowia lipolytica with inhibition zones 32 mm and 39 mm for both tested concentrations, respectively (Fig. 3a, b).
The obtained results reveal that antifungal effect of Gly-Thz-rimantadine is not due to rimantadine or amantadine core but it is probably because of specific conformation of the compound which allows penetration in fungi cell.
Compounds Fmoc-Gly-Thz-Thz-OH-rimantadine, Gly-Thz-amantadine and Fmoc-Gly-Thz-Thz-OH-amantadine do not show any activity against model strains of bacteria Bacillus cereus and Escherichia coli and fungi Yarrowia lipolytica.
Conclusions
Four adamantane derivatives were synthesized and tested for their antiviral and antimicrobial activity. The results reveal that presence of special bulky groups do not leads to improvement of antiviral activity. The rimantadine analogue with thiazole ring showed moderate activity against influenza virus A/Hongkong/68. The remaining compounds have no effect. Gly-Thz-rimantadine shows very good antifungal activity at two tested concentrations (10 mM and 60 mM) against model strain fungi Yarrowia lipolytica. Our results show very specific zone of inhibition of Gly-Thz-rimantadine which is perhaps due to morphological changes of fungi cell but not to complete death of microorganisms. Other compounds have no antifungal activity. All compounds do not show any activity against model strains G+ and G− microorganisms.
References
Angelova T, Rangelova N, Yuryev R, Georgieva N, Müller R (2012) Antibacterial activity of SiO2/hydroxypropyl cellulose hybrid materials containing silver nano particles. Mater Sci Eng C 32:1241–1246. https://doi.org/10.1016/j.msec.2012.03.015
Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K (1999) Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997-1998. Virology 254:115–123. https://doi.org/10.1006/viro.1998.9529
Bennett JE, Dolin R, Martin Blaser M (2015) Principles and practice of infectious diseases, 8th edn. Elsevier, Philadelphia
Bozdaganyan ME, Orekhov PS, Bragazzi NL, Panatto D, Amicizia D, Pechkova E, Nicolini C, Gasparini R (2014) Docking and molecular dynamics (MD) simulations in potential drugs discovery: an application to influenza virus M2 protein. Am J Biochem Biotech 10:180–188. https://doi.org/10.3844/ajbbsp.2014.180.188
Brotchie J (2010) Antidyskinetic actions of amantadine in Parkinson’s disease: are benefits maintained in the long term? Expert Rev Neurother 10:871–873. https://doi.org/10.1586/ern.10.70
Charalampopoulos D, Rastall RA (2009) Prebiotics and probiotics science and technology. Springer, New York
Chen JJ, Swope DM (2007) Pharmacotherapy for Parkinson’s disease. Pharmacotherapy 27(12 Pt 2):161S–173S. https://doi.org/10.1592/phco.27.12part2.161s
Escherichia coli (2019) Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases
Gaiday A, Levandovskiy I, Byler K, Shubina T (2008) Mechanism of influenza a M2 ion-channel inhibition: a docking and QSAR study. In: Computational science—ICCS 2008. 8th international conference, Kraków, Poland, June 23–25, 2008. Proceedings, Part II, pp 360–368. https://doi.org/10.1007/978-3-540-69387-1_40
Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, Deeva E, Yanvarev D, Ivanov A, Smirnova O, Kochetkov S, Chupakhin O, Kukhanova M (2010) Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1, 2, 4-triazine-derived inhibitor of influenza A and B virus replication. Antimicrob Agents Chemother 54:2017–2022. https://doi.org/10.1128/AAC.01186-09
Knorr R, Trzeciak A, Bannwarth W, Gillessen D (1989) New coupling reagents in peptide chemistry. Tetrahedron Lett 30:1927–1930. https://doi.org/10.1016/S0040-4039(00)99616-3
Kolocouris A, Johnson B, Tzitzoglaki C, Gay NC, Busath DD (2013) Amantadine analogs that inhibit Mdck cell infection by influenza a with M2 (S31N). Biophys J 104:277a. https://doi.org/10.1016/j.bpj.2012.11.1555
Long J, Wright E, Molesti E, Temperton N, Barclay W (2015) Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Research. https://doi.org/10.12688/f1000research.6085.2
Madzak C (2015) Yarrowia lipolytica: recent achievements in heterologous protein expression and pathway engineering. Appl Microbiol Biotechnol 99:4559–4577. https://doi.org/10.1007/s00253-015-6624-z
Moorthy HN, Poongavanam NS, Pratheepa V (2014) Viral M2 ion channel protein: a promising target for anti-influenza drug discovery. Mini Rev Med Chem 14:819–830
Rey-Carrizo M, Torres E, Ma C, Barniol-Xicota M, Wang J, Wu Y, Naesens L, DeGrado WF, Lamb RA, Pinto LH, Vázquez S (2013) 3-Azatetracyclo [5.2. 1.15, 8.01, 5] undecane derivatives: from wild-type inhibitors of the M2 ion channel of influenza A virus to derivatives with potent activity against the V27A mutant. J Med Chem 56:9265–9274. https://doi.org/10.1021/jm401340p
Ryan KJ, Ray CG (2004) Sherris medical microbiology, 4th edn. McGraw Hill, New York
Shibnev VA, Garaev TM, Finogenova MP, Shevchenko ES, Burtseva EI (2012) Search for new drugs: some pathways to overcoming drug resistance of influenza a virus to adamantane derivatives. Pharm Chem J. https://doi.org/10.1007/s11094-012-0723-2
Singleton P (1999) Bacteria in biology, biotechnology and medicine, 5th edn. Wiley, Hoboken
Smirnova TD, Danilenko DM, Eropkin M, Deeva EG, Kiselev OI (2011) Influence of rimantadine, ribavirine and triazavirine on influenza A virus replication in human monolayer and lymphoblastoid cell lines. Antibiot Khimioter 56:11–16
Smith PW, Whittington AR, Sollis SL, Howes PD, Taylor NR (1997) Novel inhibitors of influenza sialidases related to zanamivir. Heterocyclic replacements of the glycerol sidechain. Bioorg Med Chem Lett 7:2239–2242. https://doi.org/10.1016/S0960-894X(97)00399-5
Spilovska K, Zemek F, Korabecny J, Nepovimova E, Soukupa O, Windisch M, Kuca K (2016) Adamantane—a lead structure for drugs in clinical practice. Curr Med Chem 23:3245–3266. https://doi.org/10.2174/0929867323666160525114026
Stankova IG, Simeonov MF, Maximova V, Galabov AS, Golovinsky EV (1999) Synthesis and anti-virus activity of some nucleosides analogues. Zeitschrift für Naturforschung C 54:75–83. https://doi.org/10.1515/znc-1999-1-214
Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF (2008) Structural basis for the function and inhibition of an influenza virus proton channel. Nature 451:596–599. https://doi.org/10.1038/nature06528
Tenaillon O, Skurnik D, Picard B, Denamur E (2010) The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. https://doi.org/10.1038/nrmicro2298
Vogt RL, Dippold L (2005) Escherichia coli O157:H7 outbreak associated with consumption of ground beef, June-July 2002. Public Health Rep 120:174–178. https://doi.org/10.1177/003335490512000211
Wang J, Wu Y, Ma C, Fiorin G, Wang J, Pinto LH, Lamb RA, Klein ML, Degrado WF (2013) Structure and inhibition of the drug-resistant S31 N mutant of the M2 ion channel of influenza A virus. Proc Natl Acad Sci USA 110:1315–1320. https://doi.org/10.1073/pnas.1216526110
Zarubaev VZ, Golod EL, Anfimov PM, Shtro AA, Saraev VV, Gavrilov AS, Logvinov AV, Kiselev OI (2010) Synthesis and anti-viral activity of azolo-adamantanes against influenza A virus. Bioorg Med Chem 18:839–848. https://doi.org/10.1016/j.bmc.2009.11.047
Zoidis G, Fytas C, Papanastasiou I, Foscolos GB, Fytas G, Padalko E, De Clercq E, Naesens L, Neyts J, Kolocouris N (2006) Heterocyclic rimantadine analogues with antiviral activity. Bioorg Med Chem 14:3341–3348. https://doi.org/10.1016/j.bmc.2005.12.056
Zoidis G, Kolocouris N, Kelly JM, Prathalingam SR, Naesens L, De Clercq E (2010) Design and synthesis of bioactive adamantanaminoalcohols and adamantanamines. Eur J Med Chem 45:5022–5030. https://doi.org/10.1016/j.ejmech.2010.08.009
Acknowledgements
All strains of microorganisms, included in the Bulgarian National Collection, was kindly provided by the National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC), Sofia, Bulgaria. We are grateful to South-West University “Neofit Rilski”, Blagoevgrad for financial support by Project RPY-A3/19.
Author information
Authors and Affiliations
Contributions
IS and DD contributed to the study conception and design of aimed compounds as well as in the writing, review and editing of the manuscript. Synthesis, isolation and characterization of newly synthesized compounds is realized by IS, KC and RC. Material preparation, data collection and interpretation of antiviral analysis is done by LM and AG. Material preparation, data collection and interpretation of antimicrobial analysis is realized by DM.
Corresponding author
Ethics declarations
Conflict of interest
Authors Ivanka Stankova, Kiril Chuchkov, Radoslav Chayrov, Luchia Mukova, Angel Galabov, Desislava Marinkova and Dancho Danalev declare that they have no conflict of interest. This work is not related to any experiments with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stankova, I., Chuchkov, K., Chayrov, R. et al. Adamantane Derivatives Containing Thiazole Moiety: Synthesis, Antiviral and Antibacterial Activity. Int J Pept Res Ther 26, 1781–1787 (2020). https://doi.org/10.1007/s10989-019-09983-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09983-4