Abstract
Context
Freshwater wetlands, including those in coastal regions, are among the most important, albeit threatened, environments worldwide. Beyond protection, restoration is urgently required to halt the trend of wetland loss. Restoring natural hydrology offers potential to achieve this by landscape-scale rehabilitation of wetland habitat and connectivity for aquatic biodiversity, including freshwater fishes.
Objectives
This study assessed the response of a fish community, across pre-, during and post-restoration periods, to hydrological restoration works within an internationally significant coastal freshwater wetland complex with a history of flow diversion and drainage.
Methods
Biannual sampling of the fish community occurred across five zones of the wetland complex over the pre-, during and post-restoration periods spanning an eight-year timeframe (2012–19).
Results
The study revealed a coastal freshwater wetland harbouring an abundant (179,557 fish caught in this study) and regionally diverse (19 species) fish community, with the catch numerically dominated by native freshwater specialists and diadromous species. Fish community composition and abundance along with species diversity and total abundance responded significantly according to an interaction between zones and the three periods of restoration. Water quality and habitat parameters varied significantly in space and time over the study period, and helps to partially explain the responses of the fish community.
Conclusions
This study provides a practical demonstration on the application of landscape-scale restoration of wetland hydrology and associated rehabilitation of aquatic habitat and connectivity to benefit freshwater fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are among the most important ecosystems in the world (Dudgeon et al. 2006; Vörösmarty et al. 2010; Batzer and Sharitz 2014) as they provide a myriad of benefits including critical ecological services such as carbon sequestration, filtration of water, nutrient cycling, and a variety of habitats and resources along with basin-wide hydrological services (Mitsch and Gosselink 2015; Wu et al. 2019). It has been estimated that, although only representing ≈3% of the world’s surface area, wetlands account for almost half of the global monetary value of ecosystem services (Davidson et al. 2019). Further, wetlands occurring in coastal systems (i.e. at the transition between land and ocean: Kjerfve 1994) deliver 43.1% of the total global monetary value of all wetlands, despite representing less than 10% in terms of area (Davidson et al. 2019). Whilst certain types of coastal wetlands, such as mangroves and coral reefs, are well understood and studied, coastal freshwater wetlands (CFWs or freshwater lagoons sensu Ramsar) have received less attention (Boon 2012; Saintilan et al. 2018).
Coastal freshwater wetlands are distinguished as being transitional and complex ecosystems (Pérez-Ruzafa et al. 2011). They primarily receive freshwater inputs from rainfall, terrestrial runoff, streamflow and groundwater, although some may often experience periods when salinities are elevated (Pérez-Ruzafa et al. 2011, 2019; Herbert et al. 2015). Similarly, there is a continuum of connectivity with nearby ecosystems (i.e. rivers, estuaries and oceans), which can result in the hydrological isolation of CFWs. In this way, CFWs vary dynamically in space and time, and this variation influences habitat suitability for freshwater fishes for which CFWs, like other freshwater wetlands, afford cover, refuge, food resources, and spawning and nursery habitats (Copp 1997; Zeug and Winemiller 2008). Their proximity to marine ecosystems also ensures that CFWs often support critical life stages of diadromous species and, at times, estuarine and marine species. Indeed, CFWs can support a higher fish abundance and biomass compared to nearby riverine, estuarine and marine environments (Gray et al. 2011; de Andrade-Tubino et al. 2020).
Despite their importance to biodiversity and ecosystem services, wetlands are under critical threat (Strayer and Dudgeon 2010; Davidson 2014; Kingsford et al. 2016). It is estimated that 87% of global wetlands have been lost over the past three centuries (70% decline since the year 1900), with the trend in wetland extent and condition continuing to decline (Davidson 2014; Davidson et al. 2018). Coastal lagoons, including CFWs, are particularly at risk given the overlap with global human populations, with the most severe impacts being salinisation and sea-level rise, human development and pollution (cf. eutrophication), as well as hydrological alteration (Barbier et al. 2011; van Dijk et al. 2015; Kingsford et al. 2016). These multiple pressures on coastal lagoons threaten ecological integrity, with wetland-dependent species, including freshwater fishes, being detrimentally impacted. It is therefore unsurprising that one quarter of all wetland-dependent species (including those inhabiting coastal) have been assessed globally as threatened with extinction (Gardner and Finlayson 2018).
Although the consequences of impacts such as sea-level rise have been investigated (Carrasco et al. 2016; Runting et al. 2017), the influences of hydrological alteration in coastal wetlands are comparatively less studied. As with other wetland types, hydrological alterations, such as artificial drainage, diversion of flows, disconnection of tidal influence or detrimental changes in groundwater conditions will likely reduce or eliminate suitable habitat and resources thereby disrupting connectivity across the broader ecosystem. For this reason, hydrological restoration of coastal wetlands presents as an opportunity to reinstate landscape processes and connectivity to aid biodiversity recovery. Specific examples include the reinstatement of tidal influence with coupled benefits to vegetation, fish and waterbird communities in estuarine coastal lagoons (Howe et al. 2010; Boys and Pease 2017; Perillo et al. 2018) and the rehabilitation of CFWs through reinstatement of natural flowpaths and inundation regimes (Boon 2011; Raulings et al. 2011). Ensuring restoration of broader connectivity of CFWs to adjacent ecosystems such as estuaries or even other coastal wetlands is a key strategy in supporting the dispersal of species (especially those with limited dispersal capabilities) and therefore their regional recovery (Gimmi et al. 2011; Verheijen et al. 2018). In acknowledging the dynamism and complexity of CFWs, these landscape-scale restoration efforts must account for multiple influences on hydrology and the nature of hydrological impact (Hua et al. 2016; Zhao et al. 2016).
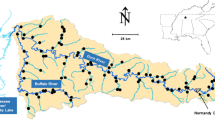
Hydrological restoration works have been recently implemented, triggering landscape-scale changes to inundation patterns and flows, across the Long Swamp Wetland Complex (hereafter, Long Swamp) – a large (≈1100 ha) coastal freshwater wetland system situated within the internationally recognised Glenelg Estuary and Discovery Bay Ramsar site in southwest Victoria, Australia (Fig. 1; Bachmann 2020; DELWP 2017). Prior to this time, for a period of up to eight decades, the combined effects of the diversion of flows through drainage (via two artificial outlets), and the interception of groundwater recharge (through adjacent land-use change) led to a reduction in the duration, extent and frequency of wetland inundation and flows, in turn triggering a process of drying and terrestrialisation across the wetland complex (Bachmann et al. 2018; Bachmann 2020). Guided by the observed impacts of the natural earlier closure of one of the artificial outlets (at White Sands), trial structures were later constructed over 2014 to 2015 to regulate and ultimately block the other artificial outlet (at Nobles Rocks) (Fig. 1). As has been utilised elsewhere, applied historical ecology (e.g. Grossinger et al. 2007; Beller et al. 2016) provided historical context on the ecology and hydrology of Long Swamp to guide the restoration works and identify clear objectives (Bachmann 2020). This restoration work, motivated by the local community and driven by a not-for-profit organisation collaborating with a range of stakeholders (i.e. government agencies, Traditional Owners, local community), aimed to increase wetland inundation and facilitate flows across the wetland complex towards the natural outlet (Bachmann 2020). In spite of the impacts associated with past hydrological change, up until that time the wetland complex continued to maintain a range of freshwater habitats, which are known to support regionally important freshwater fishes (DELWP 2017). It was therefore critical to consider how freshwater fishes would respond to the restoration process and its impacts upon the hydrology of the wetland complex.
Location of the 15 sampling sites (dots) across the Long Swamp Wetland Complex (dark grey) in the lower reaches of the Glenelg River Basin (light grey), southern Australia. Also shown is representative habitat (right three panels) and restored Bully Lake behind the final Nobles Rocks restoration structure (bottom panel)
This study aimed to assess changes in the fish community abundance and composition of Long Swamp in response to rehabilitation through large-scale restoration of hydrological drivers. This was explored through biannual sampling of the fish community across five zones during three periods defined as ‘pre-’, ‘during’ and ‘post-’restoration. It was predicted that: (i) the freshwater fish composition would be diverse in the pre-restoration period, and (ii) substantial changes in the fish community would be experienced both during restoration, due to declines in diadromous species (resulting from the deliberate closure of the artificial outlet), and post-restoration, when greater connectivity and habitat availability would see a shift towards a more homogenous fish community. The outcomes of this study are expected to provide guidance for practitioners planning and implementing landscape-scale wetland rehabilitation projects in other regions.
Materials and methods
Study location and restoration summary
Long Swamp consists of an extended (≈15 km) and generally narrow (but in some areas up to 2–3 km wide) series of freshwater wetlands, impounded by an adjacent coastal sand dune system, in the lower reaches of the Glenelg River Basin (Fig. 1). Combined archaeological and sedimentary data indicate that the wetland complex was mostly estuarine prior to the ocean stabilising nearer to its current elevation a few thousand years ago at which time it transitioned to a predominantly freshwater system (Head 1987, 1988). The wetland complex consists of the permanent, groundwater-fed freshwater Lake Momboeng in the east. Discharges from the lake slowly flow in a westerly direction along an ill-defined water course known as Eel Creek, which is interspersed by freshwater wetlands such as Bully Lake near Nobles Rocks, before reaching the defined lower section of Eel Creek and outflowing into Oxbow Lake and the Glenelg River estuary (Reynolds 2007). The groundwater spring discharges from Lake Momboeng are supplemented along the entire length of the wetland complex by localised rainfall and additional groundwater discharges from the shallow, unconfined tertiary limestone aquifer in the form of springs, seeps and direct groundwater expression (Bachmann 2020). The estuary, Oxbow Lake and (to some extent) Eel Creek are under tidal influence, but the river mouth intermittently closes through a combination of low flows from the Glenelg River, as well as sand accumulation driven by wind and ocean swells (Fig. 2).
Relevant hydrological data across the study area: a water level (m, Australian Height Datum: AHD) in Long Swamp and mean monthly rainfall (Nelson, station number 090059), and b mean daily river flow (ML day−1, black line) and status of river mouth (closed = grey bars) for the lower reaches of the Glenelg River Basin. Water level data provided by in situ loggers (HOBO U20); mean monthly rainfall (Nelson, station number 090059) obtained from the Australian Bureau of Meteorology; mean daily river flow (Glenelg River at Dartmoor, station number 238206) provided by the Victorian Department of Environment, Land, Water and Planning; mouth opening status courtesy of the Estuary Entrance Management Support System, Glenelg Hopkins Catchment Management Authority
Long Swamp has a complex history of hydrological alteration, as the two artificial outlets, present until recently, were established through the coastal dune at White Sands and Nobles Rocks during the 1930s and 1940s (Bachmann 2020). The White Sands artificial outlet naturally closed around 2004–05, whereas more recent regulation and closure of the Nobles Rocks outlet occurred sequentially from 2014 (across three stages) as the focal location for planned hydrological restoration works. The restoration process was informed by a detailed analysis of site history, elevation data (based on Light Detection and Ranging: LiDAR), biological records and predictive inundation modelling, as well as detailed communication and negotiation with Gunditjmara Traditional Owners, the local community and relevant government agencies. Initially, over autumn and winter 2014, temporary geo-fabric sandbag weirs were installed to commence the process of regulating outflows, with the third stage of restoration involving the construction of a more substantial geo-fabric sandbag trial structure during autumn and spring 2015, to effect complete closure of the outlet (Bachmann 2020).
By mid-2015, increases in water level behind the restoration structure resulted in Bully Lake being reinstated as a permanently inundated wetland (Fig. 2). The associated expansion of wetland habitats surrounding Bully Lake led to vegetation shifts up the elevation gradient in response to the change in hydrological regime, and all surface flows have since been re-directed along the original westerly flow path towards the Glenelg River. This has increased the trend in water availability (e.g. greater maximum water level, reduced periods of drying) across wetlands throughout Long Swamp proper, although ephemerality remains a feature in some zones with lower groundwater contributions (e.g. White Sands: Fig. 2). The shallow groundwater aquifer in the vicinity of Nobles Rocks is now being buffered (i.e. the local water table has risen permanently) because of the restoration works to close the outlet, and this change is directly linked to the ongoing retention of freshwater in Bully Lake (Bachmann et al. 2018 and Fig. 2).
Based on when restoration structures were demonstrated to be influencing the hydrology of Bully Lake and the broader movement of freshwater flows within Long Swamp, the study period was temporally segregated into three phases: pre- (before summer 2014–15), during (summer 2014–15 to summer 2015–16) and post-restoration (summer 2015–16 onwards).
Sampling design and protocol
Sampling focused on five zones across Long Swamp: Eel Creek (EC), White Sands (WS), the Middle Section (MS), Bully Lake (BL) behind Nobles Rocks structure, and Lake Momboeng (LM) (the last four zones being part of Long Swamp proper: Fig. 1). Sampling occurred biannually (autumn and spring) in the pre- (three occasions: spring 2012, autumn and spring 2014), during (twice: autumn and spring 2015), and post-restoration periods (eight occasions: autumn and spring 2016, autumn and spring 2017, autumn and spring 2018, autumn and spring 2019). In each zone, three sites were established, which were sampled using four single-wing (3 m long wing, 4 mm mesh, 0.6 m D-shaped entrance) fyke nets deployed overnight amongst all prevailing habitat on each occasion, except when water availability or depth limited the sites that could be sampled or the number of nets that could be deployed (Table 1).
All sampled fish were identified to species (McDowall 1996; Gomon et al. 2008), with native species returned alive to the water at the point of capture and alien species euthanased as per requirements of research permits. Each sampled fish species was then ascribed to the freshwater (generalist and specialist), diadromous, and estuarine and marine (i.e. solely estuarine, estuarine and marine, and estuarine-marine opportunist) functional groups (adapted from Potter et al. 2015; Whiterod et al. 2015).
At each site and during all sampling events, dissolved oxygen concentration (DO: mgL−1), electrical conductivity (EC: µScm−1), pH, and water temperature (°C) were recorded as water quality parameters using a YSI 556 multiprobe (Yellow Springs Institute, Yellow Springs, Ohio, USA). Water depth (m) and aquatic habitat cover (estimated percentage of aquatic plants covering the site) were also recorded as habitat parameters.
Statistical analyses
Fish catch data were converted to catch-per-unit-effort (CPUE: fish net−1 h−1) abundance by dividing fyke net catch data by the soak time in each fyke net. This estimate of CPUE abundance was achieved for each species in each fyke net at each site during each sampling event and used in subsequent analyses. Total CPUE abundance (i.e. summed over all species) and species diversity were also computed.
Hypotheses relating to spatial and temporal variability were investigated using a nested-factorial design. Differences in total CPUE abundance and diversity were analysed by permutational ANOVA, whereas differences in water quality and habitat parameters (hereafter, ‘environmental data’) and in CPUE abundance for functional groups and species were analysed by permutational MANOVA. The design consisted of the fixed factor Period (pre-, during, post-), the random factor Year (2012, 2014–2019) nested within Period, the random factor Season (Spring, Autumn) nested within Year and Period, and the fixed factor Zone (BL, EC, LM, MS, WS) crossed with the above three factors (noting that for the environmental data, BL was excluded from analysis due to that zone being dry in autumn 2014). Analyses were carried out in PRIMER v7, with CPUE abundance data √-transformed, diversity data normalised and EC (cf. environmental data) log-transformed, using a Bray–Curtis dissimilarity measure for CPUE abundance and a Euclidean dissimilarity measure for diversity and environmental data. Probability values were obtained with 9999 permutations of the residuals under a reduced model (because of the nested design: (Anderson and Robinson 2001)) with the significance level set at α = 0.05, including that for a posteriori pairwise comparisons. Patterns of variation in multivariate composition and abundance revealed by PERMANOVA for environmental data, functional groups and species were visualised by canonical discriminant analysis of the principal coordinates (CAP) plots (Anderson and Willis 2003).
Spatial and temporal relationships between fish and environment were further analysed using STATICO (Thioulouse et al. 2004) – a multivariate statistical method for the analysis of series of paired ecological tables. For both the most abundant (hence, ‘representative’) functional groups and species (√-transformed), three tables were produced with the zones as replicates and grouped according to period. Similarly, three environmental tables were produced that included the four water quality and two habitat descriptors. Following the three-step STATICO strategy: (i) a PCA was performed on each pair of functional groups/species and environmental tables, (ii) each pair was linked through co-inertia analysis producing a cross-table, and (iii) partial triadic analysis was used to analyse the series of cross-tables.
Temporal trends in abundance for (the representative) functional groups and species were investigated by min–max autocorrelation factor analysis (MAFA: Zuur et al. 2007) and by dynamic factor analysis (DFA: Zuur et al. 2003a). Using MAFA, the main trends in the data are extracted by producing axes that have maximum autocorrelation with time lag k. The first MAF axis represents the main trend or underlying pattern in the data associated with the highest autocorrelation at lag 1, the second axis has the second highest autocorrelation at lag 2, and so forth. Cross-correlations (or canonical correlations) between variables and trends are then computed and tested for significance. DFA is another multivariate technique used to estimate underlying common trends and effects of explanatory variables in multiple time series. DFA applies a dimension reduction whereby multiple time series are modelled as a linear combination of common trends, explanatory variables, a constant, and a noise component. Differences between MAFA and DFA are: (i) in MAFA the trends are independent and the first MAF trend is the most important one, unlike DFA in which the order of the trends is irrelevant; (ii) explanatory variables can be incorporated into DFA models, whereas in MAFA only correlations between axes and explanatory variables can be calculated; and (iii) DFA allows for comparison of alternative models. Because of their complementary features, MAFA and DFA are often used in conjunction in ecological studies (e.g. Erzini et al. 2005; Ligas et al. 2011; Vilizzi 2012). With both MAFA and DFA, the number of time points (i.e. the seven years of sampling in this study) is constrained to be larger than the number of variables, so that the five fish guilds and the species that were recorded in the highest abundance were retained for analysis. Prior to analysis, CPUE abundance and environmental data were √-transformed and centred. For MAFA, the first two MAF axes were estimated and canonical correlations between the individual variables and the two MAF axes tested for significance (α = 0.05). For DFA, a series of 18 models was fitted, ranging from the simplest (one common trend plus noise) to the most complex (one common trend, all six explanatory variables for the environmental data, plus noise) and with a diagonal covariance matrix (noting that no convergence was achieved with either two common trends or a symmetric positive-definite matrix: see Vilizzi 2012). Akaike’s information criterion (AIC) was then used as a measure of goodness of fit to compare models and select the one providing the lowest AIC value (Zuur et al. 2003b). Analyses were carried out in Brodgar 2.7.5 statistical package (Highland Statistics Ltd, Newburgh, UK) following Woillez et al. (2009).
Results
Catch summary
In total, 179,557 fish across 19 species (17 native, two alien), representing seven functional groups, were caught across Long Swamp (Table 2). Based on raw abundance, the freshwater specialist southern pygmy perch Nannoperca australis was by far the most abundant, followed by diadromous common galaxias Galaxias maculatus, the alien freshwater generalist eastern gambusia Gambusia holbrooki, and the estuarine smallmouth hardyhead Atherinosoma microstoma and Yarra pygmy perch Nannoperca obscura, and with the remaining 14 species accounting for less than 2% of the total catch. Amongst the less commonly encountered species were the estuarine and marine black bream Acanthopagrus butcheri and yelloweye mullet Aldrichetta forsteri, the estuarine-marine opportunist longsnout flounder Ammotretis rostratus, and the alien freshwater generalist goldfish Carassius auratus (all sampled as single individuals). Other species included the freshwater generalist flathead gudgeon Philypnodon grandiceps, the diadromous southern shortfin eel Anguilla australis and tupong Pseudaphritis urvillii, and the estuarine and marine Tamar River goby Afurcagobius tamarensis, western bluespot goby Pseudogobius olorum and lagoon goby Tasmanogobius lasti. Spatially, only four species (i.e. the freshwater specialists little galaxias Galaxiella toourtkoourt, N. australis and N. obscura, and G. maculatus) occurred across all zones, whereas seven species (i.e. the freshwater specialist river blackfish Gadopsis marmoratus, the freshwater generalists Australian smelt Retropinna semoni and C. auratus, and the four estuarine and marine species A. butcheri, A. forsteri, A. rostratus and sea mullet Mugil cephalus) were recorded in only one zone (Table 2). Temporally, most species were recorded across each restoration period with N. australis being the most abundant in each of the periods. At LM and MS, a higher number of species was consistently observed compared to the other zones.
Spatial and temporal variation
For total fish CPUE abundance, there were statistically significant differences among restoration periods depending on zone (Period × Zone interaction: Table 3, Fig. 3a). In the pre-restoration period, there was only a difference between EC and WS, where the lowest and highest fish abundances, respectively, amongst the zones were recorded. During the restoration period, WS was by far the zone with the highest fish abundance and differed significantly from all other zones. In the post-restoration period, fish abundance at EC was significantly lower compared to the other zones. Based on the seven most abundant species: pre-restoration, N. australis was especially abundant at WS and G. maculatus at LM (Fig. 3b); during restoration, N. australis was the most abundant at WS (Fig. 3c); post-restoration, G. maculatus was again the most abundant species at LM, whereas at EC most of the species were caught in lower abundance (Fig. 3d).
a Differences in total CPUE abundance across the five study zones sampled pre-, during and post-restoration. b Differences in CPUE abundance for the most abundant species (codes as in Table 2) sampled across the five study zones in the pre-restoration period. c Same during the restoration period. d Same in the post-restoration period. BL = Bully Lake; EC = Eel Creek; LM = Lake Momboeng; MS = Middle Section; WS = White Sands. Errors bars are ± SE
For water quality and habitat, there were significant differences among periods and among zones (Table 4). Temporally, DO was higher and pH was lower post-restoration relative to pre-restoration period (Fig. 4a). Spatially, the Eel Creek (EC) zone differed from LM and MS due to higher EC and lower DO (Fig. 4b). For both functional groups and species CPUE abundance, there were significant differences among zones (Table 5). At the functional group level (noting that estuarine and marine and estuarine-marine opportunists did not contribute significantly to the patterns), alien freshwater generalists (cf. G. holbrooki) were more abundant at BL, MS and WS, diadromous and freshwater generalists at LM, estuarine-marine and estuarine-marine opportunists at EC, freshwater specialists at WS, and solely estuarine at MS (Fig. 4c). At the species level, A. microstoma and N. obscura were more abundant at MS, G. marmoratus and G. maculatus at LM, and P. grandiceps at EC (Fig. 4d), with all other species not contributing significantly to the patterns.
For both functional groups and species (represented as for the above patterns), fish–environment associations varied according to restoration period. At the group level: pre-restoration, freshwater specialists were associated with higher pH and temperature, solely estuarine species with higher DO, and diadromous species with higher depth (Fig. 5a); during restoration, freshwater specialists were associated with higher EC, pH and temperature and with more cover, whereas diadromous and solely estuarine species with higher DO (Fig. 5c); post-restoration, alien freshwater generalists were associated mainly with higher depth and cover, whereas freshwater specialists and solely estuarine with higher DO (Fig. 5c). At the species level: pre-restoration, G. maculatus was associated with higher depth and N. australis with higher pH and temperature (Fig. 5d); during restoration, N. australis was again associated with higher pH and temperature, and G. maculatus and A. microstoma with higher depth (Fig. 5e); post-restoration, G. maculatus and A. microstoma were still associated with higher depth and G. holbrooki with more cover (Fig. 5f).
Fish-environment association plots for: functional groups (a pre-restoration, b during restoration, and c post-restoration) and species (d pre-restoration, e during restoration, and f post-restoration). Functional group and species codes as in Table 2
Profiles for the five representative functional groups indicated a sharp increase in freshwater specialists during restoration, a progressive increase of alien freshwater generalists (cf. G. holbrooki) throughout the study period (except for the last year of sampling), and an overall decrease in diadromous and solely estuarine species post-restoration following an increase pre-restoration (Fig. 6a). Based on MAFA, the first axis of variation (MAF1) indicated a downward trend from the beginning of the study through to the restoration period, except for the last year of sampling when an increase was present; the second axis (MAF2) indicated a peak during restoration and in the first-year post-restoration (Fig. 6b). The increase in the alien freshwater generalist group was highlighted by the significant negative correlation with MAF1 (Fig. 6c), whereas diadromous and estuarine species were significantly correlated with MAF2 (Fig. 6d). Based on DFA, the lowest AIC value was achieved with a model including the water quality parameters EC and temperature plus depth and cover. The DFA trend confirmed the peak during restoration (Fig. 6e) attributable to the freshwater specialists (Fig. 6f).
a CPUE abundance profiles for the functional groups sampled in the study zone. b Min–max autocorrelation factor (MAF) trends (solid line for Axis 1, dashed line for Axis 2) in functional group abundance during the study period. c Canonical correlations with MAF axis 1. d Canonical correlations with MAF axis 2. Trends associated with significant (α = 0.05) canonical correlations with MAF axis 1 or 2 in black with corresponding functional group marked with an asterisk. Functional group abbreviations as in Table 2
Profiles for the five representative species indicated a sharp increase in N. australis during restoration, a progressive increase throughout the study period (except for the last year of sampling) in G. holbrooki, and an overall decrease in A. microstoma and G. maculatus post-restoration following an increase pre-restoration (Fig. 7a). Based on MAFA, the first axis of variation (MAF1) indicated a downward trend from the beginning of the study through to restoration, except for the last year of sampling when an increase was present; the second axis (MAF2) indicated a peak during restoration and in the first-year post-restoration (Fig. 7b). The increase in G. holbrooki was highlighted by the significant negative correlation with MAF1 (Fig. 7c), whereas A. microstoma was significantly correlated with MAF2 (Fig. 7d). Based on DFA, the lowest AIC value was achieved with a model including the water quality parameters pH and temperature plus depth and cover. The DFA trend confirmed the peak during restoration (Fig. 7e) attributable to N. australis (Fig. 7f).
a CPUE abundance profiles for the representative species sampled in the study zone. b Min–max autocorrelation factor (MAF) trends (solid line for Axis 1, dashed line for Axis 2) in species abundance during the study period. c Canonical correlations with MAF axis 1. d Canonical correlations with MAF axis 2. Trends associated with significant (α = 0.05) canonical correlations with MAF axis 1 or 2 in black with corresponding species marked with an asterisk. Species codes as in Table 2
Discussion
The responses of a fish community to the landscape-scale hydrological restoration of an internationally significant coastal freshwater wetland were investigated in this study. Prior to restoration, the wetland complex already provided an important regional refuge for a diverse range of freshwater fishes but was experiencing a long-term drying trend as a result of past hydrological modifications. Despite the mosaic of permanent (and groundwater-fed) to ephemeral freshwater-dominated and densely vegetated habitats that prevailed, as well as the dynamic intermittent connectivity with the lower Glenelg River and estuary (and ocean), aquatic habitats in portions of the system had declined in extent and were slowly being displaced by terrestrial vegetation communities migrating downslope. Hence, while the newly listed Ramsar site of Long Swamp was found to support important populations of internationally and regionally threatened species, artificial drainage (which is commonly encountered globally in coastal wetlands) and resultant reductions in both freshwater habitat availability and connectivity across the landscape was a key ongoing threat. Thus, as an existing public conservation reserve, it was considered an ideal site for restoration works to reverse the observed drying trend that threatened to undermine those residual aquatic habitat values, and support the expansion and regional recovery of native fish and other dependent wetland species.
It was predicted that temporal responses in freshwater fishes would be linked to pre-defined periods of restoration. Consistently, the total CPUE abundance varied significantly due to a Period × Zone interaction. However, in contrast to predictions, there was no decline in the fish community experienced during the restoration phase, but rather the highest total CPUE abundance was revealed and a post-restoration increasing trend was largely absent. It is evident that the temporal trends were strongly regulated by significant spatial structuring in freshwater fishes (revealed most strongly in terms of functional group and species CPUE abundance and species diversity). Whilst EC had the highest overall species diversity as anticipated given its more variable hydrology and closer proximity to the estuary and ocean ecosystems (Pérez-Ruzafa et al. 2007), the average species diversity (as well as total CPUE abundance) was consistently lower there than in other zones. At the other geographic extent of the wetland complex, the diverse fish community of the permanent (groundwater-fed) lake of the LM zone was significantly differentiated. In Long Swamp proper (e.g. BL, MS, WS), zones were more similar (often due to the consistent presence of G. holbrooki), although the high abundance of N. australis recorded at WS contributed to some differences. Regardless, there was some evidence that spatial variability declined over time in Long Swamp proper supporting the prediction of a more homogenous fish community post-restoration, as a result of reduced direct oceanic connectivity for the upstream (eastern-most) sections of the wetland complex (via the former artificial ocean outlet at Nobles Rocks).
Significant changes in environmental parameters were observed between zones and over time. For instance, EC maintained significantly higher electrical conductivity (and lower dissolved oxygen: DO) than other permanently inundated freshwater zones which, coupled with the estuary connectivity, reflects the hydrological influence of the estuary. However this site was still characteristically ‘fresh’ (i.e. electrical conductivity below 2000µScm−1 during more than half of sampling events, and < 4000µScm−1 on all but one sampling occasion (autumn 2016: 10,540 and 11,800µScm−1 at two EC sites) across the study period, and is therefore less variable in terms of salinity than typical coastal wetlands (Pérez-Ruzafa et al. 2019; van Dijk et al. 2015). As hydrological restoration commenced, there were significant increases in DO across the wetland complex, presumably reflecting greater flow and turnover (i.e. mixing), water level (e.g. less deterioration of water quality), and aquatic plant growth (photosynthesis). Although habitat cover did not change significantly at specific sample points over time, the breakdown of inundated terrestrial vegetation and organic matter at the margins of the wetland areas likely resulted in the significant post-restoration decline in pH.
Whilst both wetted extent and duration of flows increased broadly following hydrological restoration, depth at the sampling sites did not change significantly, except for the BL site. Equally, the wetland area immediately behind the restoration structure (BL), and extending over an area within the zone of influence of the structure, transitioned rapidly from an invading vegetation community dominated by terrestrial (Acacia longifolia, Olearia axillaris and Leucopogon parviflorus) and wet (Leptospermum lanigerum) shrubland to an aquatic herbland (primarily Cycnogeton procerum) with an associated upslope expansion of sedgeland communities (primarily Baumea arthrophylla and B. juncea). These proximal and immediate physical changes to vegetation communities were not consistent across the landscape; but rather moderated gradually with increasing distance from the area of restoration works (Bachmann 2020). Remote sensing of vegetation dynamics associated with restoration (Taddeo and Dronova 2020), is a potential avenue for further exploration to determine changes in habitat availability that influence fish communities across the landscape.
The temporal trends in fish community observed across the study period are suggestive of a lag in the ecological response to hydrological changes caused by restoration works (cf. Thompson et al. 2018). These lagged responses were evident not only in the years post-restoration but also by the fact that the initial (during) impacts were not immediate (e.g. peak in CPUE abundance in 2015). These observed responses reflect a delay in broader water level and flow trends experienced across the wider wetland complex, which are heavily influenced in any given year by climate – because the system as a whole, including the shallow local groundwater aquifer that underpins it, is highly responsive to rainfall. Hence, water level increases and improved flows (i.e. connectivity) across the wetland complex following completion of the restoration structure have not been uniform in space and time. To illustrate this, whilst water levels increased rapidly at BL (behind the restoration structure), wider impacts associated with changes in surrounding groundwater and the rehabilitation of westerly flows from BL (including physical changes to vegetation and flow paths) are likely to take several years to emerge and stabilise, and these effects continue today. As a result, there appears to a landscape legacy in the flow ‘signal’ required for reliable fish movement, caused by a sustained shift in flow direction (and connectivity) across the bulk of Long Swamp toward the EC flowpath, is still developing. Whilst there were some significant changes in water quality and habitat cover in the areas of the wetland that experienced the most dramatic change in static inundation depth, it was also anticipated that there would be a lag in the conditions prevailing across the wetland complex for large areas where hydrological changes (such as flow duration) have been more subtle, and therefore causing a delayed influence on fish communities.
For freshwater coastal wetlands such as Long Swamp, inherent dynamism in wider regional climate and rainfall further influence landscape processes, relating to groundwater (and river) flows and connectivity and, to a lesser extent, the status of the Glenelg River mouth, that will influence fish responses. For example, rainfall was well below average during 2013, 2014 and 2015, before a period of above average rainfall in mid-late 2016, which coincided with the transition into the post-restoration period. Similarly, the river mouth was closed with greater frequency from early 2015 to mid-2016 relative to other times (e.g. open continuously from mid-2016 to late 2018) and thus impaired landscape connectivity is anticipated to have influenced migration into and dispersal within the wetland complex. Therefore, the maintenance of higher water levels and extended duration of flows and connectivity throughout the wetland seen in summer to autumn 2017, which inevitably influenced the observed shift in fish community composition, was the combined result of hydrological restoration and the corresponding natural end to a prolonged dry period – with each of these hydrological drivers likely enhancing the benefit of the other.
Unlike many freshwater systems nowadays (Darwall and Freyhof 2016), Long Swamp was dominated by native freshwater specialists (as opposed to freshwater generalists and alien species), which contributed strongly to the changes experienced over time. Nannoperca australis was widespread and abundant, with unexpected peaks in CPUE abundance experienced in the during-restoration period that were linked higher pH. This finding reflected a single sampling event (spring 2015) in one zone (WS), which was preceded by complete drying and refilling, indicating a capacity for dispersal, recolonisation and rapid population growth. This refilling also triggered the re-establishment of a thick growth of aquatic herbs which likely drove a marked increase in pH that is reflective of a spike in photosynthetic activity. The presence of the globally endangered N. obscura (Whiterod et al. 2019) is encouraging as it may serve as the future source for conservation translocations to re-establish locally extirpated populations in other parts of the species’ range (Wedderburn et al. 2019). In contrast, the globally vulnerable G. toourtkoourt (Coleman et al. 2019), a small aestivating species reliant on ephemeral habitats (Coleman et al. 2017), has shown a decline in CPUE abundance in the wetland complex over all zones and over time. However, it also remains possible, indeed probable based on habitat preferences, that the species has expanded into newly created areas of ephemerality on the edge of the recovering permanent habitats (a zone that was not routinely sampled) and is now less detectable at the standard sampling locations. Hence, this trend remains unclear and targeted future surveys are required to better understand and verify this pattern. The CPUE abundance of the large-bodied G. marmoratus, found only in the LM zone, has remained relatively stable over time, possibly reflecting sustained groundwater inputs and specific deep-water habitats provided almost exclusively in this zone. In the context of this finding, it is also worth noting that the LM zone has experienced no direct hydrological change as a result of being sufficiently distant from, as well as being both at a higher elevation and upstream of, restoration works.
The deliberate closure of the artificial outlet at Nobles Rocks was predicted to disrupt migration into the wetland complex, leading to a decline in diadromous species during restoration before increasing again with enhanced connectivity across the landscape (e.g. broader wetland, Glenelg River estuary, ocean) over time. However, the opposite trend was observed for G. maculatus (e.g. in 2015 and 2016, CPUE abundance remained stable but post-restoration declined subsequently with time). Consistent with the lagged responses mentioned above, the relatively stable G. maculatus CPUE abundance in the years during restoration largely reflected a greater proportion of adults (which persisted in the LM zone) as opposed to migrating juveniles, as the migration flowpath through EC was restricted. Over time, the limited migration and recruitment into the wetland complex by this largely annual species (cf. Egan et al. 2019) eventually led to the predicted declines in G. maculatus CPUE abundance, which were realised in the years post-restoration. In the last year of the study, the number of juveniles contributing to increases in the CPUE abundance of G. maculatus is in line with the shift in flow direction now under way, suggesting that newly sustained flows through Long Swamp and to the ocean via the Glenelg River estuary is beginning to have a post-restoration influence on fish movement and populations.
Consistent with the above finding, other diadromous species including P. urvilli were detected at BL for the first time towards the end of the study period. Equally, four estuarine and marine species made incursions, albeit as single individuals, into the lower reaches of EC during the post-restoration period. It is possible that rarer species that are either present in the Glenelg River estuary (such as pouched lamprey Geotria australis and shorthead lamprey Mordacia mordax) or regionally (e.g. the globally vulnerable Australian mudfish Neochanna cleaveri and climbing galaxias Galaxias brevipinnis) will eventually migrate into the wetland complex as landscape connectivity develops. However, barriers do remain, namely the intermittent closures of the river mouth and culvert structures in the lower reaches of the EC zone (at Beach Road, Nelson), which may constrain the benefits of the landscape hydrological restoration works being fully realised.
The period of hydrological restoration coincided with an increase in alien G. holbrooki at the BL and MS zones. First recorded in the wetland complex in 2012, the species represented a relatively minor component of the fish community prior to restoration. The expansion in rehabilitated wetland habitat (specifically, the persistence of additional shallow waters over the summer and autumn months) is anticipated to have created favourable conditions for the species. Yet, the wetter climatic phase experienced during 2016 and 2017, also naturally and concurrently improved seasonal habitat availability and connectivity across the wetland complex. Hence, as G. holbrooki is an aggressive invader that can achieve rapid population growth in newly established areas (cf. Pyke 2008), it likely would have proliferated over the study period regardless of hydrological restoration. Gambusia holbrooki is capable of significantly impacting small-bodied freshwater fishes such as the pygmy perches (Macdonald et al. 2012; Nicol et al. 2015), but it is unclear how the species is impacting fish communities across the restored wetland complex (in this respect, it is worth noting that N. obscura has shown a parallel expansion in response to restoration, especially at BL). Whilst its removal or control is not feasible in Long Swamp, it has been demonstrated that aggressive interactions between G. holbrooki and native species can be minimised by maintaining or enhancing thick aquatic vegetation (Pyke 2008) and minimising low water events (Macdonald et al. 2012), both of which have been positively influenced and sustained by the restoration process at a landscape-scale throughout Long Swamp.
Conclusions
The restoration of hydrological drivers to rehabilitate freshwater wetlands, including those in coastal regions, occur against a backdrop of dynamic conditions in these highly changeable environments, and responses therefore cannot always be accurately anticipated (Moreno‐Mateos et al. 2015). In many ways, each restoration project is a unique experience and needs to be approached as such, although some key commonalities worth considering have been revealed by this study. Firstly, uncertainty regarding the original character of wetlands can hamper restoration efforts: in this instance, deep exploration of historical reference material and extensive stakeholder engagement was necessary to help define original hydrological conditions and inform the restoration strategy (Bachmann 2020). Secondly, many, often unmanageable, drivers such as the interplay of surface and groundwater inputs, the degree and extent of terrestrialisation of wetland habitats, broader connectivity of flows (e.g. river mouth openings) and the regional climate will characterise and considerably influence the trajectory and nature of restoration.
In acknowledging these complex landscape-scale dynamics and context, realistic expectations of the timeframe (and scope) for restoration are required, as is the commitment to a monitoring regime of sufficient scale (e.g. from years to decades, across the whole system) to understand the state of the system as that trajectory of change unfolds (Simenstad et al. 2006). In the case of Long Swamp and of note, the process of ecological change within the fish community, in response to the wetland complex being rehabilitated through the restoration of hydrological processes and migration patterns five years ago (at the time of writing), appears to be still under way, but it is anticipated that this landscape legacy will be overcome in the future. Finally, short-term detrimental impacts during restoration and the possibility of contributing to unintended consequences should not discourage restoration efforts which (when carefully researched and planned) are almost always universally positive and impart much greater long-term resilience into the ecosystem. The Long Swamp example supports this perspective and demonstrates that with the best available knowledge and planning, and an acceptance of the unknown, landscape-scale restoration of hydrology can provide effective redress for the global loss and degradation of wetlands and biodiversity.
References
Anderson MJ, Robinson J (2001) Permutation tests for linear models. Australian & New Zealand J Statist 43(1):75–88
Anderson MJ, Willis TJ (2003) Cananical analysis of the principal coordinates: A useful method of constrained ordination for ecology. Ecology 84:511–525
Bachmann M (2020) The role of historical sources in the restoration of Long Swamp, Discovery Bay, Victoria. Ecol Manag Restor 21:14–25
Bachmann M, Farrington L, Veale L (2018) Long Swamp Restoration Trial Evaluation Report: 2014–2017. An assessment of site history, restoration works, eco-hydrological response and future management options. Prepared for the Glenelg Hopkins CMA, by Nature Glenelg Trust, Mumbannar, Victoria
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81(2):169–193
Batzer DP, Sharitz RR (2014) Ecology of Freshwater and Estuarine Wetlands. University of California Press, Oakland
Beller EE, Downs PW, Grossinger RM, Orr BK, Salomon MN (2016) From past patterns to future potential: using historical ecology to inform river restoration on an intermittent California river. Landscape Ecol 31(3):581–600
Boon PI (2011) The rehabilitation of coastal wetlands: why small-scale variations in microtopography are critical to success. Australasian Plant Conservation 20(2):9–10
Boon PI (2012) Coastal wetlands of temperate eastern Australia: will Cinderella ever go to the ball? Mar Freshw Res 63(10):845–855
Boys CA, Pease B (2017) Opening the floodgates to the recovery of nektonic assemblages in a temperate coastal wetland. Mar Freshw Res 68(6):1023–1035
Carrasco AR, Ferreira Ó, Roelvink D (2016) Coastal lagoons and rising sea level: a review. Earth Sci Rev 154:356–368
Coleman R, Raadik T, Pettigrove V, Hoffmann A (2017) Taking advantage of adaptations when managing threatened species within variable environments: the case of the dwarf galaxias, Galaxiella pusilla (Teleostei, Galaxiidae). Mar Freshw Res 68(1):175–186
Coleman R, Raadik T, Whiterod N (2019) Galaxiella toourtkoourt. The IUCN Red List of Threatened Species 2019: e.T122904035A123382176
Copp G (1997) Importance of marinas and off-channel water bodies as refuges for young fishes in a regulated lowland river. Regulated Rivers: Research & Management 13(3):303–307
Darwall W, Freyhof J (2016) Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In: Closs G, Krkosek M, Olden J (eds) Conservation of freshwater fishes. Cambridge University Press, Cambridge, pp 1–36
Davidson N, Fluet-Chouinard E, Finlayson C (2018) Global extent and distribution of wetlands: trends and issues. Mar Freshw Res 69(4):620–627
Davidson N, Van Dam A, Finlayson C, McInnes R (2019) Worth of wetlands: revised global monetary values of coastal and inland wetland ecosystem services. Mar Freshw Res 70:1189–1194
Davidson NC (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar Freshw Res 65(10):934–941
de Andrade-Tubino MF, Azevedo MCC, Franco TP, Araújo FG (2020) How are fish assemblages and feeding guilds organized in different tropical coastal systems? Comparisons among oceanic beaches, bays and coastal lagoons. Hydrobiologia 847(2):403–419
DELWP (2017) Glenelg Estuary and Discovery Bay Ramsar Site Management Plan. Department of Environment, Land, Water and Planning, East Melbourne, Victoria
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur‐Richard AH, Soto D, Stiassny ML (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81(2):163–182
Egan EM, Hickford MJ, Schiel DR (2019) Understanding the life histories of amphidromous fish by integrating otolith-derived growth reconstructions, post-larval migrations and reproductive traits. Aquatic Conservation: Marine and Freshwater Ecosystems 29(9):1391–1402
Erzini K, Inejih CA, Stobberup KA (2005) An application of two techniques for the analysis of short, multivariate non-stationary time-series of Mauritanian trawl survey data. ICES J Mar Sci 62(3):353–359
Gardner R, Finlayson M (2018) Global wetland outlook: state of the World’s wetlands and their services to people. The Ramsar Convention Secretariat, Gland, Switzerland
Gimmi U, Lachat T, Bürgi M (2011) Reconstructing the collapse of wetland networks in the Swiss lowlands 1850–2000. Landscape Ecol 26(8):1071–1083
Gomon MF, Bray DJ, Kuiter RH (2008) Fishes of Australia’s Southern Coast. New Holland Publishers, Sydney
Gray CA, Rotherham D, Johnson DD (2011) Consistency of temporal and habitat-related differences among assemblages of fish in coastal lagoons. Estuar Coast Shelf Sci 95(4):401–414
Grossinger RM, Striplen CJ, Askevold RA, Brewster E, Beller EE (2007) Historical landscape ecology of an urbanized California valley: wetlands and woodlands in the Santa Clara Valley. Landscape Ecol 22(1):103–120
Head L (1987) The Holocene prehistory of a coastal wetland system: Discovery Bay, southeastern Australia. Hum Ecol 15(4):435–462
Head L (1988) Holocene vegetation, fire and environmental history of the Discovery Bay region, south-western Victoria. Aust J Ecol 13(1):21–49
Herbert ER, Boon P, Burgin AJ et al (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10):1–43
Howe AJ, Rodríguez JF, Spencer J, MacFarlane GR, Saintilan N (2010) Response of estuarine wetlands to reinstatement of tidal flows. Mar Freshw Res 61(6):702–713
Hua Y, Cui B, He W, Cai Y (2016) Identifying potential restoration areas of freshwater wetlands in a river delta. Ecol Ind 71:438–448
IUCN (2020) The IUCN Red List of Threatened Species. Version 2019–3. International Union for Conservation of Nature and Natural Resources: www.iucnredlist.org. downloaded 19 January 2020
Kingsford RT, Basset A, Jackson L (2016) Wetlands: conservation’s poor cousins. Aquatic Conservation: Marine and Freshwater Ecosystems 26(5):892–916
Kjerfve B (1994) Coastal lagoons. In: Kjerfve B (ed) Coastal Lagoon Processes. Elsevier, Amsterdam, pp 1–8
Ligas A, Sartor P, Colloca F (2011) Trends in population dynamics and fishery of Parapenaeus longirostris and Nephrops norvegicus in the Tyrrhenian Sea (NW Mediterranean): the relative importance of fishery and environmental variables. Mar Ecol 32:25–35
Macdonald JI, Tonkin ZD, Ramsey DS, Kaus AK, King AK, Crook DA (2012) Do invasive eastern gambusia (Gambusia holbrooki) shape wetland fish assemblage structure in south-eastern Australia? Mar Freshw Res 63(8):659–671
McDowall RM (ed) (1996) Freshwater Fishes of South-eastern Australia. Reed Books, Chatswood NSW
Mitsch WJ, Gosselink JG (2015) Wetlands. John Wiley & Sons, New Jersey
Moreno-Mateos D, Meli P, Vara-Rodríguez MI, Aronson J (2015) Ecosystem response to interventions: lessons from restored and created wetland ecosystems. J Appl Ecol 52(6):1528–1537
Nicol S, Haynes TB, Fensham R, Kerezsy A (2015) Quantifying the impact of Gambusia holbrooki on the extinction risk of the critically endangered red-finned blue-eye. Ecosphere 6(3):1–18
Pérez-Ruzafa A, Marcos C, Pérez-Ruzafa IM, Pérez-Marcos M (2011) Coastal lagoons:“transitional ecosystems” between transitional and coastal waters. J Coast Conserv 15(3):369–392
Pérez-Ruzafa A, Mompeán MC, Marcos C (2007) Hydrographic, geomorphologic and fish assemblage relationships in coastal lagoons. Hydrobiologia 577:107–125
Pérez-Ruzafa A, Pérez-Ruzafa IM, Newton A, Marcos C (2019) Coastal lagoons: environmental variability, ecosystem complexity, and goods and services uniformity. In: Wolanski E, Day J, Elliott M, Ramesh R (eds) Coasts and Estuaries. Elsevier, New York, pp 253–276
Perillo G, Wolanski E, Cahoon DR, Hopkinson CS (2018) Coastal Wetlands: An Integrated Ecosystem Approach. Elsevier, Amsterdam
Potter IC, Tweedley JR, Elliott M, Whitfield AK (2015) The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16(2):230–239
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu Rev of Ecolo Evol Sys 39:171–191
Raulings EJ, Morris K, Roache MC, Boon PI (2011) Is hydrological manipulation an effective management tool for rehabilitating chronically flooded, brackish-water wetlands? Freshw Biol 56(11):2347–2369
Reynolds M (2007) Preliminary Hydrological Investigation of Long Swamp. La Trobe University, Melbourne, Nelson
Runting RK, Lovelock CE, Beyer HL, Rhodes JR (2017) Costs and opportunities for preserving coastal wetlands under sea level rise. Conserv Lett 10(1):49–57
Saintilan N, Rogers K, Kelleway J, Ens E, Sloane D (2018) Climate change impacts on the coastal wetlands of Australia. Wetlands 38:1–10
Simenstad C, Reed D, Ford M (2006) When is restoration not?: Incorporating landscape-scale processes to restore self-sustaining ecosystems in coastal wetland restoration. Ecol Eng 26(1):27–39
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J North Am Benthol Soc 29(1):344–358
Taddeo S, Dronova I (2020) Landscape metrics of post-restoration vegetation dynamics in wetland ecosystems. Landscape Ecol 35(2):275–292
Thioulouse J, Simier M, Chessel D (2004) Simultaneous analysis of a sequence of paired ecological tables. Ecology 85:272–283
Thompson RM, King AJ, Kingsford RM, Mac Nally R, Poff NL (2018) Legacies, lags and long-term trends: Effective flow restoration in a changed and changing world. Freshw Biol 63(8):986–995
van Dijk G, Smolders AJ, Loeb R, Bout A, Roelofs JG, Lamers LP (2015) Salinization of coastal freshwater wetlands; effects of constant versus fluctuating salinity on sediment biogeochemistry. Biogeochemistry 126(1–2):71–84
Verheijen BH, Varner DM, Haukos DA (2018) Effects of large-scale wetland loss on network connectivity of the Rainwater Basin. Nebraska Landscape Ecol 33(11):1939–1951
Vilizzi L (2012) Abundance trends in floodplain fish larvae: the role of annual flow characteristics in the absence of overbank flooding. Fundam Appl Limnol 181(3):215–227
Vörösmarty CJ, McIntyre PB, Gessner MO et al (2010) Global threats to human water security and river biodiversity. Nature 467(7315):555–561
Wedderburn S, Whiterod N, Gwinn D (2019) Determining the Status of Yarra Pygmy Perch in the Murray–Darling Basin. Report to the Murray-Darling Basin Authority and the Commonwealth Environmental Water Office. The University of Adelaide and Aquasave–Nature Glenelg Trust, Adelaide,
Whiterod N, Hammer M, Raadik T et al (2019) Nannoperca obscura.The IUCN Red List of Threatened Species 2019: e.T39301A123379189
Whiterod NS, Hammer MP, Vilizzi L (2015) Spatial and temporal variability in fish community structure in Mediterranean climate temporary streams. Fundam Appl Limnol 187(2):135–150
Woillez M, Rivoirard J, Petitgas P (2009) Using min/max autocorrelation factors of survey-based indicators to follow the evolution of fish stocks in time. Aquat Living Resour 22(2):193–200
Wu Y, Zhang G, Rousseau AN (2019) Quantitative assessment on basin-scale hydrological services of wetlands. Science China Earth Sciences:1–13
Zeug S, Winemiller K (2008) Relationships between hydrology, spatial heterogeneity, and fish recruitment dynamics in a temperate floodplain river. River Res Applic 24(1):90–102
Zhao Q, Bai J, Huang L, Gu B, Lu Q, Gao Z (2016) A review of methodologies and success indicators for coastal wetland restoration. Ecol Ind 60:442–452
Zuur AF, Fryer RJ, Jolliffe IT, Dekker R, Beukema JJ (2003a) Estimating common trends in multivariate time series using dynamic factor analysis Environmetrics: The Official Journal of the International Environmetrics Society 14(7):665–685
Zuur AF, Ieno EN, Smith GM (2007) Analysing Ecological Data. Springer, Netherlands
Zuur AF, Tuck ID, Bailey N (2003b) Dynamic factor analysis to estimate common trends in fisheries time series. Canadian Journal of fsheries and Aquatic Sciences 60(5):542–552
Acknowledgements
This study was partially funded by the Glenelg Hopkins Catchment Management Authority (Glenelg Hopkins CMA), which was supplemented by internal funding and volunteer effort from Nature Glenelg Trust. We thank landowners and Parks Victoria for access to some sampling sites. Field assistance was provided by Luke Albury, Caleb Bachmann, Dale Bachmann, Marie Bisson, Travis Brown, Tim Carew, Daniel Deppeler, Tom Ferguson, Ruan Gannon, Dane Handreck, Toni Haywood, Lachie Hetherington, Tom Hiatt, Sheryl Holliday, Lauren Kivisalu, Callum La Spina, Ryan Little, Adam Mattner, Mitchell McMaster, Braden Mitchell, Dan McLeod, Patrick Pickett, Brien Roberts, Dave Ryan, Eddie Saievi, Michelle Sargent, Lu-Wei Spinks, Rose Thompson, Jonathan Tuck, Liam Turner, Rob Veale, Gaye Veale, Elijah Wood and Cory Young. Thanks to Jarred Obst (Glenelg Hopkins CMA) for the provision of information on the status of the Glenelg River mouth. We thank the Editor-in-Chief Jianguo (Jingle) Wu and two anonymous reviewers for constructive feedback on the manuscript. All sampling was conducted in accordance with necessary permits (Victorian Fisheries Authority General Research Permit (RP1172)) and approvals (Wildlife and Small Institutions Animal Ethics Committee Approval (09.19); National Parks Act, Wildlife Act 1975 and Flora and Fauna Guarantee Act 1988 Research Permit (10009076)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Whiterod, N.S., Brown, L., Bachmann, M. et al. Long and lasting: spatial patterns and temporal trends in a fish community responding to landscape-scale hydrological restoration of a coastal freshwater wetland complex. Landscape Ecol 36, 1511–1532 (2021). https://doi.org/10.1007/s10980-021-01219-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01219-5